Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Shengtai Yan and Version 3 by Bruce Ren.
In order to assess the possibility of silica production via smoldering of moist rice husk, experiments of washed (moist) rice husk (7 kg with moisture content of 51%) in a newly designed smoldering apparatus was performed. The temperature inside the fuel bed during smoldering was recorded, and characteristics of ash were analyzed. Results showed that the highest temperature in the middle of the naturally piled fuel bed was about 560.0 °C, lower than those in most of combustors. Some volatiles from the lower part of the fuel bed adhere to its upper ash during piled smoldering. Silica content and specific surface area of ash from smoldering of washed (moist) rice husk were 86.4% and 84.9 m2/g, respectively.
- smoldering
- rice husk
- high moisture content
- silica
- specific surface area
1. Introduction
Smoldering is slow, low-temperature, and flameless burning of porous fuels, which is an important and complex phenomenon [1][2][1,2]. The application of it in the field of waste-to-energy conversion such as sludge treatment [3], recovery of resources from waste streams [4], and biomass energy conversion [5] has attracted lots of attention in recent years. The main advantages are its low temperature of the solid phase [6] and self-sustainability in a fuel bed with high moisture content (75–80 wt.%) [7]. From an environmental point of view, these characteristics avoid the ash-related slagging/corrosion [8], making nutrients recovery easy via recycling of ash directly to farms [9] and reducing the pollution of solid waste. As to energy consumption, it makes the complete burning of moist solid waste possible [10], reducing the energy consumption for drying fuel.
Rice husk is a typical biomass waste [11], accounting for 14–25% of the grain’s overall mass [12]. In 2021, approximately 150 million tons of rice husk were produced around the world, with China contributing approximately 40 million tons. Nowadays most rice husk is directly buried or open burned [13] due to its low nutritive value for humans compared with rice grain and rice bran [14]. Direct burying results in soil pollution, because of its slow decomposition owing to its hard surface resulting from its high silicon and high lignin content [15]. Open burning leads to air pollution because of the release of fine dust and incomplete combustion gases of CO, NOx, CH4, poly-cyclic hydrocarbons (PAH) and soot [16].
There is great potential in producing silica from rice husk due to its high content of amorphous silica (around 18–23% [17]) and ash (around 85–95% [18]). Silica is an important inorganic material and is widely used in various fields such as fertilizer, insulator, adsorbent and catalyst [19]. It is characterized by high mechanical strength, good chemical stability, high-temperature resistance, easy dispersion in solvents, etc [20][21][20,21]. With the widespread application of silica, a variety of methods have been adopted to produce it, such as precipitation, plasma synthesis, chemical vapor deposition, micro emulsion processing, combustion synthesis and hydrothermal technique [22][23][22,23]. At present, most popular mass producing methods are precipitation from alkaline silicates and hydrothermal treatment of sand with lye [24]. However, both methods are expensive, intensive energy input, and environmentally harmful due to the production of dust, nitrogen and sulfur oxides, etc in the process of obtaining silica [25][26][25,26].
Producing silica from thermochemical conversion of rice husk has received considerable attention due to its economic and environmental advantages [27]. As to lab-scale production, Dizaji et al. [28] prepared silica by burning raw rice husk and pretreated rice husk (water washing at 50 °C for 2 h) in a muffle furnace at 600 °C for 4 h. The specific surface area was around 45.0 and 240.0 m2/g, respectively. Abu Bakar et al. [29] prepared silica by burning rice husk (unleached/acid-leached) in a muffle furnace (600 °C for 2 h). The purity of silica from unleached and acid-leached rice husk was 95.8 and 99.6 wt.% (XRF results), respectively, and specific surface area was 116.0 and 218.0 m2/g, respectively. Almeida et al. [30] prepared a mixture of silica and carbon by pyrolysis of raw rice husk in a tubular furnace. The obtained silica was black, in a mixture of amorphous and crystalline, with purity of 81.6 wt.% and specific surface area of 114.0 m2/g. Schliermann et al. [31] obtained ashes produced from water washed (50 °C for 2 h) rice husk using ÖKOTHERM® furnaces. The ashes are post-treated with acid and then thermally treated at 650 °C using a muffle furnace. The specific surface area of silica is about 150–200 m2/g. As to industrial production, Fernandes et al. [32] investigated characteristics of ash from burning rice husk in a grate furnace, a fluidized bed, and a suspension/entrained combustor. The silica content in these three types of ash was 90.0, 96.7, 93.6 wt.%, and the specific surface area was 39.3, 11.4, 26.7 m2/g, respectively. The specific surface area of silica from mass production tends to be lower than that prepared in a laboratory, which may be related to none pretreatment of rice husk and the high burning temperature in industrial combustors. It is recorded that high combustion temperature results in the transformation of amorphous silica to crystalline material [33].
