Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Anna Wróblewska.
Occult infection with hepatitis C virus (OCI) is defined as the presence of HCV-RNA in hepatocytes, and/or PBMCs in individuals, who are HCV-RNA negative in serum by conventional diagnostic tests (with LoD 15 IU/mL). Depending on the presence or absence of anti-HCV in serum, two types of OCI are distinguished—seronegative and seropositive. Occult viral persistence by definition escapes clinical diagnostic schemes and can last for many years after spontaneous or treatment-induced sustained virological response (SVR).
- occult hepatitis C virus infection
- extrahepatic complications
- lymphotropism
- viral persistence
1. Introduction
It is estimated that 58 million people globally have chronic hepatitis C virus infection (CHC) and 1.5 million new infections occur every year. Treatment schemes with direct-acting antivirals (DAA) display over 95% effectiveness in HCV eradication and the number of treated patients is increasing rapidly, following improvements in worldwide accessibility of these drugs [1]. The therapeutic end-point for DAA therapy is currently recognized as the sustained virological response (SVR) in serum assessed at 12 weeks after the end of treatment using RNA nucleic acid testing (NAT) technologies with a lower limit of detection (LoD) of 15 IU/mL. Despite successful HCV clearance, some patients fail to experience improvement in clinical and immunological parameters. The risk of HCC recurrence or clinical relapse of extrahepatic HCV complications still exists after therapeutically induced SVR [2].
Beginning from 2004, independent research groups started to find small amounts of HCV-RNA in liver tissue, serum, and in peripheral blood mononuclear cells (PBMCs) of patients, who were never diagnosed with HCV infection or who achieved therapeutically induced SVR [3,4,5][3][4][5]. Since then, a widely accepted definition of occult HCV infection (OCI) has been developed. It has been defined by the presence of HCV-RNA in hepatocytes, and/or PBMCs in individuals, who are HCV-RNA negative in serum by conventional diagnostic tests (with LoD 15 IU/mL). Depending on the presence or absence of anti-HCV in serum, two types of OCI are distinguished—seronegative and seropositive [6]. Occult viral persistence by definition escapes clinical diagnostic schemes and can last for many years after spontaneous or treatment-induced SVR. However, its true clinical and epidemiological impact remains to be elucidated.
2. Immune Landscape of OCI
Patients with OCI exhibit distinct immune profiles from individuals who reached spontaneous or treatment-induced SVR, patients with CHC, and healthy controls. In comparison with healthy individuals, OCI patients show elevated expression of IFN-α, pro-inflammatory cytokine interferon gamma-induced protein 10 (IP-10), and Myxovirus resistance protein 1 (MxA) [41][7]. Additionally, occult HCV infection is associated with higher expression levels of IL-6, IL-8, IL-12, TNF-α, and macrophage inflammatory protein 1b compared to SVR individuals [42][8]. It was suggested that immunity skewed to Th2 type response is associated with HCV persistence. Indeed, CHC patients exhibit elevated expression of IL-10, an immunosuppressive cytokine, in their PBMC in comparison to individuals with self-limited hepatitis. Additionally, both in CHC and OCI high expression of IL-10 was accompanied by low or undetectable levels of serum IL-12 and IFN-γ, cytokines involved in T-cell mediated immunity and viral clearance [41][7]. Patients with seropositive OCI and those with CHC had significantly higher serum levels of Th1 (IL-2, IFN-γ) and Th2 (IL-4, IL-10) type cytokines in comparison with healthy subjects. However, patients with occult infection had even lower levels of IL-2 and IFN-γ as well as elevated concentrations of IL-4 and IL-10 when compared to chronically infected individuals [43][9]. The differences in immune setting between OCI and CHC is further depicted by the results of ex vivo stimulation of PBMC with mitogens [10]. When lymphatic viral load is low or undetectable as found in OCI HCV replication is augmented. On the other hand, in CHC, or sporadic cases of OCI with high PBMC viral load, ex vivo mitogen stimulation leads to suppression of replication. Additionally, viral replication after mitogen treatment inversely correlated with the intracellular level of IFN-α mRNA, but not IFN-γ or TNF- α expression, which suggests that IFN-α pathways play a key role in controlling viral replication during OCI [44][11].
It was shown that HCV-specific CD4+ and CD8+ cellular immune responses exist in PBMC of seropositive patients with OCI and in 52% seronegative and aviremic individuals with HCV-RNA persisting in liver tissue [45,46][12][13] (Figure 1). These responses were more frequent and the numbers of reactive cells were higher in seronegative OCI than in CHC and cryptogenic liver disease [45][12]. The magnitude of T-cell reactivity inversely correlated with HCV-RNA level in patients’ liver tissue [45][12]. On the other hand, in seropositive OCI HCV-specific T-cell responses were lower than in SVR individuals but indistinguishable from those in CHC patients [46][13]. Additionally, the expression of low-density lipoprotein receptors, involved in HCV entry, was similar in lymphocytes and monocytes of OCI and CHC patients [47][14].
