Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ying Zhang and Version 2 by Dean Liu.
Hepatocellular carcinoma (HCC) is the third-largest cause of cancer death worldwide, while immunotherapy is rapidly being developed to fight HCC with great potential. Nucleic acid drugs are the most important modulators in HCC immunotherapy.
- biocompatible cationic polymers
- hepatocellular carcinoma
- gene intervention
- immunotherapy
1. mRNA Vaccine for HCC
Beside to minimize the unwanted immune response, BCPs can amplify the immune response and active the immune cells to promote the immunotherapy of HCC. Based on the development of mRNA vaccine in malignant tumors in the past decades, the SARS-CoV-2 mRNA vaccine was fast passed through the clinic trials and played a special role in helping to slow down the COVID-19endemic [1][125]. Therefore, mRNA vaccines are attracting more and more attention compared to conventional vaccines due to their high potency, safety, ability for rapid development, and low cost [2][123]. Currently, various mRNA therapeutics have reached a milestone at high speed in the immuno-oncology field. For a long time, the major interest in the use of mRNA was on the development of cancer vaccines using mRNA encoding tumor antigens to active lymphocytes in vivo. Due to the smart design of both the structures of mRNAs as well as gene carriers that improve their in vivo stability and targeting, the therapeutic potential of mRNA in cancers can be considered as endless. Eventually, a tremendous amount of novel immunotherapeutic approaches concentrates on the use of mRNA beyond their use as the source of tumor antigens [3][126]. Synthetic custom mRNA provides a template for protein with interested sequences, and proteins lay the footstone for a broad range of pharmaceutical applications, including various modalities of cancer immunotherapy. Nucleoside modification and elimination of double-stranded RNA can avoid the immunomodulatory activity of mRNA and increase/prolong the productions of protein therapeutics. With the help of nanoparticle-based formulations that increase transfection efficiency and facilitate lymphocytes or tumor targeting, nucleoside-modified mRNA enables efficient transport of cytokines, chemokines, costimulatory receptors, antigens, or therapeutic antibodies [4][127]. The identification of suitable specific antigens to the tumor for cancer vaccines is still a challenge. Alternative processing of mRNA may offer the potential of a broadened target space and analysis of mRNA processing events in cancer cells with an emphasis on mRNA splicing have been extensive investigated. Of course, many bottlenecks must be overcome for this new avenue to have clinical translation [5][128]. Matsui and co-authors confirmed that Heat Shock Protein 70 (HSP70) was highly expressed in HCC by immunohistochemical staining. They have delivered a HSP70 mRNA to dendritic cell (DC) for treating unresectable or recurrent HCC. The phase I and II trials have verified the safety and efficacy of this DC therapy. Especially, the OS of the DC group was significantly longer than the control groups [6][129].
2. Adaptive Immunotherapy for HCC
Adaptive immunity-based therapy, including checkpoint blockade inhibition, CAR-T, TCR-T, and B cells, are widely developed for the treatment of cancer. TCR-T is extensively developed for cancer therapy, while TCR engages with both tumor intracellular and surface antigenic peptides embedded in the major histocompatibility complex (MHC) comparing to CAR [7][130]. B cells are associated with survival and immunotherapy response, and B-cell-based therapy has been developed recently, while the generation of good practice manufactured B cells is still facing various obstacles [8][9][131,132]. Therefore, there is still a long way to go for both TCR-T and B-cell therapies to clinic applications. More importantly, they are seldom exploited for liver cancer, and biocompatible polymers are not involved much in these therapies currently. Herein, rwesearchers mainly discuss the CAT-T and checkpoint blockade therapies in the domain of adaptive immunotherapy.
2.1. Check-Point Blockade Based Immunotherapies
Agents to inhibit the immune checkpoint receptors or their ligands have revolutionized the treatment of diverse malignant tumors. Many tumors are recognized by adaptive immunity, but these adaptive responses can be blocked by immunosuppressive mechanisms within the tumor. A few novel approaches are striving to expand actions of immunotherapy, which include targeting alterative immune checkpoints [10][133]. Currently, the checkpoints of programmed cell death protein 1(PD-1)/programmed cell death ligand 1(PDL1) and cytotoxic T lymphocyte antigen-4 (CTLA4) are widely exploited for cancer immunotherapy. Drake and co-authors have systematic review the cancer immunotherapy as melanoma, lung and kidney cancer [11][170], they have presented the mechanism of action as to check-point inhibition by specific antibody. As shown in Figure 3, rwesearchers a also plotted out the common mechanisms of action of checkpoint-based cancer immunotherapies with help of the reference [11][170], that also applied to HCC.