Pretreatment of rice husk is an effective way to increase the purity and specific surface area of silica and typical pretreatments for rice husk are acid-leaching and water-washing [29][34][35][29,34,35]. Moisture content of the treated rice husk is normally high. According to our pre-experiments, moisture content of rice husk is around 50% after washing. The moist rice husk is not suitable to be burned directly in a normal combustor (the moisture content in a fluidized bed combustor needs to be <35% [36], for a suspension burner <15% [37]). Smoldering might be a good choice for thermochemical conversion of moist rice husk directly to silica. Yet, to the best of our knowledge there are no experiments using smoldering in literature.
2. Characteristics of Smoldering Process
2.1. Temperature inside Fuel Bed
Temperature inside the washed (moist) and unwashed fuel bed is shown in Figure 1. The temperature history at one spot of the batch fuel bed can be divided into two stages―drying and oxidation. At the drying stage, the temperature first increases and then stabilizes at a temperature of about 60 °C. This is similar to the temperature of smoldering pine bark particle [38][39] and sewage sludge [4], which is different from the temperature (around 100 °C) of smoldering corn stalk powder [39][40] and corn flour [6]. Supplement experiments show this temperature is always around 60 °C in natural piled rice husk. In our experiments of smoldering branches, there is even no obvious plat temperature at the preheating period, which is similar to smoldering of unwashed rice husk. It implies temperature in the fuel bed at drying stage might be related to the porosity inside the fuel bed, materials, particle size, air flow, etc. The detailed analysis of this will be left for future work. At the oxidation stage, the temperature first increases rapidly and then increases at a stable rate. It drops quickly at the end stage of oxidation. For both cases the highest temperatures are around 560.0 °C, being much lower than those in most combustors (>700 °C) [40][41][41,42]. The low temperature is favorable to maintain the amorphous state of the silica [42][43].
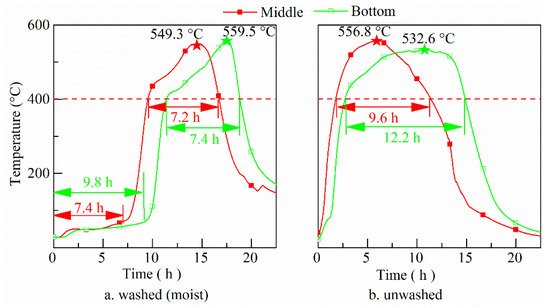

Figure 1. History of temperature of middle and bottom layer of washed (a) and unwashed (b) fuel bed (red and green pentacles for the maximum temperatures of middle and bottom layers, respectively).
Comparing the temperature development of the washed (moist) fuel bed with the unwashed fuel bed, the former has a longer duration of drying stage. The lower the part of the zone in the fuel bed, the longer the drying stage lasts. At oxidation stage, the duration of temperature > 400 °C of the moist fuel bed is shorter than for the unwashed fuel bed. This happens due to more heat generated in the process of smoldering is used to dry the moist rice husk and heat transfer rate to dry fuel is bigger than those for unwashed rice husk. The oxidation duration is also affected by bulk density of the fuel bed. In our supplementary experiments, smoldering of unwashed rice husk with bulk density of 170 kg/m3 was performed in a small apparatus. It was found that the maximum temperature becomes higher (around 600 °C) than for naturally piled rice husk. The bigger bulk density decreases the porosity inside the fuel bed, reducing thermal dispersions [38][39]. Besides, another possible reason is that the dwell time of gaseous species is extended resulting in longer duration.
