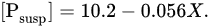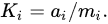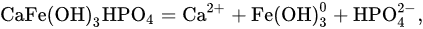
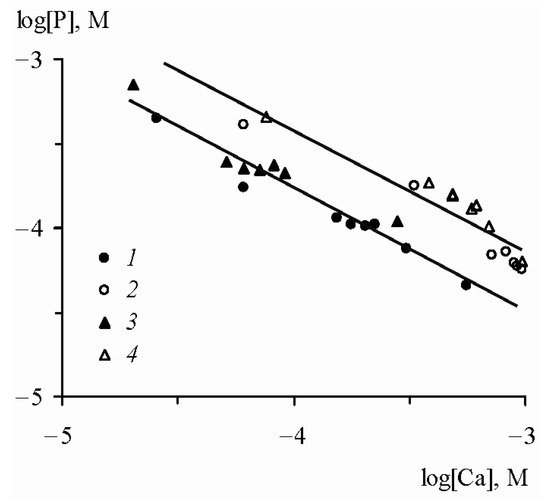
an inverse relationship between the logarithms of the concentrations of mineral phosphorus and calcium is observed (
Figure 2).
Figure 2. Relationship between logarithms of the concentrations of dissolved phosphates and calcium in the interaction of FePO
4 and AlPO
4 with fresh waters
[17]. FePO
4: (
1) water from the Moscow River, (
2) water from the Don River mouth; AlPO
4: (
3) water from the Moscow River, (
4) water from the Don River mouth.
In equilibrium with iron-calcium and aluminum-calcium phosphates, the concentration of dissolved mineral phosphorus is significantly higher than its content in river and ground waters. Therefore, it should be assumed that the presence of these solid phases is possible only where high local concentrations of dissolved phosphorus can be maintained for a long time. These can be bottom sediments with an extremely slow rate of water exchange or soils in which a high concentration of dissolved phosphorus is provided by the destruction of organic matter during the biological cycle. In all other cases, iron-calcium and aluminum-calcium phosphates must be replaced by hydroxides containing adsorbed phosphorus.
At sufficiently high concentrations of phosphates, arising, for example, during the destruction of animal or bacterial biomass, the silicate phosphatization reaction can occur, in which the silicon of the solid phase is replaced by phosphorus from the solution. This process was experimentally studied by us, using rock-forming minerals of different structural types (hornblende, orthoclase, labradorite, kaolinite, and montmorillonite) and background buffer solutions with variable concentrations of orthophosphates (0.25–6.0 mM), maintaining the pH at ~1.8, 3.7, 4.9, 6.8, 7.8, and 8.8
[18][19][20][18,19,20].
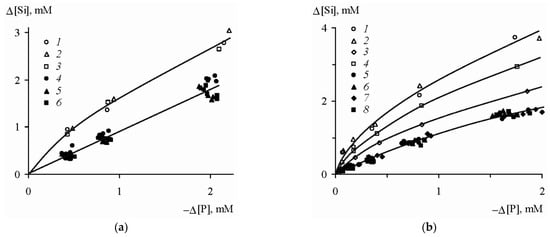
The results of the experiments demonstrated the following features. First, all the samples were characterized by approximately equivalent variations in the concentrations of phosphorus and silicon in the solution in the pH range of 3.7–8.8:
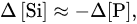
whereas at pH 1.8 the supply of the dissolved silicon was 1.3–2 times higher than the removal of the phosphates (
Figure 3), which was likely explained by the change in the stoichiometry of the phosphatization reaction. Second, the amount of phosphorus absorbed by the silicates was linearly dependent on its final concentration in the solution,
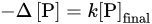
with almost the same values of the proportionality coefficient
k for the different minerals, slightly decreasing with a decrease in the acidity of the medium (
Table 4).
Figure 3. Correlation between variations in the concentrations of phosphorus and silicon in the solution upon phosphotization of silicates
[19]. (
a) pH 1.8: (
1) hornblende, (
2) orthoclase, (
3) labradorite; pH 3.7–8.8: (
4) hornblende, (
5) orthoclase, (
6) labradorite. (
b) pH 1.8: (
1) kaolinite, Glukhovetsk, (
2) the same, Podol’sk, (
3) montmorillonite, Askania, (
4) the same, near Askania; pH 3.7–8.8: (
5) kaolinite, Glukhovetsk, (
6) the same, Podol’sk; (
7) montmorillonite, Askania, (
8) the same, near Askania.