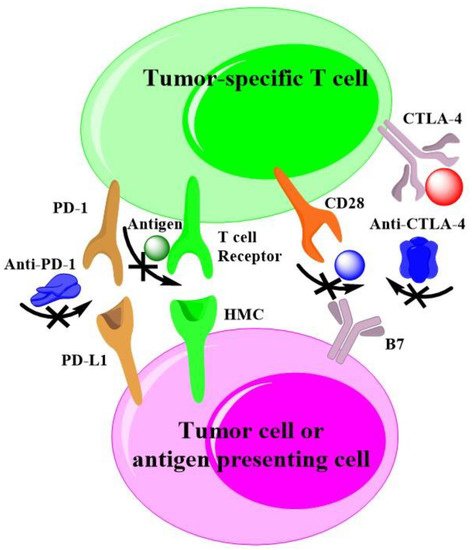
Figure 3. Checkpoint-based cancer immunotherapies.
As early as 1987, Brunet and co-authors validated a protein belonging to the immunoglobulin superfamily, named CTLA-4. It is mainly expressed in activated lymphocytes and contributes to T-cell-mediated cytotoxicity in inducible models of the process to taking part in cell–cell recognition [12][134]. With the recognition of immunotherapy, CTLA-4 has been widely investigated in preclinic and clinic. Alegre and co-authors believe CTLA-4 ligation raised the threshold amount for T-cell activation and arrested T-cell cycle progression [13][135]. Zappasodi and co-author researched the effect of CTLA-4 blockade on the metabolic fitness of intratumor T cells in relation to the glycolytic capacity of cancer cells, finding that CTLA-4 blockade promotes metabolic fitness and the infiltration of immune cells. Notably, the responses of tumor-specific CD8+ T cell are correlated with the phenotypic and functional destabilization of tumor-infiltrating regulatory T cells [14][136]. Yang and co-authors reported CTLA-4 expression in B-1a cells as a substantial function in maintaining self-tolerance by modulating these early-developing B cells that express an enriched repertoire for autoreactivity, showing that the CTLA-4 regulation of B-1a cells is a key immune regulatory mechanism [15][137]. Recently, CTLA-4 has become a major targeting site for cancer therapy. Consequently, monoclonal antibodies (mAbs) and CTLA-4-siRNA were developed to inhibit the expression. Esmaily and co-authors silenced CTLA-4 in tumor-infiltrating T cells by siRNA-loaded chitosan–lactate, which resulted in tumor regression and increased mice survival. Compared to the treatment of tumor-bearing mice with DC vaccine, the combination of siRNA-loaded NPs and DC vaccine exhibited synergistic antitumor effects [16][138]. However, the clinical trial with CTLA-4 inhibitors alone for advanced HCC are disappointed. For example, the administration of tremelimumab in patients with HCC revealed a partial response rate of 17.6% and disease inhibition rate of 76.4% [17][139]. Probably, biocompatible polymers will act as indispensable roles to enhance the immunotherapy with CTLA-4 siRNA.