2.2. Absorption of Volatiles by the Upper Ash
A one-dimensional simplified illustration on temperature field of the whole fuel bed after formation of a thin layer of ash at top surface is shown in Figure 2. It was drawn according to the history of temperature inside the fuel bed, our previous experiments [39][40] and the characteristic temperature profile in a forward smoldering system in the literature [4]. Due to the longer drying time of the moist fuel bed than an unwashed one shown in Figure 1, the former has a thinner layer of high temperature area (reaction zone) than the latter after a short time, as illustrated in the curve of Figure 2. The amount of the unreacted rice husk (without pyrolysis) is proportional to the marked area of the left side. During their devolatilization, part of the volatiles can be absorbed by the upper ash due to its low temperature. As a result, the ash of the moist fuel bed has a higher tendency to absorb volatiles from its lower part than the ash of fuel with less moisture.
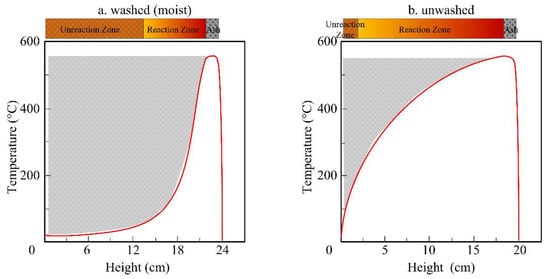

Figure 2. Schematic of spatial temperature distributions of washed (a) and unwashed (b) fuel bed.
3. Physical Properties and Mass Loss Characteristic of Rice Husk Ash
3.1. Physical Properties of Ash
Photos of ash before and after grinding from washed and unwashed smoldering as well as washed and unwashed burning are shown in Figure 3. These ashes are gray, soft, and almost retains the shape of rice husk itself. The whitest ash stems from washed burning, followed by unwashed burning. As for the other two ashes, the difference in the whiteness is negligible. The main reason is that temperature in smoldering (560 °C) is significantly lower than in muffle furnace (600 °C) and the duration of this temperature in smoldering is shorter. Other possible reasons are the removal of impurities like dust by washing or absorption of volatiles by the upper ash. The higher the whiteness, the higher silica content in ash [43][44]. It is worth mentioning that there are always some black particles in the ash. These might be the rice husk with incomplete combustion. Bridge forming in the fuel bed that makes the cooling of the related particle faster than the dense piled should be the reason.


Figure 3. Photos of four types of rice husk ash (circles show black particles in ash).
3.2. Mass Loss Characteristic of Rice Husk Ash
Thermogravimetric (TG) and derivative thermogravimetric (DTG) curves of four types of ash (washed and unwashed smoldering, washed and unwashed burning) are shown in Figure 4. It is seen from TG data that total mass-loss of four ashes is <5%. The mass loss from 50 to 950 °C for the above four types of ash are 4.2 wt.%, 3.1 wt.%, 2.5 wt.%, and 2.3 wt.%, respectively. The lower the combustion temperature and oxidation duration, the higher total mass loss.
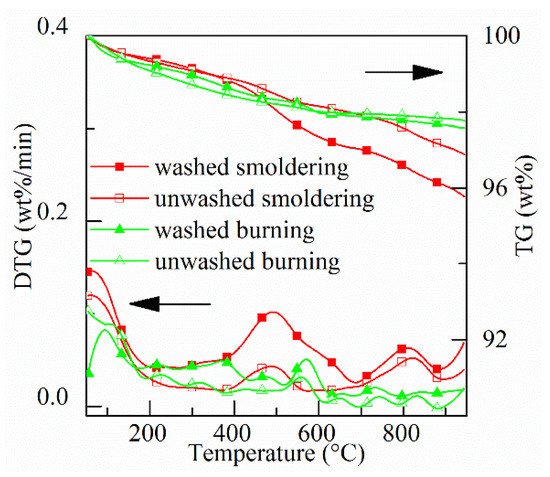

Figure 4. TG & DTG curves of four types of ash.
As shown in the DTG curves, there are three stages of mass loss: drying, oxidation, and combustion of residual carbon. Mass loss at each stage is shown in Table 1. At the drying stage (<200 °C), more water and longer drying time are there for burning ash than those for smoldering ash. This implies that the absorption of condensed materials decreases the capability of moisture absorption of ash. At the oxidation stage (200–700 °C), more mass is lost at lower temperatures for the two smoldering ashes than those for the burning ashes. For smoldering ashes, especially for smoldering of moist rice husk, mass loss occurs in the range 400–560 °C. In theory, the mass in this range is burned out during smoldering due to the maximum temperature inside the fuel bed is around 560 °C. The mass loss in this range indicates the oxidation of volatiles and carbon. At the stage of combustion of residual carbon (>700 °C), the mass loss of ash from smoldering is higher compared with burning ash due to more carbon in ash. The reason for the formation of residual carbon is that the melted silica obstructs the transport of oxygen to carbon [44][45]. At a higher temperature, the residual carbon can be burned out. Besides, the mass loss at this stage might also relate to the evaporation of KCl [45][38], the decomposition of carbonates [41][42].