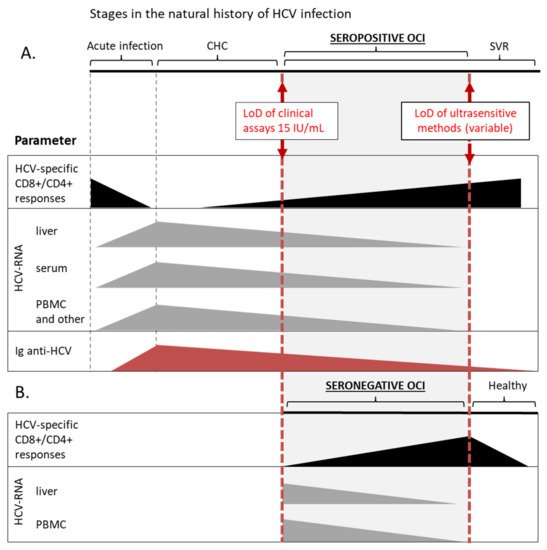

Figure 1. Schematic overview of OCI as one of the stages in the natural history of HCV infection. This chart summarizes data on the detection of basic parameters at different stages of HCV infection, including seropositive (A) and seronegative (B) OCI. Solid boxes represent a range of relative values of a particular parameter, that can be detected with current methods, and are not organized as a timeline. HCV-specific CD8+/CD4+ responses are marked with black color, the presence of HCV-RNA in different compartments with grey, and red triangles represent the level of anti-HCV immunoglobulins. In a patient with certain diagnosis different parameters can assume various values within the range, e.g., in a patient with seropositive OCI HCV-RNA can be detected in PBMC and not in the serum. LoD, lower limit of detection; CHC, chronic hepatitis C; SVR, sustained virological response; PBMC, peripheral blood mononuclear cells.
Antiviral therapy with DAAs induces profound changes in immune balance and was shown to evoke a general inflammatory state [48,49,50][15][16][17]. Reaching SVR generally leads to improvement in immune cell functions and homeostasis as well as amelioration of extrahepatic complications and this effect is inversely correlated with the severity of the disease before therapy [2]. Likewise, a complete viral clearance or occult HCV persistence after DAA seems to be determined by the patient’s immune responsiveness at baseline and by the individual ability to induce a general inflammatory response after viral suppression by DAAs [51,52][18][19]. In our study, a significant and transient elevation of neutrocyte-to-lymphocyte ratio, a marker of systemic inflammation, which occurred during and shortly after DAA therapy, predicted elimination of the replicating residual HCV in PBMC at the follow up after SVR [53][20]. Additionally, patients without HCV-RNA (−) strand showed a greater increase in neutrocyte counts during therapy [53][20]. Many pretreatment parameters are also linked with OCI occurrence after SVR. High viral load and ALT level, prolonged prothrombin time, low albumin, Child B score, raised bilirubin, and treatment experience predisposed to OCI after DAA therapy [54][21]. Basal liver necroinflammation and fibrosis scores were higher for patients who were later diagnosed with OCI after reaching treatment-induced SVR [55][22]. Moreover, as mentioned above, polymorphisms within immune-related genes such as chemokine ligand CXCL10 [56][23] or IL28 [34,37][24][25] determine viral persistence. In seronegative patients with hemophilia or kidney disease, OCI is linked with elevated biochemical parameters associated with systemic inflammation, such as cholesterol, triglyceride, low-density lipoprotein, and C-reactive protein level as well as with 25-hydroxyvitamin D deficiency [37,39][25][26]. These data show that OCI may actually reflect specific characteristics of the host immune system, including low-level inflammation and reduced responsiveness to stimulation, which allows the virus to persist even after apparent therapeutically-induced resolution of viremia. It remains to be studied what are the exact determinants of immunological dysfunction associated with occult viral persistence.
Taken together, it is now established that the virus replicating at low levels in PBMC is not neutral to the immune system but instead continues to stimulate and modulate immunological functions long after apparent viral clearance. The picture of OCI that emerges from these data suggest that it is a condition in-between chronic infection and a state of sustained response, where the immune system is able to restrict viral replication but the cellular immunity is still impaired and unable to completely clear the virus. The term ‘occult infection’ actually covers a range within the natural history of HCV infection, being, by definition, arbitrary restricted by the limits of methods for HCV-RNA detection (Figure 1). Nevertheless, OCI appears to be a separate disease entity distinct from both CHC and acute HCV infection, still lacking a complete description and uniform diagnostic criteria.
3. Clinical Consequences of OCI
The sustained virologic response and elimination of HCV from serum is viewed as a true cure of CHC, and it associates with the improvement of liver histology and significantly better clinical outcomes, even in patients with advanced liver fibrosis [57][27]. In comparison to CHC OCI is considered to be a milder form of infection with a smaller number of infected hepatocytes, associated with less pronounced hepatic injury, as well as more sporadic extrahepatic complications [58][28]. However, there are studies that show that there is still an association between OCI and hepatic necroinflammation or liver fibrosis both in treated CHC patients who reached SVR [55,58][22][28] and in those with seronegative OCI [4,59][4][29]. The persistence of residual hepatic HCV-RNA is frequent among patients with cryptogenic liver disease [4,60[4][30][31][32],61,62], which suggests that occult hepatitis C virus infection can be the underlying cause of liver damage. There is also evidence that increased risk of HCC still exists after therapeutically induced SVR [63][33] and that the residual amounts of virus present in the liver contributing to a prolonged liver injury can stimulate carcinogenesis [64,65,66][34][35][36].
