Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Nora Tang and Version 5 by Vivi Li.
Wastewater is actively used for irrigation of vegetable and forage crops in arid lands due to water scarcity and cost advantages. The objective of this review was to assess the effect of wastewater (mixture sources) reuse in irrigation on soil, crop (vegetable and forage crops), animal products, and human health.
- forage crops
- vegetable crops
- toxic metals
1. Wastewater Quality for Reuse in Irrigation of Agricultural Crops
High demands for water in dry lands plus frequent drought encourage policymakers and governments to assess alternative management practices to sustain water resources and achieve greater production [1]. Water scarcity and unreliable rainfall (Table 1) make water management practices, such as rainfall water harvesting and the use of non-conventional water of lower quality (saline and wastewater), viable investment for water supply and food production in drylands [2][3][4]. Sustainable land management requires comprehensive attention and a long-term vision to both bio-physical and socio-economic aspects [5]. The upgrade of water supply systems coupled with public awareness to reduce residential water use and saving as well as the reuse of domestic waste and greywater have been increased recently [2][6][7]. In fact, the direct use of treated and untreated municipal wastewater for irrigation purposes has been increased recently worldwide to compensate the water shortage crises. In 2018, the total annual wastewater (109 m3/year) in Mexico was 5.71, India 1.23, China 1.26, and Jordan 0.1 (Table 1). Interestingly, several countries have developed wastewater irrigation systems for direct reuse. Worldwide, the total irrigated area equipped for direct use of wastewater is about 8.42 million ha; of this amount, untreated wastewater is 4.14 million ha and treated wastewater is 4.28 million ha [8].
Table 1. Total cultivated area, annual precipitation, total volume of wastewater used for irrigation and total area equipped for wastewater irrigation for some countries that use wastewater for irrigation [8].
| Country | Rain-Fed Cultivated Area (1000 ha) | Irrigated Cultivated Area (1000 ha) | Annual Precipitation in Volume (109 m3/Year) | Annual Precipitation in Depth (mm/Year) | Wastewater (Treated and Non-Treated) for Irrigation (109 m3/Year) | Total Irrigated Area Equipped for Direct Use of Treated Wastewater (1000 ha) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| India | 76,742 | 92,575 | 3560 | 1083 | 1.23 | 1.32 | |||||||
| Pakistan | 12,710 | 18,590 | 393 | 494 | 1.02 | 32.5 | |||||||
| Iran | 8544 | 8893 | 86 | 228 | 0.33 | 240 | |||||||
| ] | [ | 25 | ] | China | 40,190 | 95,486 | 6192 | 645 | 1.26 | 3618 | |||
| Total Suspended Solids | TSS (mg L−1) | 2–987 | 2–312 | 0–21 | - | [6][23][26] | Australia | 29,008 | 2298 | 4133 | 534 | 0.14 | - |
| Sodium Adsorption Ratio | SAR | 2–90 | 1–21.9 | 3.0–8.0 | 9–13 | [2][11][25] | Japan | 1463 | 2957 | 630 | 1668 | 0.11 | - |
| Jordan | 197 | ||||||||||||
| Turbidity | T (NTU) | 3–444 | - | Not detected | 1–10 | [11][6][23][26] | 83 | 9.9 | 111 | 0.103 | 3.7 | ||
| Biological Oxygen Demand (5 days) | BOD5 (mg L−1) | 135–4450 | 10–942 | 0.0–225 | 60–300 | [2][9][11][6][23][26][27] | Palestine | 62 | 124 | 2.4 | 402 | 0.013 | - |
| Chemical Oxygen Demand | COD (mg L−1) | 15–4155 | 5–1700 | Not detected | 120–500 | [2][11][6][23][26][27] | Iraq | 3107 | 2143 | 93.7 | |||
| Fat, Oil and Grease | 216 | FOG (mg L−1 | 1.08 | ) | 8– | - | |||||||
| 232 | - | Not detected | 8 | [ | 11 | ][26] | Saudi Arabia | 2641 | 954 | 127 | |||
| Anionic surfactants | 59 | ASR (mg L−1 | 0.53 | ) | 1–80.5 | 51.92 | |||||||
| 0.3–1.0 | Not detected | 0.1–100 | [ | 11 | ] | [6][26] | Turkey | 18,974 | 4206 | 466 | 593 | 0.05 | 9.16 |
| Methylene Blue Active Substances | MBAS (mg L−1) | 1.6–118 | 0.3–39 | Not detected | 25 | [11] | Bahrain | 4.0 | 0.6 | 0.06 | 83 | 0.009 | |
| Total Coliforms | TC (CFU 100 mL−1) | 1000–1.9 × 108 | 200–2 × 10 | 1.25 | |||||||||
| 7 | 0.0–2.0 | 10–1000 | [ | 11 | ] | [6][25][26] | Algeria | 7658 | 858 | 212 | 212 | 0.01 | 1.2 |
| Egypt | |||||||||||||
| Fecal Coliforms | FC (CFU 100 mL−1) | 200–2 × 107 | 10–4 × 106 | 0.0–2.0 | 2–1000 | [11][6][25][26] | 1339 | 2497 | 18.1 | 75 | 0.29 | 35.5 | |
| Escherichia coli | E. coli (CFU 100 mL−1) | 1000–8 × 108 | 408–4 × 105 | 0.0–1.0 | 1000–105 | [2][11][6][25 | Morocco | 7815 | 1711 | 155 | 346 | 0.01 | - |
| Argentina | 31,400 | 2301 | 1643 | 591 | 0.091 | 20 | |||||||
| Mexico | 16,276 | 6331 | 1489 | 758 | 5.71 | 70 | |||||||
| Bolivia | 4449 | 278 | 1259 | 1146 | 0.016 | 1.56 | |||||||
| Brazil | 55,107 | 8411 | 14,995 | 1761 | 0.008 | - |
Wastewater and greywater reuse in irrigation are essential sources for sustainable water management in arid lands, promoting the preservation of the limited freshwater resources [9][10]. Municipal wastewater is defined as water (99.9%) and suspended and dissolved organic (e.g., lignin, fats, soaps, synthetic detergents, proteins) and inorganic solids (heavy metals) collected from homes and industries (Figure 1). Greywater (50–80% of residential wastewater) is mainly comprised of water collected separately from sewage flow that originates from clothes washers, bathtubs, showers, and sinks, without wastewater from toilets [11]. The use of greywater for irrigation would reduce the demand on water resources and alleviate the pressure on wastewater treatment plants [11].