Table 4. Proportionality coefficient
k in Equation (4) as a function of solution pH.
| Granites |
3 |
600 |
| Limestones |
2 |
480 |
| Basalts |
20 |
1300 |
| Carbonaceous shales |
3 |
– |
| Mica schists |
4 |
550 |
| Gypsum-bearing clays |
1 |
– |
Another important factor of phosphorus migration is the climate, which affects the rate of weathering and, consequently, the intensity of the phosphorus mobilization from rocks. For example, the runoff of dissolved phosphorus from the territory of Karelia (NW Russia, temperate climate) due to the pure weathering of crystalline rocks is 2 mg/m
2 yr
[39], while the average intensity of dissolved phosphorus removal during the weathering of crystalline rocks for three catchments in Brazil (humid tropical climate) is 5 times higher: 10 (5–14) mg/m
2 yr
[40].
The presence of areas with slow water exchange in catchments leads to a decrease in the phosphorus content in the waters of the primary hydrographic network. Indeed, Conley et al.
[41] showed an exponential dependence of the concentration of total dissolved phosphorus ([P
total], μg/L) on the relative area of lakes (
S, %) in catchments:
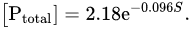
Data on the content of dissolved phosphorus in the waters of the primary hydrographic network, to which catchments with an area ≤50 km
2 were assigned, were collected during observations that lasted for at least a year and were systematized in
[42]. Based on the differences in the sources of phosphorus input, the conditions of runoff formation, and the processes in the catchments, all catchments were divided into four groups: (1) natural (forest) catchments; (2) mixed agricultural–forest catchments with land use <50%; (3) agricultural catchments with land use >50%; (4) urban catchments. For a number of catchments, the group could not be determined due to the lack of the necessary data.
Table 9 shows that the values of the arithmetic and median mean concentrations of mineral and total phosphorus in solution for all the accounted catchments differed several times, indicating the positive asymmetry of the probability distribution functions, which corresponds to the lognormal law. When the small catchments were combined into groups, the asymmetry of the probability distribution functions for the phosphorus concentrations remained. Therefore, the average median concentrations of mineral and total phosphorus, equal to 31 and 95 μg/L, can be considered as the global average concentrations of these forms of dissolved phosphorus in the waters of the primary hydrographic network under modern conditions.
Table 9. The average content and concentration range of dissolved phosphorus (μg/L) in the waters of the primary hydrographic network
[42].
| Component |
Number of Catchments |
Arithmetic Mean |
Median Mean |
Minimum |
Maximum |
| Forest catchments |
| P | min |
67 |
15 |
7 |
0 |
114 |
| P | total |
40 |
58 |
28 |
3 |
806 |
| Mixed agricultural–forest catchments |
| P | min |
23 |
88 |
48 |
6 |
435 |
| P | total |
24 |
142 |
90 |
17 |
589 |
| Agricultural catchments |
| P | min |
34 |
218 |
116 |
2 |
1145 |
| P | total |
26 |
535 |
250 |
7 |
3255 |
| Urban catchments |
| P | min |
6 |
708 |
700 |
101 |
1572 |
| P | total |
5 |
1605 |
1500 |
163 |
3300 |
| All accounted catchments, including catchments of unknown type |
| P | min |
137 |
115 |
31 |
0 |
1572 |
| P | total |
103 |
301 |
95 |
3 |
| 3300 |
|
The lowest concentrations of the dissolved forms of mineral and total phosphorus in the surface waters (7 and 28 μg/L) were observed in the forest landscapes with the least anthropogenic impact. As the economic activity intensified, the phosphorus content increased. For the mixed agricultural–forest catchments, the average concentrations of P
min and P
total were 48 and 90 μg/L, while for the agricultural catchments they increased to 116 and 250 μg/L. An even higher content of dissolved phosphorus was characteristic of the urban catchments, where the average concentrations of P
min and P
total reached 700 and 1500 μg/L. In general, there was a tendency towards an increase in the concentrations of P
min and P
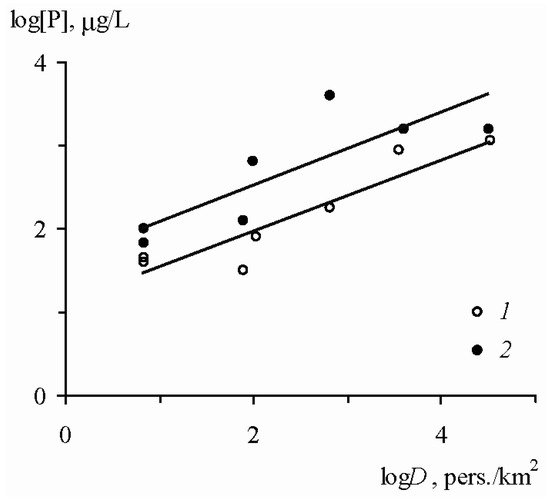
total in the surface waters of the small catchments as the population density increased (
Figure 4).