The PD-1/PDL1 axis is another targeting site for cancers, as well as HCC immunotherapy. PD-1 plays a crucial role in inhibiting immune responses and promoting self-tolerance through regulating the activity of T cells, mediating the apoptosis of antigen-specific T cells and blocking the apoptosis of regulatory T cells. PD-L1 is a trans-membrane protein that is recognized to be a co-inhibitory factor of the immune response. It can bind to PD-1, resulting in reducing the proliferation of PD-1 positive cells, inhibiting their cytokine secretion, and inducing apoptosis. The PD-1/PD-L1 axis is responsible for malignant tumor immune escape and makes a significant effect on cancer therapy [18][140]. To block the PD-1/PD-L1 axis, mAbs are exploited, and some of products have been applied in clinics [19][141]. The clinical efficacy of PD-1 suppression and its ability to augment the effector function of the tumor-specific CD8+ T cells PD-1/PD-L1 inhibition ratio have broadened the opportunities for therapy in patients with previously untreatable malignancies or ineligible to traditional therapies [20][142]. However, a clinical response to anti-PD-1 antibody is rare (<5%) for the treatment of HCC [21][143]. New methods are much urgently needed to promote the efficiency of anti-PD-1/PD-L1 axis therapy, and siRNAs to silence PD-1 or PD-L1 have great promise. Since PD-L1 overexpresses on the surface of tumors while PD-1 is an inhibitory receptor that is expressed by all T cells during activation [22][144], the targeting delivery system often employs the PD-L1 siRNA to break the PD-1/PD-L1 axis [23][145]. Zhu and co-authors developed a nanomaterial encapsulating doxorubicin and PD-L1 siRNA to evaluate its antitumor effects on HCC. The results shown that PD-L1 siRNA significantly inhibited the tumor volume through silenced the expression of PD-L1 in tumor tissue of a H22 tumor-bearing animal model. Additionally, the treatment of PD-L1 siRNA also modulated the populations of matured dendritic cells and cytotoxic T cells in tumor tissues [24][146].
2.2. CAR-T Cell Therapy for HCC
CAR-T cell therapy in early clinical trials revolutionized cancer therapy, especially the patients with pre-B-cell acute lymphoblastic leukemia or B-cell lymphomas. These trials resulted in rapid FDA approvals of anti-CD19 CAR T-cell products for both acute lymphoblastic leukemia and coupled types of B-cell lymphoma [25][147], although CAR-T cell therapy has achieved successful outcomes against hematological malignancies and provided a new perspective for treating solid tumors. However, the low efficacy of CAR-T cells for solid tumors stops its further clinic applications, and it is very urgent to update CAR-T cell therapy for solid tumors [26][27][148,149]. To ouresearchers' best knowledge, there are only two mostly positive trials reports that have used GD2 CARs to target neuroblastoma [28][150] and HER2 CARs for sarcoma [29][151]. The reason is not yet clear, and there is a lot of controversy. The solid tumor landscape presents unique barriers comparing to hematological malignancies. The CAR T cells must successfully traffic to solid tumor sites and successfully infiltrate the stromal elements of solid tumors in order to induce tumor-associated antigen (TAA)-specific cytotoxicity, regardless of antigen loss or heterogeneity. Additionally, T cells must surmount challenges from the microenvironment of solid tumors, such as nutritional depletion, hypoxia, the presence of suppressive cytokines, and suppressive immune cells [30][171].
Nanotechnologies with biocompatible polymers are potential solutions to crack down above matters. Parayath and co-authors delivered CAR mRNA into circulating T cells for transiently reprograming to recognize disease-relevant antigens. In mouse models of prostate cancer and hepatitis B-induced HCC, repeated infusions of these nanomedicine induce sufficient host T cells expressing tumor-specific CARs to cause tumor regression at levels similar to bolus infusions of ex vivo engineered lymphocytes [31][172]. Moffett and co-authors have developed PGA based polymers to deliver mRNA for cancer treatment and demonstrated CAR-programmed T-cells with appropriately designed mRNA nanoparticles can transiently program gene expression to improve their therapeutic potential [32][173]. Actually, smart biodegradable polymers have the potential to overcome the matter confronted the CAR-T therapy in solid tumor. A tumor microenvironment imposes barriers to the passive diffusion of CAR-T mRNA, which renders tumor penetration an unresolved obstacle to an effective active of T cells, while the tumor penetrated polymeric nanocomposites can enhance the trafficking of drugs [33][174], as well as applied for CAR-T mRNA. Hypoxia plays a crucial role in cancer progression, immune editing, and drug response, which often results in tumors escaping from immunosurveillance and CAR-T cell-mediated cytotoxicity [34][175]. Nguyen and co-authors have demonstrated that oxygen delivery through polymeric microcapsules is dependent on multiple parameters, such as polymeric shell, the shell thickness, the pressure gradient across the shell, and oil layer between the polymeric shell and the gas core [35][176]. These polymeric microcapsules have chance to promote the efficacy of the CAR-T mRNA for solid tumor. Of course, CAR T cell therapy has many challenges, such as cytokine release syndrome and neurotoxicity during treating leukemia and lymphoma [36][177]. As shown in Figure 4, rwesearchers h have drawn a scheme with help of the reference to interpret the side effect [36][177], which should be addressed when gene intervention-based CAR T cell therapy is developed in HCC.