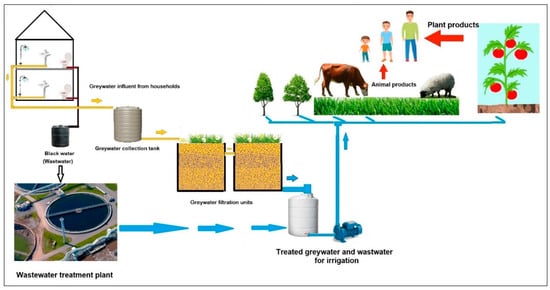
Figure 1. Schematic diagram for wastewater and greywater reuse in agriculture.
Numerous wastewater treatment and quality assessment methods have been used worldwide [12]. Microbial contaminants, such as total and fecal coliforms, normally exceed the recommended levels due to unrestricted entry of untreated wastewater into the environment; therefore, the use of suitable purification treatments with high removal efficiency for microbial agents are critical [13]. In addition, wastewater treatment plants might emit several microbiological contaminants in the air, including mesophilic, psychrophilic and coliform bacteria [14]. However, the abundance of these microbes in the air and the purified wastewater depends on the treatment plant capacity and purification system [14]. In Iran, the assessment of wastewater treatment systems (activated sludge, stabilization ponds, wetlands, and low and medium pressure UV disinfection systems) for microbial removal revealed that the active sludge systems was not efficient in reducing coliforms (total and fecal) compared to stabilization pond systems [15]. Udayanga et al. [16] found that thermal processing of sewage sludge, especially pyrolysis, valorized the carbon rich organic fraction of the sludge, while successfully reducing its volume. Ozonation-based disinfection methods can effectively remove antibiotic resistant bacteria from aqueous solutions [17]. However, the process efficiency was affected by the ozone dose as well as the wastewater solids and pH [17]. Biological treatments of wastewater normally remove microbial pollutants but these methods fail to eliminate numerous chemical compounds, such as pharmaceuticals [14]. Therefore, additional treatment process is necessary to remove these pollutants, including membrane filtration, adsorption, coagulation, electrochemical treatment, or advanced oxidation [14]. For example, adsorption onto activated carbons has been selected as the procedure to remove different chemical contaminant at the industrial scale [13]. Overall, wastewater treatment systems involve a combination of physical, biological, and chemical processes to purify the effluent efficiently [13].
The use of wastewater for irrigation of food crops is controversial. The economic and energetic assessment of wastewater reuse as viable complementary sources of water represent a possible opportunity [6]. In the arid regions, research studies have recommended the reuse (with precautions) of wastewater for irrigation in urban landscape and forage crop production [2][10][18]. However, analysis of this type of water depends on its source. The use of wastewater for irrigation might generate substantial environmental contamination and toxicology problem especially when farmers use untreated wastewater [19][20]. Wastewater contains toxic microorganisms and heavy metals such as Ni, Cd, Cr, and Pb that can induce severe risks to humans and the environment [19].