Further confirmation of the association of OCI with liver pathology came from a study on an animal model of Hepacivirus infection. This report clearly showed that occult re-infection induces liver damage with hepatic lymphocyte infiltration and fibrosis, even in the absence of robust viral replication and in the presence of specific antiviral T-cell responses [67][37]. Wang et al. analyzed groups of 60 patients treated with DAAs, 50 with pegylated IFN, and a group of 30 individuals who spontaneously resolved HCV infection [55][22]. They tested PBMC samples and paired liver biopsies from treated patients for the presence of OCI using qPCR and RNAscope in situ hybridization assay, respectively. These authors unequivocally showed that OCI is positively associated with a higher degree of hepatic fibrosis and necroinflammation after treatment and that significant post-therapeutic regression of liver fibrosis occurs only in non-OCI patients, and not in individuals with OCI. Another interesting observation was a higher incidence of OCI among patients treated with IFN-free regimens in comparison to individuals treated with IFN, and to those who spontaneously resolved infection [55][22]. Although all patients included in this study were ethnically Chinese and the results need to be confirmed on larger groups of other human subpopulations, this work underlines the clinical significance of OCI and the need for post-treatment monitoring of CHC patients.
Existing clinical data show a link between OCI and extrahepatic HCV-related diseases such as glomerulonephritis, cryoglobulinemia, or lymphoproliferative disorders. Specifically, occult infection was found in 34 of 87 seronegative, aviremic patients with autoimmune-mediated glomerulonephritis as compared to 1 OCI case among 26 patients from the control group with hereditary glomerulonephritis [68][38]. The presence of OCI in these patients correlated with worse kidney function and faster progression to end-stage kidney disease in comparison to HCV-RNA negative individuals [68][38]. It was also shown that viral antigen NS3 and HCV particles are present in kidney tissue of seropositive and seronegative patients with glomerulonephritis, being likely the cause of renal injury [69,70,71][39][40][41]. Likewise, occult HCV infection may be a hidden cause of many cases of mixed cryoglobulinemia in patients with no evidence of past or present HCV infection [72,73][42][43]. Replicative HCV-RNA strand was found in PBMC of five out of nine patients with mixed cryoglobulinemia who reached SVR after antiviral therapy and remained HCV-RNA negative both in serum and liver tissue. The presence of HCV-RNA associated with persistence of B-cells bearing chromosomal translocation t(14;18), which is frequently found in B-cell non-Hodgkin’s lymphomas, and mixed cryoglobulinemia syndrome [74][44]. HCV infection is known to associate with abnormal proliferation of lymphocytes, and residual HCV-RNA was found in 12 out of 32 (37.5%) seronegative patients with malignant lymphoproliferative disorders, who were HCV-RNA negative using standard tests in serum [75][45]. A similar prevalence of seronegative OCI (27 OCI out of 77–35%) in a group of patients with lymphoproliferative diseases was found by Kisiel et al. [76][46] Interestingly the majority of HCV-positive patients had HCV-RNA in their bone marrow and in nine individuals, viral persistence was confined only to this compartment, with no signs of infection in PBMC or serum [76][46].
So far significant associations of OCI with clinical effect were shown in analyses with small groups of patients, and further large-scale studies are needed to confirm these data. To reliably distinguish patients with and without residual HCV-RNA in numerous samples, a reliable method of OCI detection is of primary importance.
4. Epidemiological Significance of OCI
Residual HCV-RNA has been detected in populations free of markers of ongoing HCV infection and previous exposure to the virus. Persisting HCV was found in high-risk groups such as injection drug users—9.6–18.2% [77[47][48],78], patients with beta-thalassemia major—3.3–6.3% [38[49][50],79], hemodialysis patients—4.8–45% [80[51][52],81], or infectious liver disease-free subjects undergoing phlebotomy—1.27% [82][53]. A recent meta-analysis of studies on populations from the Middle East and Eastern Mediterranean countries estimated the pooled rate of OCI in patients diagnosed with cryptogenic liver disease to be 20.8% [83][54]. OCI was also shown to co-exist with HIV or HBV infections. These viruses share common transmission routes with HCV and co-infections with evident markers of ongoing infection in serum are prevalent in high-risk populations. OCI is frequent in HIV-infected individuals with the prevalence ranging from 9.2% to 11.4%, and reaching 31% in HIV/HBV co-infected patients reported in one study on patients from Georgia [84,85,86][55][56][57]. In addition, simultaneous occult HBV infection and OCI were identified in 1.1% of Iranian HIV-infected individuals [86][57]. Although in HCV/HBV coinfected liver cells HCV inhibits HBV replication, seronegative OCI was also found in active HBV carriers [82][53].
Unexpectedly OCI was also identified in an apparently healthy group from the general population—3.3% [87][58], and random blood donors from Spain, Mexico, and China—0.1–3.4% [88,89,90][59][60][61]. In Egypt, which has one of the highest HCV infection rates, the pooled OCI prevalence estimated by meta-analysis of studies on healthy populations reached 4.79% [83][54]. In fact, it is possible that OCI can be prevalent in other groups where the presence of HCV-specific T-cell responses without detectable viremia and seroconversion are frequently found, such as healthcare workers, prisoners, household contacts of CHC patients, and their spouses [91][62]. It was shown that such responses can be hallmarks of not only past exposure and resolution of HCV infection but can also indicate ongoing OCI [45,92][12][63].