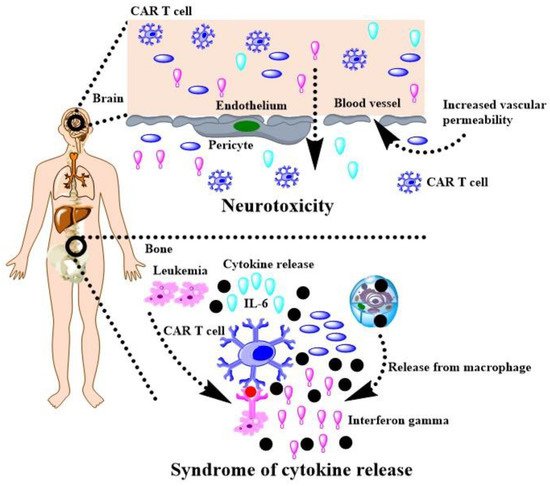
Figure 4. CAR T cell therapy for cancers and its challenges.
3. Innate Immunotherapy for HCC
Beside the adoptive immunotherapy, innate immunotherapy also has bright future for HCC treatment, especially the therapies based on natural killer (NK) cells, macrophages, and neutrophils.
So far, T cells-based cancer immunotherapies, including immunological checkpoint blockade and adoptive cellular therapy, have attracted the main attentions of immunotherapies. However, NK cells are receiving renewed interest recently since they present the considerable advantages of not relying on antigen specificity [37][178]. Several groups have successfully developed NK cell functions directed against glioblastoma [38][152], neuroblastoma [39][153], lung cancer [40][154]. Compelling evidence suggests that NK cells play an irreplaceable role in the immune function of the liver and immunotherapy against HCC, indicating that NK cells might be an ideal target to prevent HCC [41][155]. NK cells are essential components of innate immunity against tumor and vary in phenotype, and the functions have been described in HCC patients, who show disruption of NK activating receptor/ligand axis. The CAR-engineered NK cells provide unique opportunities to create CAR-NK with multiple specificities with potentially less adverse effects [42][156]. Nath and co-authors have verified that NK Cell recruitment and activation are regulated by CD47 expression in the tumor microenvironment [43][157], which make it possible to treat cancers with CD47 mRNA targeting delivery to NK cells. Au and co-authors have developed tri-specific natural killer cell nano-engagers for targeted chemoimmunotherapy, which employed biodegradable poly(ethylene glycol)-block-poly(lactide-co-glycolide) (PEG-PLGA) to co-deliver anti-human EGFR antibody, anti-CD16, and anti-4-1BB to treat B16F10 tumor-bearing mice [44][158]. It has demonstrated that anti-cancer activities of NK-92 cell line are excellent in clinical trials. While the clinical efficacy of NK-92 cells has not reached their full potential because of reduced immune functions compared to primary NK cells. Enhancements of NK-92 functions currently rely on gene delivery (including mRNA and plasmid DNA) with limited efficiencies. To enable precise genetic modifications, CRISPR genome engineering platform for NK-92 based on the nucleofection of CAS9 ribonucleoprotein was developed [45][159]. Furthermore, polymer-stabilized CAS9 nanoparticles and modified repair templates increase genome editing efficiency to active the functions of NK cells [46][160]. Based on the crucial role of NK cell in the therapy of HCC, BCPs for gene delivery (including plasmid DNA, mRNA, miRNA, siRNA, and CRISPR/CAS9 mRNA) to NK cells to fight HCC show bright future.