Figure 4. Relationship between the average annual concentrations of dissolved forms of mineral (
1) and total (
2) phosphorus in the waters of the primary hydrographic network and the population density
D [6].
2.2. Phosphorus in River Waters
The rivers of the world carry into the ocean ~3 Gt/yr of dissolved matter and 15–20 Gt/yr of solid matter. The phosphorus runoff in the form of particulate suspended matter significantly exceeds its dissolved flux, which plays an extremely important role for biota and biogeochemical processes.
2.2.1. Phosphorus of Suspended Matter and Bed Load
The distribution function of the phosphorus content in suspensions of 77 large, medium, and small rivers of the world corresponds to a lognormal law; the arithmetic and geometric mean concentrations of phosphorus equal 1500 and 1000 µg/g
[43], respectively, which is close to the estimate
[44]: 1270 µg/g. About 3% of the phosphorus in river suspended matter is represented by bioavailable soluble/exchangeable forms that can be used by living organisms
[45][46][45,46].
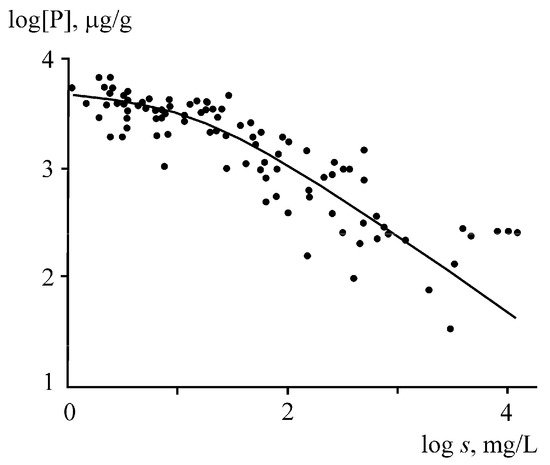
Phosphorus runoff in the form of suspended solids is affected by the ratio of fine and coarse fractions. The phosphorus content in the fine fractions of the suspended matter and bottom sediments of rivers is 2–10 times higher than that in the coarse fractions. The consequence of this is apparently a decrease in the phosphorus concentration in river suspensions, with an increase in the total content of suspended solids (turbidity), which is accompanied by an increase in the proportion of the coarse fractions (
Figure 5). The highest phosphorus concentrations (~4000 μg/g) are observed at a turbidity <20 mg/L, while at a turbidity >100 mg/L, the phosphorus concentration begins to decline sharply, reaching 400 μg/g at a suspended matter content of 1000 mg/L. The same reason leads to an inverse relationship between the concentration of phosphorus in suspended matter and the water discharge or erosion rate. At small discharges during the low-water period, the relative contribution of fine suspensions increases and the phosphorus concentration reaches its maximum values, while in the high-water period, the bulk of suspended solids are represented by coarse suspensions with low phosphorus content.
Figure 5. Relationship between the phosphorus content in suspended matter and the turbidity (
s) of river waters
[43].