So far, the potential epidemiological significance of these infections remains to be determined, as there is no data unequivocally confirming transmission of HCV infection from persons with OCI. It is known, however, that residual HCV persisting in patients’ PBMCs is infectious in vivo [93][64], and in a chimpanzee model [94][65]. In fact, only 20 copies of the virus were sufficient to transmit HCV infection in chimpanzees [95][66]. Additionally, the transmission of low levels of HCV can be masked by an over 6-month long eclipse phase where HCV-RNA remains undetectable even with the highly sensitive RT-PCR assay, before establishing a high-level viremia [96][67]. Other reports show that OCI could be responsible for the observed cases of late relapses of HCV infection in patients who reached SVR after therapy [96,97[67][68][69],98], as well as of donor liver reinfection in successfully treated CHC patients after transplantation [34,99][24][70]. Furthermore, it was found that the prevalence of occult infection among family members of patients with OCI is similar to that of relatives of CHC patients [100][71].
Thus, the risk of HCV transmission from subjects with OCI exists and detection of residual HCV-RNA in PBMC samples of blood donors raises important questions about the safety of blood testing. As the standard screening of blood donations for anti-HCV antibodies, HCV-RNA, and elevated liver enzymes are not sufficient to exclude OCI, it was suggested to include other tests such as detection of anti-HCV core antibody [101,102,103][72][73][74]. Some authors underline the necessity of screening serial samples as HCV-RNA levels seem to fluctuate over time [6,104][6][75].
References
- World Health Organization. Recommendations and Guidance on Hepatitis C Virus Self-Testing; World Health Organization: Geneva, Switzerland, 2021; p. 32.
- Comarmond, C.; Cacoub, P.; Saadoun, D. Treatment of chronic hepatitis C-associated cryoglobulinemia vasculitis at the era of direct-acting antivirals. Ther. Adv. Gastroenterol. 2020, 13, 1756284820942617.
- Pham, T.N.Q.; MacParland, S.; Mulrooney, P.M.; Cooksley, H.; Naoumov, N.V.; Michalak, T.I. Hepatitis C Virus Persistence after Spontaneous or Treatment-Induced Resolution of Hepatitis, C. J. Virol. 2004, 78, 5867–5874.
- Castillo, I.; Pardo, M.; Salas, C.; Graus, J.; Carreño, V.; Bartolomé, J.; Ortiz-Movilla, N.; Rodríguez-Iñigo, E.; De Lucas, S.; Jiménez-Heffernan, J.A.; et al. Occult Hepatitis C Virus Infection in Patients in Whom the Etiology of Persistently Abnormal Results of Liver-Function Tests Is Unknown. J. Infect. Dis. 2004, 189, 7–14.
- Radkowski, M.; Gallegos-Orozco, J.F.; Jablońska, J.; Colby, T.V.; Walewska-Zielecka, B.; Kubicka, J.; Wilkinson, J.; Adair, D.; Rakela, J.; Laskus, T. Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatol. 2005, 41, 106–114.
- Pham, T.N.; Michalak, T.I. Occult Hepatitis C Virus Infection and Its Relevance in Clinical Practice. J. Clin. Exp. Hepatol. 2011, 1, 185–189.
- Pham, T.N.Q.; Mercer, S.E.; Michalak, T.I. Chronic hepatitis C and persistent occult hepatitis C virus infection are characterized by distinct immune cell cytokine expression profiles. J. Viral Hepat. 2009, 16, 547–556.
- Radkowski, M.; Opoka-Kegler, J.; Cortes, K.C.; Bukowska-Ośko, I.; Perlejewski, K.; Pawełczyk, A.; Laskus, T. Evidence for immune activation in patients with residual hepatitis C virus RNA long after successful treatment with IFN and ribavirin. J. Gen. Virol. 2014, 95, 2004–2009.
- Mousa, N.; Eldars, W.; Eldegla, H.; Fouda, O.; Gad, Y.; Abousamra, N.; Elmasry, E.; Arafa, M. Cytokine Profiles and Hepatic Injury in Occult Hepatitis C Versus Chronic Hepatitis C Virus Infection. Int. J. Immunopathol. Pharmacol. 2014, 27, 87–96.
- Pham, T.N.; King, D.; MacParland, S.; McGrath, J.S.; Reddy, S.B.; Bursey, F.R.; Michalak, T.I. Hepatitis C Virus Replicates in the Same Immune Cell Subsets in Chronic Hepatitis C and Occult Infection. Gastroenterology 2008, 134, 812–822.
- Pham, T.N.Q.; Mulrooney-Cousins, P.M.; Mercer, S.E.; MacParland, S.A.; Inglot, M.; Zalewska, M.; Simon, K.; Michalak, T.I. Antagonistic expression of hepatitis C virus and alpha interferon in lymphoid cells during persistent occult infection. J. Viral Hepat. 2007, 14, 537–548.
- Quiroga, J.A.; Llorente, S.; Castillo, I.; Rodríguez-Iñigo, E.; Pardo, M.; Carreño, V. Cellular Immune Responses Associated with Occult Hepatitis C Virus Infection of the Liver. J. Virol. 2006, 80, 10972–10979.
- Roque-Cuéllar, M.C.; Sánchez, B.; García-Lozano, J.R.; Praena-Fernandez, J.M.; Márquez-Galán, J.L.; Roldán, A.N.; Aguilar-Reina, J. Hepatitis C virus-specific cellular immune responses in sustained virological responders with viral persistence in peripheral blood mononuclear cells. Liver Int. 2013, 34, e80–e88.
