Electrospinning has become a very popular technique for the fabrication of nanofibers due to its low cost and simple handling. Nanofiber materials have found utilization in many areas such as medicine, sensors, batteries, etc. In catalysis, these materials also present important advantages, since they present a low resistance to internal diffusion and a high surface area to volume ratio. These advantages are mainly due to the diameter–length proportion. A bibliographic analysis on the applications of electrospun nanofibers in catalysis shows that there are two important groups of catalysts that are being investigated, based on TiO2 and in carbon materials. The main applications found are in photo- and in electro-catalysis.
1. Introduction
During the last decade, new synthesis methods have been developed for nanomaterials, i.e., those in which their shape and molecular composition (or both) at a nanometer scale can be controlled. In this manner, the availability of nanomaterials with a wide range of chemical and physical properties has increased exponentially during recent years. These materials, given their extraordinary properties, have great potential application in fields such as medicine, electronics, sensors and catalysis, among others. The terms nanotubes, nanowires and nanofibers are commonly used to refer to those materials that present a very high length to width ratio with respect to nanotubes and nanorods, especially in the case of nanowires
[1][2][1,2]. The applications and synthesis methods for nanotubes and nanofibers are significantly different, and the present study will only focus on the applications of nanofibers in catalysis.
Nanofibers have several promising characteristics, such as a very large surface area to volume ratio, quite high mechanical performance and flexibility in surface functionalities and composition, in comparison with other materials
[2][3][4][2,3,4] such as pellets, monolits or powers. Subsequently, they find new and novel applications in several fields, and several synthesis methods have been described, such as self-assembly, phase separation drawing processing, template-assisted synthesis or solvent casting
[5][6][7][5,6,7]. Among all the described methods, the most simple and versatile is the electrospinning method, being subsequently the most commonly used for a majority of applications
[8][9][10][11][8,9,10,11]. In this technique, an electrostatic field is applied to a conducting fluid which is usually a polymer solution. When this electrostatic field is strong enough to overcome the surface tension of the fluid, the droplet at the tip of the spinneret becomes unstable and a tiny jet is ejected. The jet stream is collected, and by this way the fibers are obtained. A quick search in a database, such a Scopus in November 2021, shows that with the key words electrospinning and fibers in the title, keywords or abstract, almost 23,000 entries are found, published during the last two decades, demonstrating the high number of applications that are being developed for electrospun fibers, being around 4100 of those entries related with catalysts or catalysis. These data indicate that most of the applications of nanofibers are for nanomedicine, sensors and batteries, and catalysis is also a promising application for them. The software VOSViewer (Centre for Science and Technology Studies, Leiden University, The Netherlands) was used to generate a multi-dimensional map that groups bibliometric metadata into closely related clusters to analyze the most-cited entries (limited to 200) related to catalysis (
Figure 1). One cluster (green), with 14 items, includes titanium oxide and photocatalysis and electrochemistry; another cluster (blue) with 11 items includes carbon nanofibers, lithium-ion batteries, electric batteries and secondary batteries; whereas the yellow cluster with 10 items includes nanocomposites, membranes and polyacrylonitrile. Then, there is one more cluster (red), with 13 items, including drug delivery, tissue engineering and biocompatibility, indicating that one of the main applications of electrospun fibers is in medicine, as has been already mentioned
[1].
In catalysis, the fiber-based materials present several advantages with respect to other materials for several reactions and have been used as supports for many commercial catalytic processes. These materials present a low resistance to internal diffusion and quite a high surface area to volume ratio, due to the diameter–length proportion. Some years ago, Reichelt et al.
[12] reviewed the use of ceramic, metal and glass fiber materials as catalytic supports for several metal- and oxide-based active phases. They concluded that the fibers presented superior catalytic properties in terms of mass transfer and pressure drop, although this also depended on the reactor design. Although nanofibers and fibers may exhibit different properties due to the different diameter range considered, it is expected that nanofibers retain many of the advantages of conventional fibers and will present additional ones due to their nanostructure. As electrospinning equipment has become very popular in the last decade, many catalytic applications of electrospun nanofibers have been developed as shown by the bibliographic analysis results (
Figure 1). Some review papers focus on the synthesis and characterization of nano- and microfibers
[13][14][13,14] that can be used as catalysts themselves or as catalytic supports.
2. Applications of Electrospun Nanofibers in Catalysis
2.1. Carbon Fiber Applications
Table 1 summarizes the main applications of advantages of
carbon nanofibers (CNFs)CNFs in catalysis.
Table 1.