The use of fertilizers is accompanied by an immobilization of the phosphorus in the upper soil horizons, which are the main supplier of suspended matter. As a result, the phosphorus content in suspensions denudated from cultivated lands is approximately 2 times higher than in the runoff of solids from forest catchments: 2500 and 1100 μg/g, respectively
[47].
Forests prevent the erosion of the earth’s surface and should reduce phosphorus runoff. This is confirmed by the data for seven small catchments in Southern Quebec
[48], where the relationship between the concentration of suspended phosphorus ([P
susp], μg/L) and the degree of forest coverage of the territory (
X, %) was established:
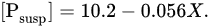
Deforestation should lead to an increase in suspended phosphorus runoff on a global scale, but it is still very difficult to quantify this effect.
It is estimated that 10 to 30% of the river runoff of solid matter is carried in the form of bed load, in which the phosphorus content is on average 800 µg/g
[49]. This value is lower than the phosphorus content in river suspended matter (1000 μg/g), which corresponds to the larger hydraulic size of the bed load.
2.2.2. Dissolved Phosphorus
In
[50][51][50,51], the average annual and long-term average annual data on the content of the dissolved forms of mineral and total phosphorus in 179 rivers of the world (>200 observation stations) are summarized. The arithmetic and median mean concentrations of dissolved mineral phosphorus are 113 and 28 μg/L, respectively, and those of total dissolved phosphorus are equal to 241 and 85 µg/L (
Table 10). The distribution of the concentrations of dissolved phosphorus obeys the lognormal law; therefore, the median mean concentrations are preferred for obtaining average values.
Table 10. The average content of dissolved phosphorus (μg/L) in the river waters of different geographic zones
[50][51][50,51].
| Geographic Zone |
Number of Stations |
Arithmetic Mean |
Median Mean |
| P | min |
Most of the natural factors affecting the content of chemical elements in river water are closely related to the geographic zonality, which determines the features and intensity of the weathering processes, biological activity, etc. In this regard, to analyze the spatial distribution of phosphorus content, all rivers were divided into four groups according to their geographical zones: Arctic region and subarctic zone, temperate zone, humid tropics and subtropics, and dry tropics and subtropics.
The minimum median mean concentrations of the dissolved forms of mineral and total phosphorus were observed in the rivers of the Arctic and subarctic belts, where the biological cycle of elements is much slower and the anthropogenic impact on the aquatic environment is not pronounced, given that there are no extensive sources of phosphorus input associated with agricultural industries, fewer large cities, and, therefore, less industrial and domestic wastewater. The highest median mean concentrations of dissolved mineral phosphorus were characteristic of the rivers of the temperate zone and the zone of dry tropics and subtropics. This is explained by the powerful anthropogenic impact on the nature of these regions, as well as the favorable conditions for the involvement of phosphorus in the biological cycle and its rapid turnover therein. A similar situation is typical for total dissolved phosphorus.
The average annual concentrations of the dissolved forms of mineral and total phosphorus for the rivers of the world correlate with the mineralization of river water (
r = 0.94 and 0.89, respectively) and with the concentration of total nitrogen (
r = 0.81 and 0.79, respectively)
[51]. The cycles of nitrogen and phosphorus are closely linked in the biological cycle of matter and liable to similar anthropogenic changes. Like phosphorus, nitrogen is used in mineral fertilizers and its concentration in wastewater also increases tens and hundreds of times. A rather close correlation between dissolved phosphorus and the mineralization of waters is interesting. It can be assumed that it arises due to an increase in the mineralization of river water in the north–south direction parallel to an increase in the population density, which is an indicator of the anthropogenic load and, in particular, of the intensity of anthropogenic phosphorus sources. Indeed, the average concentrations of mineral and total phosphorus in the river water regularly increase with an increase in the population density in the catchments (
Table 11).
Table 11. The average content of dissolved phosphorus in the water of rivers with different population densities in their catchments
[51].