The chemical composition of tertiary treated wastewater are often within the World Health Organization (WHO) allowable limits, but heavy metal concentrations (i.e., Ni, Cd, Pb, As, and Cr) has exceeded the maximum limits in several regions of the world, especially in regions that use untreated wastewater for irrigation (Table 2). Microbial levels depend on wastewater sources and might contain pathogens, such as Pseudomonas, Salmonella, Aeromonas, and Staphylococcus; therefore, should not be reused for irrigation without treatment [21]. Table 2 shows that levels of total coliforms, fecal coliforms, and Escherichia coli have also exceeded the maximum world limits in untreated wastewater. In addition, treated wastewater microbial levels (total coliforms, fecal coliforms, and Escherichia coli) and some heavy metals (Cu, Cd, Cr, and Ni) were higher than the standard limits. Akoto et al. [20] found that irrigation of farmlands with untreated wastewater contaminated the soil by Ni, Pb, Cr, and Cd and transferred these contaminants to the farm products (lettuce). A study conducted by Qishlaqi et al. [9] showed that excessive accumulation of Ni and Pb was found in wheat tissues irrigated with untreated wastewater. They concluded that strict protection measures and rigorous integrated systems are essential to alleviate the negative effect of wastewater reuse in agriculture, especially the regions irrigated with untreated wastewater. The global assessment of irrigated croplands affected by urban wastewater (treated and untreated) revealed that about 36 million ha of irrigated croplands were located in wastewater dependent catchments [22]. In addition, about 82% of these affected croplands (29.3 million ha) were located in countries (China, India, Pakistan, Mexico, and Iran) in which less than 75% of wastewater is normally treated [22]. Considering the microbial and chemical analysis of untreated and treated wastewater exceeding the world standard limits in some regions of the world as well as the recommendations of previous studies, untreated wastewater should be restricted (wastewater treatment is essential) with a periodic water analysis to reduce the transfer of heavy metals to crops, animals, and humans.
Table 2. Quality of un-treated and treated wastewater compared with fresh and allowable word standard limit for irrigation. World standards represent the range limits for the WHO and the following countries: Canada, India, Jordan, Italy, Australia, Japan, China, Slovenia, Germany, and Great Britain. Bold values indicate where wastewater variable exceeded the standards limits.
| Parameter | Symbol and Unit | Untreated Wastewater | Treated Wastewater | Fresh Water | Word Standards | Reference |
|---|---|---|---|---|---|---|
| Potential of Hydrogen (H+) | pH | 5–10 | 6–8 | 7.1–7.6 | 5.5–9.5 | [2][9][11][6][23][24] |
| Electrical conductivity | ECw (dS m−1) | 0.5–10 | 0.4–0.8 | 0.3–4.0 | 0.7–10 | [2][11][6][23][24] |
| Total Dissolved Solids | TDS (mg L−1) | 279–2444 | 100–429 | 80–154 | 450–2000 | [2][11 |
| ] | ||||||
| [ | ||||||
| 26 | ||||||
| ] | ||||||
| Bicarbonate | ||||||
| HCO | ||||||
| 3 | ||||||
| (mg L | ||||||
| −1 | ) | 2–223 | - | - | 520 | [11][26] |
| Orthophosphate | PO4 (mg L−1) | 0–52 | 0.2–3.2 | 0.03–0.8 | 30 | [24][28] |
| Nitrate | NO3 (mg L−1) | 10–52 | 0.1–7.0 | 0.0–0.004 | 45–50 | [11][6][23][24][26] |
| Sulfate | SO4 (mg L−1) | 1–22 | 0.5–28 | 0.0–0.1 | 500 | [11][29][24][25][26] |
| Total nitrogen | TN (mg L−1) | 1–61 | 0.5–17.7 | 2.0–10 | 5–50 | [2][11][6][18][24][26][27] |
| Potassium | K+ (mg L−1) | 20–39 | 1–10 | 0.0–12 | 80 | [2][9][11][18][24][25] |
| Phosphorus | P (mg L−1) | 26–38 | 0.05–1.2 | 0.01–1.0 | - | [2][9][18][25][26][27] |
| Calcium | Ca+2 (mg L−1) | 1–100 | 0.1–72 | 1.5–15 | 230–400 | [2][9][11][18][25][26] |
| Magnesium | Mg+2 (mg L−1) | 1–60 | 0.1–23 | 0.0–10 | 60 | [2][9][11][18][25][26] |
| Manganese | Mn (mg L−1) | 0.02–0.16 | 0.0002–0.06 | 0.0–0.17 | 0.2 | [9][11][27][30][31][32] |
| Iron | Fe (mg L−1) | 0.1–2.7 | 0.1–0.4 | 0.0–0.1 | 0.1–5 | [2][9][11][18][27][30][31] |
| Zinc | Zn (mg L−1) | <0.002–13.0 | 0.01–0.7 | 0.0–0.17 | 2.0 | [2][9][11][30][31] |
| chloride | Cl− (mg L−1) | 9–450 | 63–205 | 1.0–18 | 140–400 | [2][11][26][27] |