Macrophages have commonly been categorized into M1 or M2 polarized phenotypes. Pro-inflammation M1 classically activated by IFN-γ or lipopolysaccharide [47][161]. The M1-polarized macrophages secrete IL-6, TNF-α, and other tumor-inhibition cytokines. Immunosuppressive M2 alternatively activated by interleukin IL-13 or IL-4. The M2-polarized macrophages secrete alternative macrophage activation-associated chemokines and promoting angiogenesis. Tumor-associated macrophages (TAMs) promote carcinogenesis by stimulating angiogenesis, migration, invasion, and metastasis [48][162]. TAMs are abundant in the tumor microenvironment of HCC, and better understanding of tumor associated macrophages would allow for the development of novel macrophage-targeting immunotherapies [49][163]. Although it is still controversy the role of M1 in the development of HCC, most target is to polarize the macrophage from M2 to M1 in tumor environment. For example, IL-37 was drugged to suppresses HCC growth through inhibiting M2 polarization of tumor-associated macrophages [50][164]. At this juncture, gene delivery to polarize M2 to M1 in tumor environment would be especially important. Poly(glutamic acid) was used to targeted deliver mRNA to TAMs and then reprogrammed them toward an M1 phenotype, which could thwart their pro-cancer activities and unleash antitumor immunity [51][165]. Sharma and co-authors have employed single cell RNA sequencing to extensively analyze the cellular landscape of human liver from development to HCC, which provide novel targets for interventions in HCC [52][166]. Based on the extensive investigations of TAM biology, various gene therapeutics (including mRNA, siRNA, and miRNA) are ready for reprograming M2 to M1 in the HCC tumor environment if smart and safety polymeric carriers are available.
Neutrophils are the most abundant white blood cells in blood, as well as constitute a significant part of the tumor microenvironment. Neutrophils play major roles associated with inflammation and are actively involved in cancer progression and metastasis [53][54][167,179]. The ratio of circulating neutrophil-to-lymphocyte as a robust biomarker represents clinical outcome in various cancers. The phenotypes of tumor-associated neutrophil (TAN) can predict cancer development and progression. Various treatments on TANs obviously affect therapeutic efficacy [55][168]. Neutrophils have a significant impact on the tumor microenvironment through cytokines and chemokines secreted by TANs, which influence inflammatory cell recruitment and activation. Moreover, products generated by neutrophils, such as proteinases and reactive oxygen species, have specific roles in regulating cancer cell proliferation, angiogenesis, and metastasis. Therefore, TANs targeting as a tool of antitumor therapy is reliable [56][180]. Although miR-223 was targeted into neutrophils to enhance the clearance of infectious diseases [57][169] and nanoparticle targeting of adherent neutrophils to prevent vascular inflammation with more and more attention to the neutrophils exploiting in HCC therapy [58][181]. Therefore, the demands of BCPs for gene delivery to neutrophils to fight HCC are about to be expanded.
4. Intervention of Oncogenes to Modulate Tumor Immune Microenvironment
TIME often decides the tumor progressive and their response to immunotherapy [19][141]. Anti-PD1/PDL1 therapy shows bright future in the treatment of HCC while it is only response <15% patients because of the harsh tumor environment. Fortunately, intervention of oncogenes can steadily modulate TIME. Zhao and co-authors have validated PTEN mutations resulted in immunosuppressive in glioblastoma based on genomic and transcriptomic analysis [59][182], Triulzi and co-authors investigated the correction between HER2 activity and TIME, and the results shown that activated HER2 oncogene modulates recruitment and activation of tumor infiltrating immune cells [60][183]. Actually, the above oncogenes also involved in HCC and can be developed for HCC immunotherapy [61][62][184,185]. Of course, aiming at the host cell of target oncogene is the next step for the modulation of TIME to boost HCC immunotherapy. Joyce group has systematic reviewed the therapeutic targeting of the tumor microenvironment recently [63][186]. They have summarized the most advanced tumor microenvironment associated therapies, discussed the current challenges, and presented future perspectives in this evolving field. As shown in Figure 5, rwesearchers have plotted out the immune cells in tumor environment referring their work, which can be exploited as target for HCC immunotherapy. In this article, researchers hwe have reminded immunotherapies of some other tumors via gene delivery with BCPs because they are good references for HCC. For example, the modulations of KRAS in lung cancer and MYC in HCC are useful for TIME-positive changes for immunotherapy, while the process of target delivery by BCPs is not big different, which are just variational organs and corresponding physiological characteristics. However, the malignant tumors still have more common properties, and BCPs can be modified with respective ligands to achieve targeting (e.g., glycyrrhizic acid for HCC while folic acid for lung cancer). Therefore, the success applications of BCPs in other tumors can accelerate the research of HCC immunotherapy with nucleic acid delivery by BCPs.