- Roque-Cuéllar, M.C.; Sánchez, B.; García-Lozano, J.R.; Garrido-Serrano, A.; Sayago, M.; Praena-Fernández, J.M.; Roldán, A.N.; Aguilar-Reina, J. Expression of CD81, SR-BI and LDLR in lymphocytes and monocytes from patients with classic and occult hepatitis C virus infection. J. Med. Virol. 2012, 84, 1727–1736.
- Gardini, A.C.; Foschi, F.G.; Conti, F.; Petracci, E.; Vukotic, R.; Marisi, G.; Buonfiglioli, F.; Vitale, G.; Ravaioli, F.; Gitto, S.; et al. Immune inflammation indicators and ALBI score to predict liver cancer in HCV-patients treated with direct-acting antivirals. Dig. Liver Dis. 2019, 51, 681–688.
- Gardini, A.C.; Conti, F.; Foschi, F.G.; Brillanti, S.; Andreone, P.; Mazzella, G.; Ravaioli, F.; Buonfiglioli, F.; Bolondi, L.; Crespi, C.; et al. Imbalance of Neutrophils and Lymphocyte Counts Can Be Predictive of Hepatocellular Carcinoma Occurrence in Hepatitis C-related Cirrhosis Treated with Direct-acting Antivirals. Gastroenterology 2018, 154, 2281–2282.
- Debes, J.D.; van Tilborg, M.; Groothuismink, Z.M.; Hansen, B.E.; Wiesch, J.S.Z.; von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate with Development of Hepatocellular Carcinoma in Patients With HCV Infection Treated With Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517.e3.
- Meissner, E.G.; Wu, D.; Osinusi, A.; Bon, D.; Virtaneva, K.; Sturdevant, D.; Porcella, S.; Wang, H.; Herrmann, E.; McHutchison, J.; et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J. Clin. Investig. 2014, 124, 3352–3363.
- Alao, H.; Cam, M.; Keembiyehetty, C.; Zhang, F.; Serti, E.; Suarez, D.; Park, H.; Fourie, N.H.; Wright, E.C.; Henderson, W.; et al. Baseline Intrahepatic and Peripheral Innate Immunity are Associated with Hepatitis C Virus Clearance During Direct-Acting Antiviral Therapy. Hepatology 2018, 68, 2078–2088.
- Wróblewska, A.; Lorenc, B.; Cheba, M.; Bielawski, K.P.; Sikorska, K. Neutrocyte-to-lymphocyte ratio predicts the presence of a replicative hepatitis C virus strand after therapy with direct-acting antivirals. Clin. Exp. Med. 2019, 19, 401–406.
- Mekky, M.A.; Sayed, H.I.; Abdelmalek, M.O.; Saleh, M.A.; Osman, O.A.; Osman, H.A.; Morsy, K.H.; Hetta, H.F. Prevalence and predictors of occult hepatitis C virus infection among Egyptian patients who achieved sustained virologic response to sofosbuvir/daclatasvir therapy: A multi-center study. Infect. Drug Resist. 2019, 12, 273–279.
- Wang, Y.; Rao, H.; Chi, X.; Li, B.; Liu, H.; Wu, L.; Zhang, H.; Liu, S.; Zhou, G.; Li, N.; et al. Detection of residual HCV-RNA in patients who have achieved sustained virological response is associated with persistent histological abnormality. EBioMedicine 2019, 46, 227–235.
- Wang, X.; Wang, S.; Liu, Z.-H.; Qi, W.-Q.; Zhang, Q.; Zhang, Y.-G.; Sun, D.-R.; Xu, Y.; Wang, H.-G.; Li, Z.-X.; et al. Regulatory polymorphism of CXCL10 rs1439490 in seronegative occult hepatitis C virus infection. World J. Gastroenterol. 2018, 24, 2191–2202.
- Elmasry, S.; Wadhwa, S.; Bang, B.-R.; Cook, L.; Chopra, S.; Kanel, G.; Kim, B.; Harper, T.; Feng, Z.; Jerome, K.R.; et al. Detection of Occult Hepatitis C Virus Infection in Patients Who Achieved a Sustained Virologic Response to Direct-Acting Antiviral Agents for Recurrent Infection After Liver Transplantation. Gastroenterology 2017, 152, 550–553.e8.
- Ayadi, A.; Nafari, A.H.; Sakhaee, F.; Rajabi, K.; Ghaderi, Y.; Jamnani, F.R.; Vaziri, F.; Siadat, S.D.; Fateh, A. Host genetic factors and clinical parameters influencing the occult hepatitis C virus infection in patients on chronic hemodialysis: Is it still a controversial infection? Hepatol. Res. 2019, 49, 605–616.
- Nafari, A.H.; Ayadi, A.; Noormohamadi, Z.; Sakhaee, F.; Vaziri, F.; Siadat, S.D.; Fateh, A. Occult hepatitis C virus infection in hemophilia patients and its correlation with interferon lambda 3 and 4 polymorphisms. Infect. Genet. Evol. 2020, 79, 104144.
- Alberti, A. Impact of a sustained virological response on the long-term outcome of hepatitis C. Liver Int. 2011, 31, 18–22.