| Sodium | Na+ (mg L−1) | 2–667 | 1.0–136 | 2.0–19 | 69–230 | [2][11][25] |
| Copper | Cu (mg L−1) | 0.001–91 | 0.001–24 | 0.001–0.02 | 0.2 | [11][27][30][31] |
| Boron | B (mg L−1) | 0.02–0.44 | 0.001–0.04 | 0.0–0.1 | 0.7–3.0 | [2][11][6][26][33] |
| Aluminum | Al (mg L−1) | 0.0–21 | 0.0–1.5 | 0.0–0.03 | 5.0 | [2][11][25][26][33] |
| Cadmium | Cd (mg L−1) | <0.001–4.0 | <0.002–0.4 | 0.0–0.03 | 0.01 | [2][9][11][30][31][32][34] |
| Lead | Pb (mg L−1) | <0.003–84 | <0.01–1.3 | 0.003–5.0 | 5.0 | [2][9][11][30][31][32][34] |
| Chromium | Cr (mg L−1) | <0.004–42 | 0.0–4.0 | <0.008–0.8 | 0.1 | [9][11][26][30][32][33][34] |
| Arsenic | As (mg L−1) | 0.0001–6.0 | 0.001–0.002 | <0.0025 | 0.1 | [2][11][25][26][30][33] |
| Nickel | Ni (mg L−1) | 0.04–70 | 0.01–9.0 | <0.001–6.0 | 0.2 | [9][11][18][30][31][32] |
| Cobalt | Co (mg L−1) | 0.0–0.01 | 0.0–<0.0001 | Not detected | 0.05 | [25][26][27][33] |
| Selenium | Se (mg L−1) | <0.001 | <0.001 | Not detected | 0.02 | [26] |
| Vanadium | V (mg L−1) | 0.0001–0.004 | - | Not detected | 0.1 | [33] |
| Mercury | Hg (mg L−1) | 0.001 | - | Not detected | 0.0001–0.01 | [34] |
Several studies have suggested greywater reuse as an alternative to wastewater [6][7][21]. However, heavy metal levels of greywater and wastewater could be similar [2][35]. In addition, the collection of used water from kitchen and laundry only required extra cost and could be inapplicable in some residential regions. Therefore, the greywater reuse in irrigation is limited worldwide when compared to wastewater. When wastewater is considered for reuse, it is normally tested according to specific quality standards to avoid threatening the environment and humans [36][37]. These water quality standards consist of chemical and microbiological components. However, only specific variables are measured and the probability of having non-measured toxic substances is high [36][38]. In Australia, 22 organic micro-pollutants including triclosan, caffeine, paracetamol, acesulfame, and salicylic acid were found in greywater and thus the reuse of this recycled-irrigation water can act as a source of microbial pollutant to soil, plants, and groundwater [29]. Similarly, in Palestine, the antibiotic and herbicide analysis of residential greywater used for irrigation of food crop production showed that antibiotics and herbicides were presented in the reused greywater. Those toxic antibiotics (1.3 to 1592.9 ng L−1) and herbicide (3.1–22.4 ng L−1) materials included tetracycline, ciprofloxacin, atrazine, erythromycin, oxolinic acid, and trifluralin [38].
2. Effect of Wastewater Reuse on Soil and Plant
The use of wastewater in arid lands for irrigation of vegetable and forage crops is common due to the high salinity of overexploited aquifers, water scarcity, and cost considerations [20][39]. In many arid and semi-arid regions (e.g., Jordan, Iraq, Ghana Saudi Arabia, and India), irrigation is essential to overcome the prolonged drought periods during the summer. In salt-affected arid lands (due to overexploitation of aquifers), farmers are turning to wastewater as a source of low saline water [39]. The reuse of wastewater for irrigation may have several beneficial effects for plants because it increases the levels of some beneficial elements (N, P, K, Fe, Zn, Ca, and Mg) in the soil [39]. Table 2 shows that micronutrients concentration (N, P, K, and Ca) in untreated wastewater are much higher than fresh water. Higher nutrient and organic matter in the soil lead to a higher growth rates and production. The use of treated wastewater in irrigation effectively increased stem height and the dry matter of Panicum maximum compared to those irrigated with fresh water [39]. The higher performance of the wastewater treatment can be explained by its higher nutritive content, especially in N [39].
Heavy metals such as Cd, Cr, Pb, and Ni are metallic elements, have relatively higher weight than water, are extremely soluble in the aquatic environments, and consequently, they can be uptake easily by living organisms (plant, animal, human) [34]. Turner et al. [33] found that the use of greywater for irrigation gradually increased soil (B, Cr, As, and Cu) and groundwater metals (Al, As, Cr, Cu, Fe, Mn, Ni, and Zn) exceeded safe limits standards after four years.