Table 12. Change in water runoff (
Q, km
3/yr), concentration ([P
min], μg/L) and runoff (
JPmin, thous. t/yr) of dissolved mineral phosphorus in the USSR in 1936–1980
[54].
| Drainage Basin |
1936–1970 |
1970–1980 |
| Q |
[P | min | ] |
JPmin |
Q |
[P | min | ] |
JPmin |
| Arctic Ocean |
2746 |
6.2 |
16.9 |
2849 |
13.5 |
38.5 |
| Arctic and subarctic zones |
7 |
| Pacific Ocean |
866 | 76 |
6 |
| 12.8 |
11.1 |
726 |
27.2 |
19.8 |
Temperate zone |
123 |
132 |
32 |
| Atlantic Ocean |
261 |
24.6 |
6.4 |
235 |
38.2 |
Humid tropics and subtropics |
19 |
39 |
16 |
| 9.0 |
| Aral–Caspian |
381 |
29.4 |
11.2 |
315 |
45.3 |
14.2 |
Dry tropics and subtropics |
33 |
93 |
31 |
| Former USSR territory |
4250 |
10.7 |
Whole world |
182 |
113 |
28 |
| P | total |
| Arctic and subarctic zones |
3 |
235 |
19 |
| Temperate zone |
68 |
247 |
106 |
| Humid tropics and subtropics |
12 |
226 |
91 |
| Dry tropics and subtropics |
14 |
228 |
175 |
| Whole world |
97 |
241 |
85 |
| Observation Zone |
|---|
| Transformation Zone |
River Boundary |
Sea Boundary |
| Severn |
Bristol Channel of the Atlantic Ocean |
P | min |
June 1975 |
0–28 |
16–26 |
610 |
80 |
+10 |
Wastewater inflow |
[65] |
| “ |
February 1976 |
0–32 |
5–28 |
310 |
100 |
+20 |
As above |
“ |
| Clyde |
Irish Sea |
P | min |
April 1973 |
0–32 |
1–8 |
242 |
15 |
−68 |
Sorption on suspended matter |
[66] |
| “ |
March 1974 |
0–32 |
– |
40 |
242 |
~0 |
– |
“ |
| Groundwater of the provinces of temperate climate |
|
| Scheldt |
North Sea |
P | min |
January 1978 |
0–35 |
– |
| Bog landscapes |
191 |
| Mixed forest landscapes |
59.5 |
| 280 |
| Southern taiga landscapes |
57.1 |
| Forest-steppe and steppe landscapes |
75.8 |
| Groundwater of the provinces of tropical and subtropical climate |
|
| 62 |
| Wet savannah landscapes |
29.4 |
| Rainforest landscapes |
65.3 |
| Subtropical forest landscapes |
58.7 |
| ~0 |
| Landscapes of dry savannah and steppes |
127 |
| Groundwater of the provinces of arid climate |
|
| Landscapes of the temperate continental zone: |
|
| – |
[ | 67 | ] |
Rhine–
Meuse |
As above |
P | min |
“ |
0–35 |
– |
465 |
62 |
~0 |
– |
“ |
| Ems |
“ |
P | min |
October 1981 |
14–30 |
– |
– |
40 |
~0 |
– |
[68] |
| Weser |
“ |
P | min |
“ |
0–32 |
– |
520 |
62 |
~0 |
– |
“ |
| Elbe |
“ |
P | min |
“ |
0–31 |
– |
200 |
68 |
~0 |
– |
“ |
| Neva |
Baltic Sea |
P | min |
August 1990 |
0–5 |
0–3 |
13 |
31 |
−82 |
Biological consumption |
[69] |
| P | total |
“ |
0–5 |
0–3 |
36 |
17 |
−49 |
As above |
“ |
| soda waters |
| Keret |
White Sea |
P | total |
July 1992 |
0–23 |
0–18 |
5.0 |
14 |
−62 |
“ |
[70] |
| Pulonga |
As above |
P | total |
“ |
0–25 |
0–5 |
7.0 |
14 |
−48 |
“ |
“ |
| Knyazhaya |
“ |
P | min |
June 2000 |
0–26 |
– |
5.3 |
3.3 |
~0 |
– |
[71] |
| Niva |
“ |
P | total |
July 1992 |
0–20 |
– |
9.0 |
11 |
~0 |
– |
[70] |
| P | min |
“ |
0–20 |
0–10 |
| 20.6 |
| 3.8 |
1.3 |
−33 |
Biological consumption |
“ |
| “ |
June 2000 |
0–27 |
0–10 |
9.7 |
3.5 |
−46 |
As above |
[71] |
| sulphate waters |
63.3 |
| “ |
June 2016 |
0–19 |
0–13 |
5.5 |
12 |
+35 |