Table 1. Mass loss of different stages (wt.%).
| Drying | Oxidation | Combustion of Resdiual Carbon | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | O | SO | 3 | P | 2 | O | 5 | MgO | Cl | Fe | 2 | CaOO | 3 | SO | 3 | P | 2 | O | 5 | Al | 2 | O | 3 |
| MgO | Cl | Fe | 2 | O | 3 | ||||||||||||||||||
| Washed smoldering | 0.6 ± 0.1 | 2.4 ± 0.2 | 1.2 ± 0.1 | ||||||||||||||||||||
| Unwashed smoldering | 0.7 ± 0.1 | ||||||||||||||||||||||
| 1 | 86.6 | 4.16 | 2.61 | 1.25 | 1.15 | 1.08 | 0.78 | 0.73 | 0.72 | ||||||||||||||
| 1.4 ± 0.1 | 1.0 ± 0.1 | ||||||||||||||||||||||
| 2 | 86.4 | 4.20 | 2.70 | 1.26 | Washed burning | 0.9 ± 0.1 | 1.3 ± 0.1 | 0.3 ± 0.1 | |||||||||||||||
| Unwashed burning | 0.8 ± 0.2 | 1.3 ± 0.1 | 0.2 ± 0.1 |
34. Silica Content in Rice Husk Ash
3.1. Reproducibility and Reliability of XRF Measurement
Main compositions (>0.5%) from triplicate XRF measurements of ash produced by smoldering of washed rice husk are shown in Table 3. It is seen that the reproducibility is good (relative error < 10%). As to reliability, if there are elements with atomic weights <23, the absolute contents of element are not reliable as pointed in Section 2.4. For smoldering ash, residue carbon and absorbed volatiles affect this measured elemental content.
Table 2. Contents of main elements in ash from triplicate measurements (wt.%).
| NO. | SiO | 2 | K | 2 | O | CaO | Al | 2 | O | 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Washed smoldering | 86.4 | 4.19 | 2.69 | 1.26 | 1.20 | 1.12 | 0.83 | 0.71 | 0.70 | ||||||
| Unwashed smoldering | 89.0 | 4.46 | 1.34 | 1.23 | 1.14 | 0.81 | 0.75 | 1.17 | 0.91 | 0.750.71 | 0.70 | ||||
| 0.49 | 0.41 | 3 | 86.2 | 4.20 | 2.76 | ||||||||||
| Washed burning | 93.4 | 1.07 | 2.08 | 1.26 | 1.22 | 1.15 | 0.90 | 0.70 | 0.69 | ||||||
| 0.58 | 0.50 | 0.70 | 0.30 | 0.39 | 0.21 | Ave | 86.4 ± 0.20 | 4.19 ± 0.03 | 2.69 ± 0.08 | 1.26 ± 0.01 | 1.20 ± 0.05 | 1.12 ± 0.04 | 0.83 ± 0.07 | 0.71 ± 0.02 | 0.70 ± 0.02 |
3.2. Content of Silica and Other Main Compositions in Rice Husk Ash
The contents of SiO2 and others main compositions in the four types of ash are listed in Table 3. The main component is SiO2 and the contents of it in all ashes is >85%. The SiO2 content in descending order is washed burning (93.4%), unwashed burning (90.2%), unwashed smoldering (89.0%), and washed smoldering (86.4%). Contents of other compositions in descending order of the content are K2O, CaO, SO3, P2O5, MgO, Cl, Fe2O3, Al2O3 in smoldering ash, and this order holds for most elements in other ashes.