Vegetables are an essential component of our daily diet. However, the ability of vegetable growers to provide the ever-growing population with the required amount is limited by the unpredicted rainfall pattern and unsuitable irrigation systems [20]. Therefore, farmers tend to use wastewater, which is readily available alternative for irrigation in most dryland regions. However, wastewater contains a substantial amount of pollutants, such as heavy metals. Figure 2 shows the metadata analysis results of previous studies that assess the accumulation of heavy metals in wastewater irrigated vegetables; leafy-green (lettuce, spinach, parcel, mint, cabbage, pudina, and coriander), bulbs and tubers (onion, garlic, potato, radish, and carrot), and fruits (tomato, pepper, cauliflower, okra, and eggplant). Although heavy metals in wastewater were within the standard limits, the concentration of those toxic elements (Pb, Cd, Ni, Cr, As, and Zn) exceeded the allowable limits in both soil and vegetables edible parts (Figure 2). In fact, the concentration of heavy metals in vegetable edible parts increased by 3–9 fold compared to those irrigated with fresh water. For example, leafy-green from wastewater-irrigated fields increased Pb concentration by 5 fold, Cd by 7 fold, Ni by 8 fold, Cr, As, and Zn by about 6 fold compared to those irrigated with fresh water. Khan et al. [32] used wastewater for irrigation of vegetable crops (spinach, coriander, carrot, tomato, and cauliflower). They found that all tested leafy-green, root, and fruit vegetable samples were contaminated with high levels of Pb, Ni, and Cd; higher than WHO limits. Qureshi et al. [23] found that the concentration of Zn and Cr in leafy-green vegetables (lettuce and spinach) was higher than root and fruit (tomato, eggplant, radish, and carrots) vegetables. Therefore, selecting suitable crops can potentially reduce the health risk for humans. In this review, the metadata analysis showed that the concentrations of Pb and As were similar across vegetable types while leafy-green had higher Ni, Cr, and Zn than bulb, tubers, and fruit vegetables (Figure 2). Overall, although the concentration of heavy metals in wastewater used for irrigation were within the WHO limits, the long-term reuse of this recycled water led to excessive build-up of those toxic metals in the soil. Therefore, rigorous and continuous testing (wastewater, soil, plant) is required in cultivated farms to prevent the translocation of heavy metals in the food chain [40].
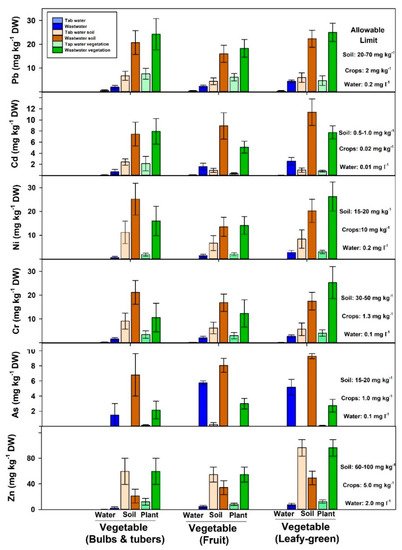
Figure 2. Heavy metals concentration in fresh and wastewater soil and vegetables [10][20][23][24][27][30][31][32][39][41][42][43][44][45][46][47][48][49][50]. Leafy-greens represent lettuce, spinach, parcel, mint, cabbage, pudina, and coriander; bulbs and tubers represent onion, garlic, radish, potato and carrot; fruits vegetables represent tomato, pepper, cauliflower, okra, and eggplant. Bars represent mean ± SE.
Soil is the key component for developing an integrated and sustainable wastewater management system. This is because the chemistry and physics of the soil can significantly affect the levels of toxic materials in the soil and consequently the quality of the crops. The reviews of previous studies, conducted on wastewater reuse in agriculture, revealed that most studies that positively recommended the reuse of wastewater were (1) short-term studies (less than 4 years) or/and (2) assuming that the analysis of wastewater only is sufficient for safe use in agriculture and thus could maintain the level of heavy metals in the crops within the recommended WHO limits [2][18][24][27]. However, long-term studies on wastewater reuse found that several heavy metals significantly increase across years leading to potential soil contamination. The long-term irrigation (~20 years) of wastewater in Shiraz, Iran increased organic matter of the soil by 20–30%, pH by 2–3 units, and heavy metals levels by more than 100%; exceeded the WHO limits [9]. Although the wastewater quality was acceptable in that study (Ni 0.19; Zn 0.06; Cd 0.004; Pb 0.33; Cr 0.1 mg kg−1), the frequent irrigation led to accumulation of contaminated soil in the top 10 cm soil; Pb 441 mg kg−1 (soil limits: 20–70 mg kg−1), Cd 3.2 mg kg−1 (limits: 0.5–1.0 mg kg−1), Ni 297 mg kg−1 (limits: 15–20 mg kg−1), Cr 29 mg kg−1 (limits: 30–50 mg kg−1) and Zn 170 mg kg−1 (limits: 60–100 mg kg−1) [9]. In Nigeria, the vertical distribution analysis and modeling of heavy metals in vegetable farms irrigated with wastewater showed that these gardens will not be suitable for human consumption after 10–20 years if the heavy metal balances (input from wastewater and output metal taken out by plant biomass or leaching) remain unchanged [51].
Heavy metals accumulation potential is different between plant species. The transfer factor is normally used to estimate the translocation of those toxic metals from the soil to plant species [52]. The transfer factor is the ratio between the heavy metal concentrations in the edible part of vegetables (mg kg−1) to the concentration of the metal in soil. Interestingly, Meng et al. [52] found that the transfer factor of heavy metals (especially, Cd and Pb) from soil to vegetables was extremely high. For example, the transfer factor of Cd for cabbage was 1.82 and for potato was 1.52. Similar results were found by Tiwari et al. [30] who found that transfer of toxic metals (As, Cd, Cr, Pb, and Ni) from soil to edible parts of vegetables (pepper, cabbage, spinach, radish, and tomato) was high and unsafe due to possible transfer in the food chain leading to health hazards for humans. They suggested that only vegetable crops that restrict heavy metals in non-edible ports may be cultivated [30]. Overall, to guarantee food safety and the safe use of wastewater for irrigation, urgent attention is necessary to apply appropriate permanent monitoring and pollution control [52].