- Pardo, M.; López-Alcorocho, J.M.; Rodríguez-Iñigo, E.; Castillo, I.; Carreno, V. Comparative study between occult hepatitis C virus infection and chronic hepatitis C. J. Viral Hepat. 2007, 14, 36–40.
- Berasain, C.; Betés, M.; Panizo, A.; Ruiz, J.; Herrero, J.I.; Civeira, M.; Prieto, J. Pathological and virological findings in patients with persistent hypertransaminasaemia of unknown aetiology. Gut 2000, 47, 429–435.
- Bokharaei-Salim, F.; Keyvani, H.; Monavari, S.H.R.; Alavian, S.M.; Madjd, Z.; Toosi, M.N.; Alizadeh, A.H.M. Occult hepatitis C virus infection in Iranian patients with cryptogenic liver disease. J. Med. Virol. 2011, 83, 989–995.
- Yaghobi, R.; Kazemi, M.J.; Geramizadeh, B.; Hosseini, S.A.M.; Moayedi, J. Significance of Occult Hepatitis C Virus Infection in Liver Transplant Patients with Cryptogenic Cirrhosis. Exp. Clin. Transplant. 2020, 18, 206–209.
- Keyvani, H.; Bokharaei-Salim, F.; Monavari, S.H.; Esghaei, M.; Toosi, M.N.; Fakhim, S.; Sadigh, Z.-A.; Alavian, S.M. Occult Hepatitis C Virus Infection in Candidates for Liver Transplant With Cryptogenic Cirrhosis. Zahedan J. Res. Med Sci. 2013, 13, e11290.
- Finkelmeier, F.; Dultz, G.; Peiffer, K.-H.; Kronenberger, B.; Krauss, F.; Zeuzem, S.; Sarrazin, C.; Vermehren, J.; Waidmann, O. Risk of de novo Hepatocellular Carcinoma after HCV Treatment with Direct-Acting Antivirals. Liver Cancer 2018, 7, 190–204.
- Comar, M.; Molin, G.D.; D’Agaro, P.; Crocè, S.L.; Tiribelli, C.; Campello, C. HBV, HCV, and TTV detection by in situ polymerase chain reaction could reveal occult infection in hepatocellular carcinoma: Comparison with blood markers. J. Clin. Pathol. 2006, 59, 526–529.
- Esaki, T.; Suzuki, N.; Yokoyama, K.; Iwata, K.; Irie, M.; Anan, A.; Nakane, H.; Yoshikane, M.; Nishizawa, S.; Ueda, S.; et al. Hepatocellular Carcinoma in a Patient with Liver Cirrhosis Associated with Negative Serum HCV Tests but Positive Liver Tissue HCV RNA. Intern. Med. 2004, 43, 279–282.
- Hanafy, A.S.; Seleem, W.M.; Basha, M.; Marei, A.M. Residual hepatitis C virus in peripheral blood mononuclear cell as a risk factor for hepatocellular carcinoma after achieving a sustained virological response: A dogma or fiction. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1275–1282.
- Manickam, C.; Martinot, A.J.; Jones, R.A.; Varner, V.; Reeves, R.K. Hepatic immunopathology during occult hepacivirus re-infection. Virology 2017, 512, 48–55.
- Castillo, I.; Martinez-Ara, J.; Olea, T.; Bartolomé, J.; Madero, R.; Hernández, E.; Bernis, C.; Aguilar, A.A.; Quiroga, J.; Carreño, V.; et al. High prevalence of occult hepatitis C virus infection in patients with primary and secondary glomerular nephropathies. Kidney Int. 2014, 86, 619–624.
- Cao, Y.; Zhang, Y.; Wang, S.; Zou, W. Detection of the hepatitis C virus antigen in kidney tissue from infected patients with various glomerulonephritis. Nephrol. Dial. Transplant. 2009, 24, 2745–2751.
- Bataille, S.; Kaplanski, G.; Boucraut, J.; Halfon, P.; Camus, C.; Daniel, L.; Burtey, S.; Berland, Y.; Dussol, B. Membranoproliferative Glomerulonephritis and Mixed Cryoglobulinemia after Hepatitis C Virus Infection Secondary to Glomerular NS3 Viral Antigen Deposits. Am. J. Nephrol. 2012, 35, 134–140.
- Kong, D.; Wu, D.; Wang, T.; Li, T.; Xu, S.; Chen, F.; Jin, X.; Lou, G. Detection of viral antigens in renal tissue of glomerulonephritis patients without serological evidence of hepatitis B virus and hepatitis C virus infection. Int. J. Infect. Dis. 2013, 17, e535–e538.
- Casato, M.; Lilli, D.; Donato, G.; Granata, M.; Conti, V.; Del Giudice, G.; Rivanera, D.; Scagnolari, C.; Antonelli, G.; Fiorilli, M. Occult hepatitis C virus infection in type II mixed cryoglobulinaemia. J. Viral Hepat. 2003, 10, 455–459.
- Sikorska-Wiśniewska, M.; Sikorska, K.; Wróblewska, A.; Liberek, T.; Perkowska-Ptasińska, A.; Dębska-Ślizień, A. Recurrence of Cryoglobulinemia Secondary to Hepatitis C in a Patient with HCV RNA (−) Negative in the Serum. Case Rep. Nephrol. Dial. 2021, 11, 110–115.