Main advantages and applications of CNFs.
| Main Properties of Carbon Materials as Catalytic Supports |
Main Applications of CNFs |
Advantages of CNFs in Electrocatalysis & Catalysis |
| Abundant source |
Electrochemical energy storage |
Do not agglomerate |
| High surface area |
Biosensing devices |
Blinder-free |
| Mechanical stability |
Drug delivery |
High stability |
| |
Catalysis |
Good Dispersion and isolation of active species |
| |
|
Prevent active phase oxidation |
2.2. TiO2 Fibers Applications
It is well-known that titanium oxide is widely used as photocatalyst for many environmental and energy applications. TiO
2 oxide is also used in catalysis
[15][16][17][47,48,49], for processes such as the selective reduction of nitrogen oxides
[18][50] and has found other applications such as in white pigment, as a food additive and as a component of sensors and other devices. These important applications underline the interest in the development of TiO
2-based materials. It has been demonstrated that the performance is improved when primary TiO
2 particle sizes are at the nanoscale
[15][16][17][47,48,49], but, unfortunately, these small sizes create several disadvantages, such as the presence of suspended nanoparticles when the photocatalyst is used in a liquid effluent; thus, several works can be found that describe the immobilization of TiO
2 nanoparticles for their use as photocatalyst.
Subsequently, several synthesis methods for the preparation of TiO
2 nanomaterials have been developed, and electrospinning has proved to be one of the most useful due to its versatility. Kim and coworkers
[19][51] prepared highly crystalline hollow TiO
2 fibers can be prepared by electrospinning, and with a uniform wall thickness. These authors first prepared polymer fibers by electrospinning and then used them as sacrificial template. By atomic layer deposition, a layer of TiOx was deposited on the polymeric fibers, then the polymeric material was removed by heat treatment, obtaining the crystalline hollow TiO
2 fibers with good crystallinity and controlled shape (diameter and wall thickness). A different approach for the preparation of TiO
2 fibers was followed by Nikfarjam
[20][52] et al. They prepared a solution with a Ti precursor (titanium tetraisopropoxid), ethanol and acetic acid, that was mixed with the polymer PVP (polyvinyl alcohol). The mixture was electrospun with an aluminum foil as cathode. After calcination to decompose the polymer matrix, TiO
2 nanofibers were obtained. These fibers were used for gas-sensing applications. This approach of preparing fibers by the electrospining of Ti precursor-PVP solutions and subsequent heat treatment was used by other authors
[21][22][23][24][25][53,54,55,56,57], demonstrating that this is a suitable technique. For some applications, such as for anode materials for Li-ion batteries, it is necessary to prepare mixed oxide fibers, such as TiNb
2O
7, and titanium niobium oxide nanofibers can be also prepared thought this procedure
[26][58], involving the preparation of a precursor solution (titanium butoxide and niobium ethoxide) in ethanol/acetic acid that is mixed with a polymeric solution, electrospun, and then heated in air for polymeric matrix removal.
2.3. Zeolite and Other Ordered Mesoporous Fibers Applications
Porous materials such as zeolite or MOFs have been extensively studied for many applications in catalysis, as adsorbents and for drug delivery [1][27][28][1,69,70], among other applications. As has been discussed for other materials, such as TiO2 catalysts, zeolite nanoparticles have been found to have higher catalytic activity as compared to the correspondent micromaterial counterparts. In general, nanoscaled active phases catalysts [29][30][71,72] exhibit better mechanical properties, are more economical and present excellent activity and selectivity results, and these advantages also include nanoscaled ordered mesoporous materials. Despite these advantages, the use of nanomaterials in catalysis is limited due to their unavoidable agglomeration and, as has been discussed in the case of TiO2 photocatalysts, when nanoparticles are used they can lynch to the liquid effluent. These disadvantages that nanoparticles present can be overcome with the use of nanofibers and, subsequently, several routes for the preparation of zeolite and other mesoporous materials fibers have been described, with electrospinning being one of the most extended procedures [31][73]. Hashaikeh et al. [31][73] have reviewed the applications and synthesis methods for zeolite and mesoporous inorganic nanofibers materials. The general procedure for synthetizing the mesoporous fibers would include [31][32][33][34][35][73,74,75,76,77] the preparation of a solution containing the organic structure directing agent in a solvent that, after heat treatment and stirring, gives rise to a gel that is mixed with polymer (PVA) and electrospun. The gel can be replaced by nanoparticles [35][77], which can be commercial ones. By using coaxial electrospinning [35][77], hollow fibers can be also obtained. With this procedure, two needles that contain two solutions form the core and the sheath, separately. A paraffin oil or a similar organic solvent can be used as an inner solution, then, it can be removed, forming the hollow fibers. Multilayer fibers with several needles and solutions can be subsequently produced with this procedure [36][37][38][78,79,80]; thus, the possible combinations are almost infinite. Scheme 12 summarized the general procedure for the synthesis of ordered mesoporous inorganic fibers by electrospinning.