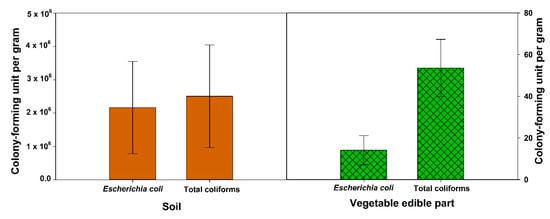
Although treated wastewater is bacterially safe and has a positive impact on plant growth, microbiological contamination of vegetable crops irrigated with wastewater has been reported in many regions of the world [53][54] Escherichia coli is a coliform group of bacteria that is used to represent the bacterial pathogens in the reused wastewater and their behavior is expected to reflect enteric pathogens [11]. In this review, the mean total Escherichia coli in wastewater-irrigated soil was found to be about 2 × 106 (CFU g−1) and about 15 (CFU g−1) in vegetable edible parts (leaf, bulb, tuber, and fruit) (Figure 3). In addition, the mean total coliforms were about 1.4 × 106 (CFU g−1) and about 55 (CFU g−1) in vegetable edible parts (Figure 3). Qureshi et al. [23] found that all vegetables irrigated with wastewater had different levels of microbial loading in their edible part. The highest level of contamination of total coliform were found in spinach, radish, and eggplant while the lowest concentrations were found in lettuce and tomatoes. In addition, the Escherichia coli counts on lettuce, tomatoes, eggplant, and carrot were higher than spinach. They concluded that the tertiary-level of wastewater treatment does not fully remove pathogenic bacteria (total coliform and Escherichia coli) from the reused wastewater nor the edible parts of the vegetables [23].
References
- Pourmohammadali, B.; Hosseinifard, S.J.; Salehi, M.H.; Shirani, H.; Boroujeni, I.E. Effects of soil properties, water quality and management practices on pistachio yield in Rafsanjan region, southeast of Iran. Agric. Water Manag. 2019, 213, 894–902.
- Al-Mefleh, N.K.; Othman, Y.A.; Tadros, M.J.; Al-Assaf, A.; Talozi, S. An assessment of treated greywater reuse in irrigation on growth and protein content of Prosopis and Albizia. Horticulturae 2021, 7, 38.
- Tadros, M.; Al-Mefleh, N.; Othman, Y.; Al-Assaf, A. Water harvesting techniques for improving soil water content, and morpho-physiology of pistachio trees under rainfed conditions. Agric. Water Manag. 2021, 243, 106464.
- Muriu-Ng’ang’a, F.W.; Mucheru-Muna, M.; Waswa, F.; Mairura, F.S. Socio-economic factors influencing utilisation of rain water harvesting and saving technologies in Tharaka South, Eastern Kenya. Agric. Water Manag. 2017, 194, 150–159.
- Visser, S.; Keesstra, S.; Maas, G.; De Cleen, M. Soil as a basis to create enabling conditions for transitions towards sustainable land management as a key to achieve the SDGs by 2030. Sustainability 2019, 11, 6792.
- Boano, F.; Caruso, A.; Costamagna, E.; Ridolfi, L.; Fiore, S.; Demichelis, F.; Gavão, A.; Pisoeiro, J.; Rizzo, A.; Masi, F. A review of nature-based solutions for greywater treatment: Applications, hydraulic design, and environmental benefits. Sci. Total Environ. 2020, 711, 134731.
- Friedler, E.; Hadari, M. Economic feasibility of on-site greywater reuse in multistorey buildings. Desalination 2006, 190, 221–234.
- FAO. AquaStat, Food and Agriculture Organization of the United Nations. 2018. Available online: http://www.fao.org/nr/water/aquastat/data/query/results.html (accessed on 15 October 2021).
- Qishlaqi, A.; Moore, F.; Forghani, G. Impact of untreated wastewater irrigation on soils and crops in Shiraz suburban area, SW Iran. Environ. Monit. Assess. 2008, 141, 257–273.
- Al-Hamaiedeh, H.; Bino, M. Effect of treated grey water reuse in irrigation on soil and plants. Desalination 2010, 256, 115–119.
- WHO. Guidelines for the Safe Use of Wastewater, Excreta and Greywater; World Health Organization: Geneva, Switzerland, 2006; Volume 2.
- Lisak, G. Reliable environmental trace heavy metal analysis with potentiometric ion sensors-reality or a distant dream. Environ. Pollut. 2021, 289, 117882.
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155.
- Michałkiewicz, M. Comparison of wastewater treatment plants based on the emissions of microbiological contaminants. Environ. Monit. Assess. 2018, 190, 640.
- Aghalari, Z.; Dahms, H.; Sillanpää, M.; Sosa-Hernandez, J.; Parra-Saldívar, R. Effectiveness of wastewater treatment systems in removing microbial agents: A systematic review. Glob. Health 2020, 16, 13.
- Udayanga, W.; Veksha, A.; Giannis, A.; Lisak, G.; Chang, V.; Lim, T. Fate and distribution of heavy metals during thermal processing of sewage sludge. Fuel 2018, 226, 721–744.