Table 3. Main compositions in 4 types of ash.
| Types | SiO | 2 | K | |||||
|---|---|---|---|---|---|---|---|---|
| Unwashed burning | ||||||||
| 90.2 | 4.12 | 1.22 | 0.82 | 0.94 | 0.83 | 0.61 | 0.36 | 0.19 |
3.3. Effect of Production Method on Silica Content
The purity of silica in ash is affected by three main factors: absorption of volatiles by upper ash, combustion temperature and pretreatment of rice husk. Volatiles adhering to the surface of upper-ash smoldering decreases the SiO2 content. Incomplete burn out of solid organics at low combustion temperature also decreases SiO2 content in ash. The pretreatment way of washing can remove some water soluble inorganics, such as K, Cl and dust [46][47][46,47]. The removal of water soluble inorganics can increase the silica content [35]. According to Table 3, the measured silica content in ash of washed smoldering is similar to or lower than that in unwashed smoldering, while the content of water-soluble ions (Ca, S, P, Mg, Cl, Fe, Al) is higher than that in unwashed smoldering. The lower silica content for washed smoldering is related to the shorter oxidation duration in the moist fuel bed, which results in incomplete combustion of rice husk. The higher content of water-soluble ions might relate to the inaccuracy of XRF measurement. The removing of carbon results in an increase of those water-soluble ions contents as a percentage of the whole ash.
4. BET Specific Surface Area
4.1. Specific Surface Area of the Four Types of Rice Husk Ash
The BET specific surface area of ash produced in this study (washed and unwashed smoldering, washed and unwashed burning) and in literature is shown from Figure 5. Two characteristics can be seen from this data of this study: (1) The specific surface area of ash prepared from washed rice husk is higher than that from unwashed rice husk; (2) for the ash of prepared from washed rice husk, the specific surface area is lower when smoldering is used compared with burning in the muffle furnace. However, the situation is opposite for the ash prepared from unwashed rice husk.
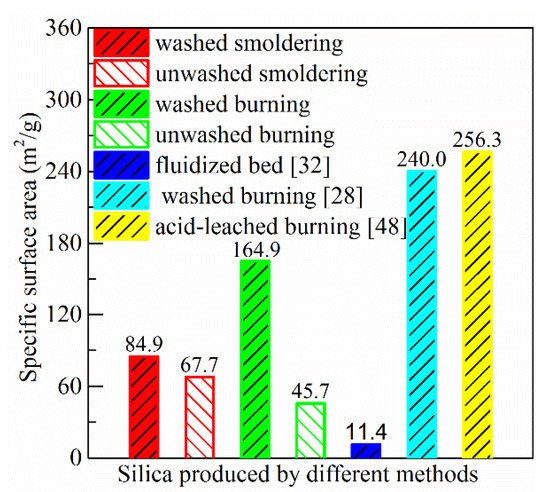

Figure 5. Specific surface area of silica produced by different methods. ([32]: no-pretreatment rice husk burned in a fluidized bed combustor; [28]: washed rice husk (50 °C tap water for 2 h) burned in a muffle furnace (600 °C for 4 h); [48]: citric acid-leached rice husk (1 wt.% at 80 °C for 3 h) burned in a muffle furnace (700 °C for 2 h)).
4.2. Factors of Specific Surface Area
It can be seen from the above characteristics that the specific surface area is affected by pretreatment conditions and combustion temperature. Pretreatment, such as washing, removes part of potassium. This decreases the formation of eutectic from interaction of K and Si. The decrease in amount of the eutectic partly avoids the transformation from amorphous silica to crystalline via melting in eutectic and condensing in cooling stage, and consequently increases the specific surface area [49]. Pretreatment of washing also removes soil particles which normally have lower specific surface area than amorphous silica.
As to temperature, low temperature avoids sintering/eutectic melting of the mixed components in ash of rice husk and is beneficial for silica to maintain its amorphous state and high specific surface area [50]. There is a combination effect of the two factors. It is very hard to get high-specific-surface-area ash from burning original rice husk at a high temperature (>700 °C).
4.3. Comparison of Specific Surface Area in This Study with Those of Silica Prepared Using Methods in Literature
The specific surface area of ash produced by smoldering of washed rice husk is 84.9 m2/g, which is lower than those prepared in the laboratory (99.2–293.9 m2/g) [13][29][51][13,29,51], but higher than those produced in the industry (11.4–39.3 m2/g) [32][52][32,52], as shown in Figure 5. In the laboratory, rice husk is normally washed or leached using water and acid to remove alkali and alkaline earth metals, such as the experiments performed by Dizaji [28] and Huang [48]. In the industry, no-pretreatment rice husk is burned directly in combustors. The high temperature (>700 ℃) of most combustor is not suitable to produce silica with high specific surface area.