Destruction of organic matter |
A.S. | 1 |
| chloride waters |
21.7 |
| Kolvitsa |
“ |
P | min |
June 2000 |
0–28 |
– |
5.7 |
3.3 |
~0 |
– |
[71] |
| Landscapes of the dry tropical zone |
76.7 |
| “ |
June 2016 |
0–21 |
0–13 |
5.4 |
12 |
+35 |
Destruction of organic matter |
A.S. |
| Groundwater of the mountainous areas |
| Stream in the Por’ya Inlet |
| |
| “ |
P | min |
July 2008 |
0–23 |
0–12 |
4.4 |
2.1 |
−21 |
Biological consumption |
[72] |
| High-mountain and mountain–meadow landscapes |
18.0 |
| Kuzreka |
“ |
P | min |
February 2020 |
0–23 |
0–2 |
2.5 |
6.8 |
+70 |
Destruction of organic matter |
A.S. |
| Mountain–forest and mountain–taiga landscapes |
40.1 |
| Indera |
“ |
P | min |
September 2008 |
0–24 |
0–12 |
5.6 |
2.1 |
−25 |
Biological consumption |
[72 |
| Mountain–steppe landscapes |
46.8 |
| ] |
| Chavanga |
“ |
P | min |
“ |
0–24 |
0–12 |
3.0 |
2.1 |
−46 |
As above |
“ |
| Average concentrations |
|
| Strelna |
“ |
P | min |
February 2010 |
0–10 |
0–10 |
4.3 |
2.5 |
−18 |
“ |
A.S. |
| Groundwater of the leaching areas: |
56.9 |
| Onega |
“ |
P | min |
August 2004 |
0–21 |
0–12 |
11 |
4.7 |
−20 |
“ |
“ |
| permafrost |
| “ |
| 22.6 |
| June 2011 |
0–18 |
0–2 |
1.4 |
0.5 |
−43 |
“ |
[ | 73] |
| temperate climate |
98.2 |
| “ |
January 2017 |
0–6 |
– |
6.9 |
12 |
~0 |
– |
| tropical and subtropical climate |
71.8 |
| mountainous areas |
34.9 |
| Groundwater of the areas of continental salinization |
62.6 |
1.8 |
3.7 |
4.9 |
6.8 |
7.8 |
8.8 |
| k |
0.57 |
0.57 |
0.54 |
0.51 |
0.46 |
0.46 |
According to the data in Table 5, the amount of silicon removed from the studied silicates and replaced by phosphates at pH 3.7–8.8 reached 6.5–11.0% of the initial silicon content in the minerals. Even more silicon (up to 9.4–19.9%) entered the solution at pH 1.8, when the process of phosphatization was accompanied by the acid leaching of silicates, which led to an additional release of silicon and violation of equivalence (3). Such large amounts of removed silicon and absorbed phosphorus, which were much higher than the limiting values of the sorption removal of phosphates, definitely indicated the occurrence of a chemical reaction which replaced the silicate with a phosphate mineral.
Table 5. Amount of silicon passed into solution in the experiments on phosphatization of silicates at the maximum initial concentration of phosphates 1
| total |
|---|
| <1 |
21 |
81 |
| 1–10 |
28 |
76 |
| 10–50 |
34 |
157 |
| 50–100 |
39 |
139 |
| 100–200 |
193 |
120 |
| 200–700 |
556 |
598 |
According to
[52], for large rivers there is only a weakly expressed tendency towards an increase in the runoff of dissolved mineral phosphorus with an increase in the population density in the catchments. However, if one takes into account the presence of a directly proportional dependence of phosphorus removal from catchments on the value of specific water discharge, a significant correlation (
r = 0.78) is found between the runoff of dissolved mineral phosphorus and the population density in the catchments, normalized to the specific water discharge.