- Foroughi, M.; Khiadani, M.; Kakhki, S.; Kholghi, V.; Naderi, K.; Yektay, S. Effect of ozonation-based disinfection methods on the removal of antibiotic resistant bacteria and resistance genes (ARB/ARGs) in water and wastewater treatment: A systematic review. Sci. Total Environ. 2021, 9, 151404.
- Al-Ajmi, A.; Salih, A.; Kadim, I.; Othman, Y. Chemical constituents and heavy metals contents of barley fodder produced under hydroponic system in GCC countries using tertiary treated sewage effluents. J. Phytol. 2009, 1, 374–380.
- Khalid, S.; Shahid, M.; Natasha, I.; Sarwar, T.; Shah, A.; Niazi, N. A Review of Environmental Contamination and Health Risk Assessment of Wastewater Use for Crop Irrigation with a Focus on Low and High-Income Countries. Int. J. Environ. Res. Public Health 2018, 15, 895.
- Akoto, O.; Addo, D.; Baidoo, E.; Agyapong, E.; Apau, J.; Fei-Baffoe, B. Heavy metal accumulation in untreated wastewater-irrigated soil and lettuce (Lactuca sativa). Environ. Earth Sci. 2015, 74, 6193–6198.
- Leong, J.; Oh, K.; Poh, P.; Chong, M. Prospects of hybrid rainwater-greywater decentralised system for water recycling and reuse: A review. J. Clean. Prod. 2019, 142, 3014–3027.
- Thebo, A.; Drechsel, P.; Lambin, E.; Nelson, K. A global, spatially-explicit assessment of irrigated croplands influenced by urban wastewater flows. Environ. Res. Lett. 2017, 12, 074008.
- Qureshi, A.; Hussain, I.; Ismail, S.; Khan, Q. Evaluating heavy metal accumulation and potential health risks in vegetables irrigated with treated wastewater. Chemosphere 2016, 163, 54–61.
- Rodda, N.; Salukazana, L.; Jackson, S.; Smith, M. Use of domestic greywater for small-scale irrigation of food crops: Effects on plants and soil. Phys. Chem. Earth. 2011, 36, 1051–1062.
- Finley, S.; Barrington, S.; Lyew, D. Reuse of domestic greywater for the irrigation of food crops. Water Air Soil Pollut. 2009, 199, 235–245.
- Eriksson, E.; Auffarth, K.; Henze, M.; Ledin, A. Characteristics of grey wastewater. Urban Water 2020, 4, 85–104.
- Christou, A.; Eliadou, E.; Michael, C.; Hapeshi, E.; Fatta-Kassinos, D. Assessment of long-term wastewater irrigation impacts on the soil geochemical properties and the bioaccumulation of heavy metals to the agricultural products. Environ. Monit. Assess. 2014, 186, 4857–4870.
- Hussain, S.; Abdul Aziz, H.; Isa, M.; Ahmad, A.; Van Leeuwen, J.; Zou, L.; Beecham, S.; Umar, M. Orthophosphate removal from domestic wastewater using limestone and granular activated carbon. Desalination 2011, 271, 265–272.
- Turner, R.; Warne, M.; Dawes, L.; Thompson, K.; Will, G. Greywater irrigation as a source of organic micro-pollutants to shallow groundwater and nearby surface water. Sci. Total Environ. 2019, 669, 570–578.
- Tiwari, K.; Singh, N.; Patel, M.; Tiwari, M.; Rai, U. Metal contamination of soil and translocation in vegetables growing under industrial wastewater irrigated agricultural field of Vadodara, Gujarat, India. Ecotoxicol. Environ. Saf. 2011, 74, 1670–1677.
- Khan, A.; Javid, S.; Muhmood, A.; Mjeed, T.; Niaz, A.; Majeed, A. Heavy metal status of soil and vegetables grown on peri-urban area of Lahore district. Soil Environ. 2013, 32, 49–54.
- Khan, M.; Malik, R.; Muhammad, S. Human health risk from Heavy metal via food crops consumption with wastewater irrigation practices in Pakistan. Chemosphere 2013, 93, 2230–2238.
- Turner, R.; Warne, M.; Dawes, L.; Vardy, S.; Will, G. Irrigated greywater in an urban sub-division as a potential source of metals to soil, groundwater and surface water. J. Environ. Manag. 2016, 183, 806–817.
- Kinuthia, G.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 8434.
- Al-Habahbeh, K.A.; Al-Nawaiseh, M.B.; Al-Sayaydeh, R.S.; Al-Hawadi, J.S.; Albdaiwi, R.N.; Al-Debei, H.S.; Ayad, J.Y. Long-term irrigation with treated municipal wastewater from the Wadi-Musa region: Soil heavy metal accumulation, uptake and partitioning in olive trees. Horticulturae 2021, 7, 152.
- Leal, L.; Soeter, A.; Kools, S.; Kraak, M.; Parsons, J.; Temmink, H.; Zeeman, G.; Buisman, C. Ecotoxicological assessment of greywater treatment systems with Daphnia magna and Chironomus riparius. Water Res. 2012, 46, 1038–1044.