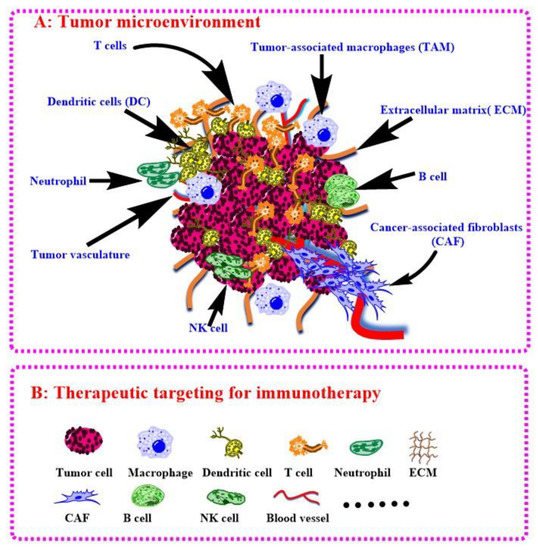
Figure 5. Tumor microenvironment (A) and therapeutic targeting for immunotherapy (B).
The MYC gene is widely investigated in HCC, and its high expression can worsen the TIME, which is not conducive to anti-PD1 therapy. RWesearchers believe that MYC gene inhibitor (MYCi)-based drugs can promote the responsiveness of anti-PD1/PDL1 treatment mainly for the following reasons. First, MYC gene directly or indirectly regulates about 15% of human genes [64][65][187,188], many of which are on the joints of immune-related signaling pathway. Second, MYC gene can regulate immune-related signaling pathways and ameliorate TIME. Casey and co-authors found that the MYC gene regulates the expression of CD47, PD-L1, and genes associated with immune signaling pathways, which ultimately makes cancer patients resistant to PD1 treatment [66][189]. Third, anti-PD1/PDL1 therapy was found to be promoted after the use of MYCi to block MYC gene expression. Han and co-authors screened a MYCi and found MYCi increased the responsiveness of anti-PD1 therapy by inhibiting MYC gene expression [67][190]. There are a lot of investigations on the activation and inhibition of MYC gene, which makes it easy for us to choose gene drugs. In this case, it is feasible to use MYCi and PDL1-siRNA as drug to target tumors for combination therapy. MYC gene is closely related to tumor development and treatment, and it is regarded as the most promising drug target to promote HCC immunotherapy because MYC is disordered protein and lack of available drug identification site. Looking for drugs acting on MYC protein has been a major problem in drug research and development, and MYC gene regulation has been widely studied in order to develop reliable gene drugs. Ma and co-authors showed that lncRNA HOTAIR activates MYC gene expression through negative regulation miRNA-130a [68][191]. Shigeyasu and Cho found that PVT1 lncRNA activates MYC gene expression [69][70][192,193]. Yu and co-author found that circBIRC6 can positively regulate MYC gene expression [71][194]. In the case of MYC gene inhibition, Tai and co-authors found that miR-342-3p inhibits MYC gene activity by inhibiting the expression of E2F1[72][195]. Weissmiller and co-authors found that the SMARCB1 gene directly inhibits MYC gene expression [73][196]. The above investigations have verified that TIME regulation by MYCi can promote anti-PD1/PDL1 treatment. At this juncture, the delivery system become especially important and biocompatible polymeric gene carriers will play an irreplaceable role for immunotherapy of HCC.