The intensification of economic activity is accompanied by an increase in the phosphorus content in river runoff. Systematic observations carried out in 1936–1980 on the territory of the USSR showed a noticeable increase in the concentration and runoff of dissolved mineral phosphorus over time (
Table 12). The same was established for other large rivers of the world, including the coastal parts of the sea basins into which these rivers flow
[53].
Environmental protection measures can not only stop the increase in dissolved phosphorus concentrations but also cause its significant decrease. In particular, due to a reduction in the volumes of municipal wastewater and the use of phosphorus-containing detergents, the total phosphorus runoff into Lake Erie decreased from 27.9 to 10.5 thous. t/yr during 1968–1981
[55]. The deepening of wastewater treatment and a decrease in its volume led to a decrease in the phosphorus runoff into the Rhine and Elbe rivers from 51.1 and 20.5 thous. t/yr, respectively, in 1983–1987 to 20.5 and 12.5 thous. t/yr in 1993–1997
[56].
2.3. Phosphorus in Groundwater in the Zone of Active Water Exchange
The surface waters of the primary hydrographic network, rivers and lakes, are in direct hydrodynamic connection with groundwater, which plays an important role in the formation of the chemical composition of the continental runoff of dissolved matter. The greatest influence is exerted by the groundwater of the zone of active water exchange, the discharge of which is the main source of river runoff during the low-water period. The phosphorus content in groundwater is of the same order of magnitude as in the waters of the primary hydrographic network.
The average content of dissolved mineral phosphorus in the groundwater of the Critical Zone varies within the same order of magnitude: from 18 to 191 µg/L (
Table 13). The maximum concentrations (191 and 127 μg P/L) were found in the waters of bog landscapes and steppes (dry savannah). The lowest phosphorus content was observed in the waters of permafrost zones and mountainous areas, in which the fluorine mobilization from rocks is impeded by the low temperature and relatively high water velocity, respectively. The concentrations of dissolved mineral phosphorus in the groundwater in areas of leaching and continental salinization, despite the significant difference in their mineralization, are relatively equal, amounting to 56.9 and 62.6 μg/L, respectively.
Table 13. The content of dissolved phosphorus in groundwater of the Critical Zone
[57].
| Groundwater Type |
[P | min | ], μg/L |
| Groundwater of the provinces of permafrost |
|
| Northern bog landscapes |
26.3 |
| Tundra landscapes |
| Average for groundwater of the Critical Zone |
| 58.0 |
2.4. Integral Characteristic of the Phosphorus River Runoff
2.4.1. Phosphorus Runoff in the Composition of Suspended Matter and Bed Load
The average phosphorus concentrations in the suspended matter and bed load of world rivers are 1000 and 800 μg/g, respectively
[49]. The most detailed calculations of the global runoff of suspended matter give a value of 15.5 Gt/yr
[58][59][58,59]. The mass of the bed load, according to various estimates, is from 10 to 30% of the mass of suspended matter, and 20% can be taken as an average value. Hence, the total continental runoff of suspended and drawn phosphorus is equal to 18.0 Mt/yr. This value is in close agreement with earlier estimates, 16.1
[44] and 20.4
[60] Mt P/yr; however, these did not take into account the runoff of bed load.
Phosphorus is also carried out from land via ice runoff and coastal abrasion. Here, phosphorus is mainly contained in the lithogenic material, while the contribution of its dissolved forms is negligible. The phosphorus content in the products of glacial erosion and coastal abrasion can be taken to equal that in the rocks of the land surface: 690 μg/g. A.P. Lisitsyn
[61] estimates the removal of the solid products of ice runoff and coastal abrasion to be 1.5 and 0.5 Gt/yr, which corresponds to a phosphorus mass of 1.4 Mt/yr.
2.4.2. Dissolved Phosphorus in River Runoff
A detailed assessment of river phosphorus runoff was made in
[50][51][50,51], where data for more than 100 medium and large rivers of the world were used and a correction for the value of the accounted water runoff for each continent was applied (
Table 14). The total volume of continental water runoff in these works was taken to be equal to 38,500 km
3/yr. The more correct value is 41,700 km
3/yr
[27], which would mean that the river runoff of dissolved mineral and total phosphorus increases to 1.6 and 4.5 Mt/yr, respectively.