- Li, F.Y.; Wichmann, K.; Otterpohl, R. Review of the technological approaches for grey water treatment and reuses. Sci. Total Environ. 2009, 407, 3439–3449.
- Craddock, H.; Panthi, S.; Rjoub, Y.; Lipchin, C.; Sapkota, A.; Sapkota, A. Antibiotic and herbicide concentrations in household greywater reuse systems and pond water used for food crop irrigation: West Bank, Palestinian Territories. Sci. Total Environ. 2020, 699, 134205.
- Abdoulkader, B.A.; Mohamed, B.; Nabil, M.; Alaoui-Sossé, B.; Eric, C.; Aleya, L. Wastewater use in agriculture in Djibouti: Effectiveness of sand filtration treatments and impact of wastewater irrigation on growth and yield of Panicum maximum. Ecol. Eng. 2015, 84, 607–614.
- Chung, B.Y.; Song, C.H.; Park, B.J.; Cho, J.Y. Heavy metals in brown rice (Oryza sativa L.) and soil after long-term irrigation of wastewater discharged from domestic sewage treatment plants. Pedosphere 2011, 21, 621–627.
- Zavadil, J. The effect of municipal wastewater irrigation on the yield and quality of vegetables and crops. Soil Water Res. 2009, 4, 91–103.
- Gupta, N.; Khan, D.; Santra, S. Heavy metal accumulation in vegetables grown in a long-term wastewater-irrigated agricultural land of tropical India. Environ. Monit. Assess. 2012, 184, 6673–6682.
- Shilpi, S.; Seshadri, B.; Sarkar, B.; Bolan, N.; Lamb, D.; Naidu, R. Comparative values of various wastewater streams as a soil nutrient source. Chemosphere 2018, 192, 272–281.
- Dhanasekarapandian, M.; Chandran, S.; Kumar, V.; Surendran, U. Assessment of heavy metals in soil, paddy straw and SEM analysis of the soil for the impact of wastewater irrigation in Girudhumal sub basin of Tamil Nadu, India. Glob. Nest J. 2019, 21, 310–319.
- Nazir, R.; Khan, M.; Masab, M.; Rehman, H.; Rauf, N.; Shahab, S.; Ameer, N.; Sajed, M.; Ullah, M.; Rafeeq, M.; et al. Accumulation of heavy metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water Collected from Tanda Dam kohat. J. Pharm. Sci. Res. 2015, 7, 89–97.
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem. Toxicol. 2010, 48, 611–619.
- Mzini, L.; Winter, K. Analysis of grey-water used for irrigating vegetables and possible effects on soils in the vicinity of Umtata Dam, Eastern Cape. Water SA 2015, 41, 115–120.
- Amin, N.-U.; Ahmad, T. Contamination of soil with heavy metals from industrial effluent and their translocation in green vegetables of Peshawar, Pakistan. RSC Adv. 2015, 5, 14322–14329.
- Khan, M.J.; Jan, M.T.; Farhatullah, N.; Khan, M.A.; Perveen, S.; Alam, S.; Jan, A.U. The effect of using wastewater for tomato. Pak. J. Bot. 2011, 43, 1033–1044.
- Joon, N.; Ek, P.; Zevenhoven, M.; Hupa, L.; Miró, M.; Bobacka, J.; Lisak, G. Online microcolumn-based dynamic leaching method for investigation of lead bioaccessibility in shooting range soils. Chemosphere 2020, 256, 127022.
- Abdu, N.; Abdulkadir, A.; Agbenin, J.O.; Buerkert, A. Vertical distribution of heavy metals in wastewater-irrigated vegetable garden soils of three West African cities. Nutr. Cycl. Agroecosyst. 2011, 89, 387–397.
- Meng, W.; Wang, Z.; Hu, B.; Wang, Z.; Li, H.; Goodman, R.C. Heavy metals in soil and plants after long-term sewage irrigation at Tianjin China: A case study assessment. Agric. Water Manag. 2016, 171, 153–161.
- Ahemad, M.; Malik, A. Bioaccumulation of heavy metals by zinc resistant bacteria isolated from agricultural soils irrigated with wastewater. Bacteriol. J. 2011, 2, 12–21.
- Williams, J.; Dimbu, P. Research article effect of Abbatoir waste water on soil microbial communities. Sch. Acad. J. Biosci. 2015, 5, 452–455.
- Siggins, A.; Burton, V.; Ross, C.; Lowe, H.; Horswell, J. Effects of long-term greywater disposal on soil: A case study. Sci. Total Environ. 2016, 557, 627–635.
- Malkawi, H.I.; Mohammad, M.J. Survival and accumulation of microorganisms in soils irrigated with secondary treated wastewater. J. Basic Microbiol. 2003, 43, 47–55.
- Disha, A.; Al Harun, M.; Akter, S.; Billah, S.; Abdullah-Al-Noman, M. Reusing greywater for cultivation of Capsicum frutescens and Calendula officinalis. J. Environ. Manag. 2020, 272, 111088.
More