- Giannini, C.; Giannelli, F.; Zignego, A.L. Association between mixed cryoglobulinemia, translocation (14;18), and persistence of occult HCV lymphoid infection after treatment. Hepatology 2006, 43, 1166–1167.
- Lotfi, A.; Mohamed, E.; Shalaby, A.; Eissa, N.; El-Dabaa, D.S.; Sallam, E.; Kamel, A.M.; Abdelaziz, M.M.; El-Afifi, H.; Abdel-Moneim, A.M. Occult hepatitis C virus infection in patients with malignant lymphoproliferative disorders. Int. J. Immunopathol. Pharmacol. 2020, 34.
- Kisiel, E.; Radkowski, M.; Pawelczyk, A.; Horban, A.; Stańczak, J.; Bukowska-Ośko, I.; Cortés, K.C.; Kazmierczak, J.; Popiel, M.; Laskus, T. Seronegative hepatitis C virus infection in patients with lymphoproliferative disorders. J. Viral Hepat. 2013, 21, 424–429.
- Sheikh, M.; Bokharaei-Salim, F.; Monavari, S.H.; Ataei-Pirkooh, A.; Esghaei, M.; Moradi, N.; Babaei, R.; Fakhim, A.; Keyvani, H. Molecular diagnosis of occult hepatitis C virus infection in Iranian injection drug users. Arch. Virol. 2018, 164, 349–357.
- Donyavi, T.; Bokharaei-Salim, F.; Khanaliha, K.; Sheikh, M.; Bastani, M.N.; Moradi, N.; Babaei, R.; Habib, Z.; Fakhim, A.; Esghaei, M. High prevalence of occult hepatitis C virus infection in injection drug users with HIV infection. Arch. Virol. 2019, 164, 2493–2504.
- Ayadi, A.; Nafari, A.H.; Irani, S.; Mohebbi, E.; Mohebbi, F.; Sakhaee, F.; Vaziri, F.; Siadat, S.D.; Fateh, A. Occult hepatitis C virus infection in patients with beta-thalassemia major: Is it a neglected and unexplained phenomenon? J. Cell. Biochem. 2019, 120, 11908–11914.
- Kahyesh-Esfandiary, R.; Sadigh, Z.; Esghaei, M.; Bastani, M.; Donyavi, T.; Najafi, A.; Fakhim, A.; Bokharaei-Salim, F. Detection of HCV genome in peripheral blood mononuclear cells of Iranian seropositive and HCV RNA negative in plasma of patients with beta-thalassemia major: Occult HCV infection. J. Med Virol. 2019, 91, 107–114.
- Barril, G.; Castillo, I.; Arenas, M.D.; Espinosa, M.; Garcia-Valdecasas, J.; Garcia-Fernandez, N.; González-Parra, E.; Alcazar, J.M.; Sánchez, C.; Diez-Baylón, J.C.; et al. Occult Hepatitis C Virus Infection among Hemodialysis Patients. J. Am. Soc. Nephrol. 2008, 19, 2288–2292.
- Abdelmoemen, G.; Khodeir, S.A.; Saif, S.A.-; Kobtan, A.; Abd-Elsalam, S. Prevalence of occult hepatitis C virus among hemodialysis patients in Tanta university hospitals: A single-center study. Environ. Sci. Pollut. Res. 2017, 25, 5459–5464.
- De Marco, L.; Manzini, P.; Trevisan, M.; Gillio-Tos, A.; Danielle, F.; Balloco, C.; Pizzi, A.; De Filippo, E.; D’Antico, S.; Violante, B.; et al. Prevalence and Follow-Up of Occult HCV Infection in an Italian Population Free of Clinically Detectable Infectious Liver Disease. PLoS ONE 2012, 7, e43541.
- Hedayati-Moghaddam, M.R.; Soltanian, H.; Ahmadi-Ghezeldasht, S. Occult hepatitis C virus infection in the Middle East and Eastern Mediterranean countries: A systematic review and meta-analysis. World J. Hepatol. 2021, 13, 242–260.
- Gatserelia, L.; Sharvadze, L.; Karchava, M.; Dolmazashvili, E.; Tsertsvadze, T. Occurrence of occult HCV infection among Hiv infected patients in Georgia. Georgian Med. News 2014, 226, 37–41.
- Bokharaei-Salim, F.; Keyvani, H.; Esghaei, M.; Zare-Karizi, S.; Dermenaki-Farahani, S.-S.; Hesami-Zadeh, K.; Fakhim, S. Prevalence of occult hepatitis C virus infection in the Iranian patients with human immunodeficiency virus infection. J. Med Virol. 2016, 88, 1960–1966.
- Jamshidi, S.; Bokharaei-Salim, F.; Esghaei, M.; Bastani, M.; Garshasbi, S.; Chavoshpour, S.; Dehghani-Dehej, F.; Fakhim, S.; Khanaliha, K. Occult HCV and occult HBV coinfection in Iranian human immunodeficiency virus-infected individuals. J. Med. Virol. 2020, 92, 3354–3364.
- De Marco, L.; Gillio-Tos, A.; Fiano, V.; Ronco, G.; Krogh, V.; Palli, D.; Panico, S.; Tumino, R.; Vineis, P.; Merletti, F.; et al. Occult HCV Infection: An Unexpected Finding in a Population Unselected for Hepatic Disease. PLoS ONE 2009, 4, e8128.