Table 14. River runoff of dissolved forms of mineral and total phosphorus
[50][51][50,51] 1.
| Continent |
Water Runoff, km | 3 | /yr |
% of Accounted Water Runoff |
Phosphorus Runoff, thous. t/yr |
| P | min |
P | total |
| P | min |
P | total |
| Desorption from suspended matter |
| [ |
| 105 |
| ] |
| “ |
| May 1978 |
| 0–36 |
| 0–25 |
| 28 |
| 6.0 |
| +100 |
| As above |
| “ |
 an inverse relationship between the logarithms of the concentrations of mineral phosphorus and calcium is observed (Figure 2).
an inverse relationship between the logarithms of the concentrations of mineral phosphorus and calcium is observed (Figure 2).
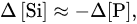 whereas at pH 1.8 the supply of the dissolved silicon was 1.3–2 times higher than the removal of the phosphates (Figure 3), which was likely explained by the change in the stoichiometry of the phosphatization reaction. Second, the amount of phosphorus absorbed by the silicates was linearly dependent on its final concentration in the solution,
whereas at pH 1.8 the supply of the dissolved silicon was 1.3–2 times higher than the removal of the phosphates (Figure 3), which was likely explained by the change in the stoichiometry of the phosphatization reaction. Second, the amount of phosphorus absorbed by the silicates was linearly dependent on its final concentration in the solution,
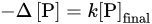 with almost the same values of the proportionality coefficient k for the different minerals, slightly decreasing with a decrease in the acidity of the medium (Table 4).
with almost the same values of the proportionality coefficient k for the different minerals, slightly decreasing with a decrease in the acidity of the medium (Table 4).
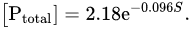 Data on the content of dissolved phosphorus in the waters of the primary hydrographic network, to which catchments with an area ≤50 km2 were assigned, were collected during observations that lasted for at least a year and were systematized in [42]. Based on the differences in the sources of phosphorus input, the conditions of runoff formation, and the processes in the catchments, all catchments were divided into four groups: (1) natural (forest) catchments; (2) mixed agricultural–forest catchments with land use <50%; (3) agricultural catchments with land use >50%; (4) urban catchments. For a number of catchments, the group could not be determined due to the lack of the necessary data.
Table 9 shows that the values of the arithmetic and median mean concentrations of mineral and total phosphorus in solution for all the accounted catchments differed several times, indicating the positive asymmetry of the probability distribution functions, which corresponds to the lognormal law. When the small catchments were combined into groups, the asymmetry of the probability distribution functions for the phosphorus concentrations remained. Therefore, the average median concentrations of mineral and total phosphorus, equal to 31 and 95 μg/L, can be considered as the global average concentrations of these forms of dissolved phosphorus in the waters of the primary hydrographic network under modern conditions.
Data on the content of dissolved phosphorus in the waters of the primary hydrographic network, to which catchments with an area ≤50 km2 were assigned, were collected during observations that lasted for at least a year and were systematized in [42]. Based on the differences in the sources of phosphorus input, the conditions of runoff formation, and the processes in the catchments, all catchments were divided into four groups: (1) natural (forest) catchments; (2) mixed agricultural–forest catchments with land use <50%; (3) agricultural catchments with land use >50%; (4) urban catchments. For a number of catchments, the group could not be determined due to the lack of the necessary data.
Table 9 shows that the values of the arithmetic and median mean concentrations of mineral and total phosphorus in solution for all the accounted catchments differed several times, indicating the positive asymmetry of the probability distribution functions, which corresponds to the lognormal law. When the small catchments were combined into groups, the asymmetry of the probability distribution functions for the phosphorus concentrations remained. Therefore, the average median concentrations of mineral and total phosphorus, equal to 31 and 95 μg/L, can be considered as the global average concentrations of these forms of dissolved phosphorus in the waters of the primary hydrographic network under modern conditions.