5. Intervention of Metabolism to Modulate Tumor Immune Microenvironment
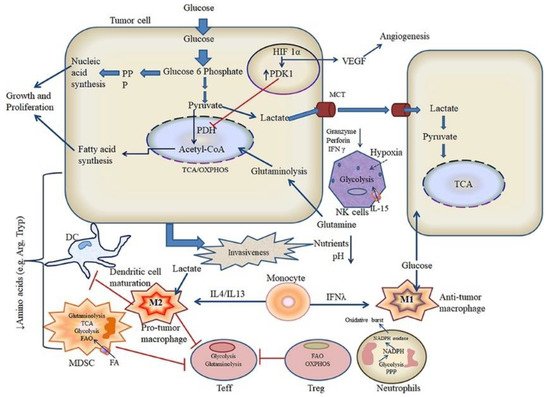
The microenvironment in cancerous tissues is immunosuppressive, whereas the microenvironment of tissues affected prognosis of immunotherapy. Although these opposing immunological states, the metabolic states in the tumor microenvironments and inflammatory diseases are similar, which show elevated levels of metabolic by-products while have low levels of nutrients compared with normal tissues. A clear understanding of the metabolic signature of HCC will enable therapeutic intervention aimed at reprograming the bioavailability of metabolites and modulating the dysregulated immunological state, promoting the immunotherapy [74][197]. As discussed above, reprograming of TAMs was widely developed for HCC therapy. Recent investigations have indicated that metabolism profiles manipulate phenotypes and functions of macrophages. On the contrary, polarization can trigger metabolic shifts in macrophages. Those discovery implicate a special role of metabolism in TAMs, and it can be target for the promotion of immunotherapy [75][198]. The research of immune metabolism has revealed that metabolic changes can result in anti-cancer immunity. Correspondingly, combination therapies with metabolic inhibitors and antibodies of immune checkpoint blockade have shown exciting results. The Rathmell lab developed strategies to shift immune cell metabolism to tune TIME, and finally to enhance immunotherapy [76][199]. Regulatory T cells (Tregs) are a subset of T cells that contribute to immunosuppressive effects in tumor microenvironment, which can promote differentiation, proliferation, secretion of immunosuppressive factors, and chemotactic recruitment of Tregs to play crucial role in immunosuppression in tumor tissues. The cell metabolism reprogramming is relative to the functional effects on Tregs. Therefore, it’s important to well understand the role of cell metabolism on the TIME for HCC immunotherapy [77][200]. The knowledge from extensive research in immune metabolism shows that targeting metabolism could help to enhance antitumor immunity [78][201]. The Locasale lab developed a computational pipeline to study metabolic programs in single cells to define the intratumor metabolic landscape. They found the expression of both glycolytic and mitochondrial network strongly correlates with hypoxia in all cell types, especially the immune cells [79][202]. Metabolic pathways could modulate the TIME and mitochondrial metabolism, which are an attractive target for cancer immunotherapy. Rosner lab have verified that BTB and CNC homology1 targets mitochondrial metabolism [80][203]. Glycolysis level correlates with immune activity in TIME, while the systematic investigation of the relevance between tumor glycolysis and tumor immunity in various tumor remains scarce. Jiang and co-authors have found glycolytic activity enhances PD-L1 expression on tumor cells, and subsequently promotes the response of anti-PD-1/PD-L1 immunotherapy [81][204]. Targeted delivery of therapeutics to mitochondria remains a great challenge due to their location in the sub-cellular compartment and complexity of the intracellular environment. Jiang and co-authors have reported a class of mitochondrion-targeted liposomal delivery carriers, which exhibits about 3.7-fold higher mitochondrion-targeted delivery efficacy than current triphenylphosphonium [82][205]. Metabolism regulation of tumor and simultaneously modulating the TIME to perform immune attack are significant for cancer prevention. Liu and co-authors have developed a novel drug vector to inhibit glycolysis of cancer cells and mitigate the immunosuppressive microenvironment [83][206]. Chaudhary and co-authors have reviewed recent literatures on metabolic reprogramming and associated signaling pathways that mediate crosstalk of tumor with immune cells [84][207]. As shown in Figure 6, they have provided a scheme as to metabolic crosstalk of tumor and immune cells in tumor microenvironment. Although they mainly discussed in oral squamous cell carcinoma, while it’s a good reference for HCC.