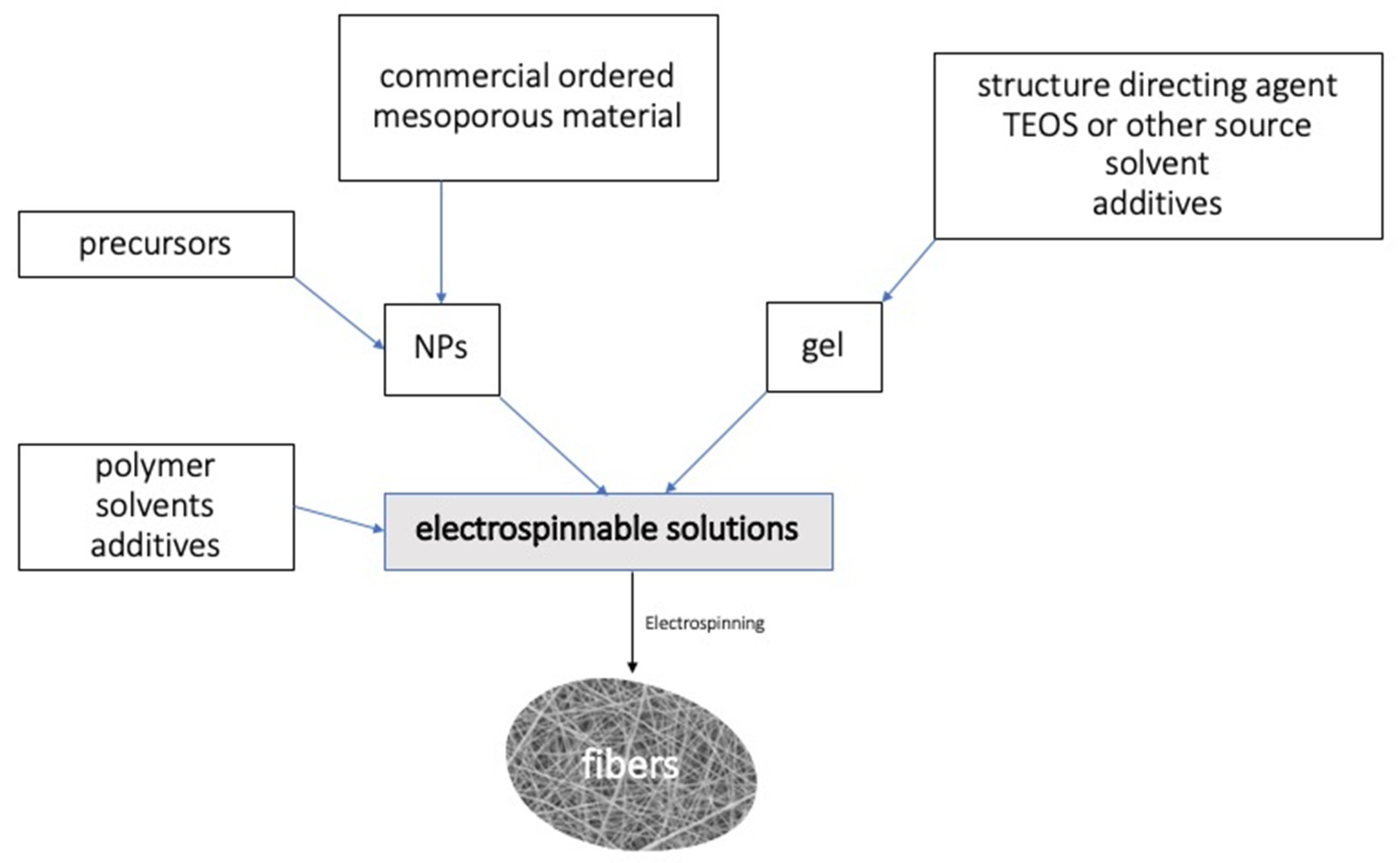
Scheme 12. General procedure for the synthesis of ordered mesoporous inorganic fibers by electrospinning. NPs (NanoParticles). TEOS (TetraEtlyl OrthoSilicate).
3. Conclusions
It has been performed a bibliographic analysis that showed how there are two important groups of electrospun-fiber-catalysts that are being investigated, based on TiO
2 and in carbon materials, being the main applications in photo- and in electro-catalysis. Along other materials such as mixed oxides can be prepared by electrospinning and are also useful catalysts, as have been
analyzreviewed. The
includreviewed studies
' results have shown that the possibilities of electrospun fibers are quite advantageous due to several factors:
- -As fibers, they present a low resistance to internal diffusion and a high surface area to volume ratio, due to their diameter–length proportion;
- -As nanomaterials, the activity and selectivity of isolated nanoscaled catalytically active phases is higher, as has been demostrated for many catalytic systems;
- -The possibilities that allow the electrospinning procedure are almost infinite since this equipment has become very popular, due to its low cost and simple handling. In addition, there is the possibility to prepare multilayered fibers with modulated properties. It has been also discussed how, with the control of the electrospinning parameters, such as voltage, being applied, the materials’ properties such as the diameter of the fibers can be modulated and the synthesis methods are quite reproducible.
Althought most of the applications that have been explored to date for these materials are focused in medicine and for the manufacture of sensors and batteries, the superior properties of these materials indicate that they can be considered a new class of catalysts that are going to be more important for the improvement of several catalytic processes and for the development of new ones.