- Quiroga, J.A.; Avellon, A.; Bartolomé, J.; Andréu, M.; Flores, E.; González, M.I.; González, R.; Pérez, S.; Richart, L.A.; Castillo, I.; et al. Detection of hepatitis C virus (HCV) core–specific antibody suggests occult HCV infection among blood donors. Transfusion 2016, 56, 1883–1890.
- Martínez-Rodríguez, M.D.L.L.; Noguez, U.; Arroyo-Anduiza, C.I.; Mata-Marin, J.A.; Benitez-Arvizu, G.; Portillo-López, M.L.; Ocaña-Mondragón, A. Prevalence and risk factors of Occult Hepatitis C infections in blood donors from Mexico City. PLoS ONE 2018, 13, e0205659.
- Lin, H.; Chen, X.; Zhu, S.; Mao, P.; Zhu, S.; Liu, Y.; Huang, C.; Sun, J.; Zhu, J. Prevalence of Occult Hepatitis C Virus Infection among Blood Donors in Jiangsu, China. Intervirology 2016, 59, 204–210.
- Abdelwahab, S.F. Cellular immune response to hepatitis-C-virus in subjects without viremia or seroconversion: Is it important? Infect. Agents Cancer 2016, 11, 23.
- Roque-Cuéllar, M.C.; Sánchez, B.; García-Lozano, J.R.; Praena-Fernandez, J.M.; Roldán, A.N.; Aguilar-Reina, J. Cellular immune responses and occult infection in seronegative heterosexual partners of chronic hepatitis C patients. J. Viral Hepat. 2011, 18, e541–e549.
- MacParland, S.A.; Pham, T.N.Q.; Guy, C.S.; Michalak, T.I. Hepatitis C virus persisting after clinically apparent sustained virological response to antiviral therapy retains infectivity in vitro. Hepatology 2009, 49, 1431–1441.
- Veerapu, N.S.; Park, S.-H.; Tully, D.C.; Allen, T.; Rehermann, B. Trace amounts of sporadically reappearing HCV RNA can cause infection. J. Clin. Investig. 2014, 124, 3469–3478.
- Katayama, K.; Kumagai, J.; Komiya, Y.; Mizui, M.; Yugi, H.; Kishimoto, S.; Yamanaka, R.; Tamatsukuri, S.; Tomoguri, T.; Miyakawa, Y.; et al. Titration of Hepatitis C Virus in Chimpanzees for Determining the Copy Number Required for Transmission. Intervirology 2004, 47, 57–64.
- Barreiro, P.; Vispo, E.; Maida, I.; Aguilera, A.; Fernández-Montero, J.V.; de Mendoza, C.; Labarga, P.; Soriano, V. Very late HCV relapse following triple therapy for hepatitis C. Antivir. Ther. 2014, 19, 723–724.
- Hara, K.; Rivera, M.M.; Koh, C.; DeMino, M.; Page, S.; Nagabhyru, P.R.; Rehermann, B.; Liang, T.J.; Hoofnagle, J.H.; Heller, T. Sequence Analysis of Hepatitis C Virus From Patients With Relapse After a Sustained Virological Response: Relapse or Reinfection? J. Infect. Dis. 2014, 209, 38–45.
- Boschi, C.; Colson, P.; Tissot-Dupont, H.; Bernit, E.; Botta-Fridlund, D.; Aherfi, S. Hepatitis C Virus Relapse 78 Weeks After Completion of Successful Direct-Acting Therapy. Clin. Infect. Dis. 2017, 65, 1051–1053.
- Cortés-Mancera, F.M.; Restrepo, J.C.; Osorio, G.; Hoyos, S.; Correa, G.; Navas, M.C. Occult hepatitis C virus infection in a re-transplanted patient with liver failure of unknown etiology. Rev. Col. Gastroenterol. 2010, 25, 76. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-99572010000100016&lng=en (accessed on 29 October 2021).
- Castillo, I.; Bartolomé, J.; Quiroga, J.A.; Barril, G.; Carreño, V. Hepatitis C virus infection in the family setting of patients with occult hepatitis C. J. Med. Virol. 2009, 81, 1198–1203.
- Quiroga, J.A.; Castillo, I.; Llorente, S.; Bartolomé, J.; Barril, G.; Carreño, V. Identification of serologically silent occult hepatitis C virus infection by detecting immunoglobulin G antibody to a dominant HCV core peptide epitope. J. Hepatol. 2009, 50, 256–263.
- Castillo, I.; Bartolomé, J.; Quiroga, J.A.; Barril, G.; Carreño, V. Diagnosis of occult hepatitis C without the need for a liver biopsy. J. Med. Virol. 2010, 82, 1554–1559.
- Barril, G.; Quiroga, J.A.; Arenas, M.D.; Espinosa, M.; Garcia-Fernandez, N.; Cigarrán, S.; Herrero, J.A.; Del Peso, G.; Caro, P.; Agudo, R.G.; et al. Impact of Isolated Hepatitis C Virus (HCV) Core-Specific Antibody Detection and Viral RNA Amplification among HCV-Seronegative Dialysis Patients at Risk for Infection. J. Clin. Microbiol. 2014, 52, 3053–3056.
- Michalak, T.I.; Pham, T.N. Anti-HCV core antibody: A potential new marker of occult and otherwise serologically silent HCV infection. J. Hepatol. 2009, 50, 244–246.
More
