Magnesium alloys are a strong candidate for various applications in automobile and aerospace industries due to their low density and specific strength. Micro-alloying magnesium with zinc, yttrium, and cerium enhances mechanical properties of magnesium through grain refinement and precipitation hardening.
- thermodynamic modeling
- magnesium
- phase diagram
- liquidus projection
1. Introduction
Micro-alloying magnesium with RE such as zinc and yttrium resulted in promising mechanical properties [1][2][3][4][5][6][7][8][9][27,31,35,40,44,46,55,58,60]. Furthermore, the addition of RE elements to Mg-Zn promote activation of prismatic slip and increase the stacking fault energy, therefore weakening the texture of magnesium alloys [3][10][11][12][13][4][5][14][15][16][17][35,36,37,38,39,40,44,51,61,66,72]. Micro-alloying magnesium with zinc increases its fluidity in casting [18][77], whereas yttrium addition has a remarkable effect on aging precipitation and high solid solution strengthening [19][20][21][78,79,80]. Moreover, cerium tends to precipitate a thermally high stable compound (Mg2Ce) in magnesium rich region, which improve microstructure stability at elevated temperatures. Diluting zinc in Mg-Ce alloy significantly improves stretch formability by modifying the basal plane texture through solid solution hardening mechanism [22][23][24][25][26][81,82,83,84,85]. Moreover, the highest zinc in Mg-Ce alloy improves yield strength and ultimate tensile strength through precipitation of intermetallic compounds. Whereas the ratio of Ce/Zn increases, grain refinements and loss of formability occurs [27][22][23][24][25][26][28][29][30][71,81,82,83,84,85,86,87,88]. Mg-Zn-Y alloys display promising mechanical properties because of precipitates of thermally stable ternary compounds (W-Mg3Y solid solution, I-Mg3YZn6, and LPSO-phase Mg12ZnY) as well as high solubility of yttrium in magnesium.
To better understand phase stability, phase relation, and the effect of precipitation on age hardening, knowledge of binary and ternary phase diagrams is essential. Additionally, accurate prediction of phase diagram plays an important role in materials development and alloy design. Phase diagram is a tool used to predict the equilibrium phase(s) and phase(s) percentage at certain temperatures for specified alloys and simulate the phase consistency and solidification process of individual alloys. Moreover, the percentage of the predicted phase(s) that exist in the microstructure can be calculated.
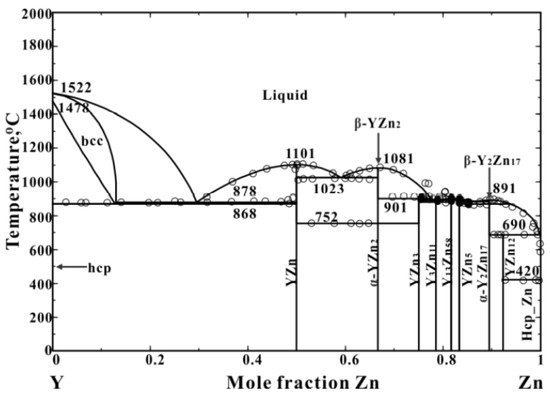
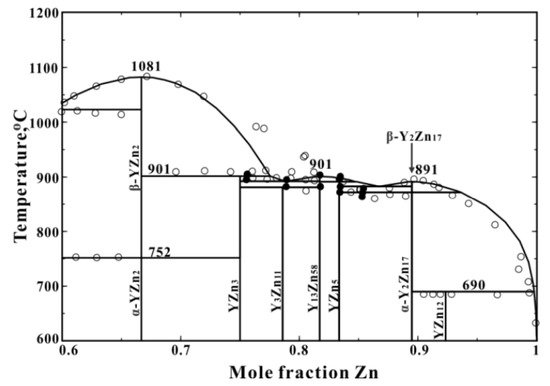
2. Zinc-Yttrium Phase Diagram
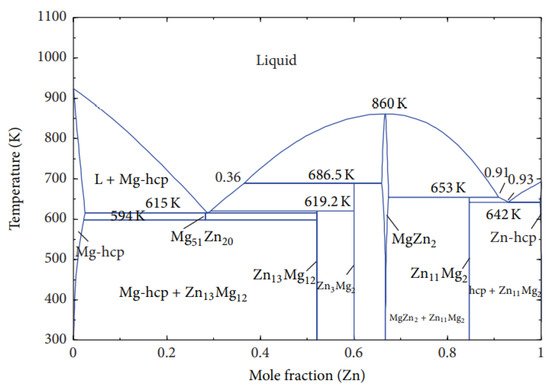
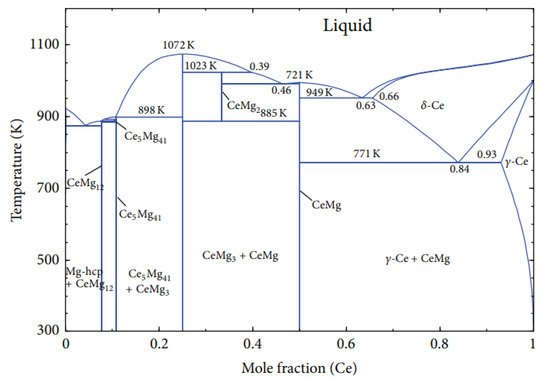
3. Mg-Zn, Mg-Y, and Mg-Ce Phase Diagrams
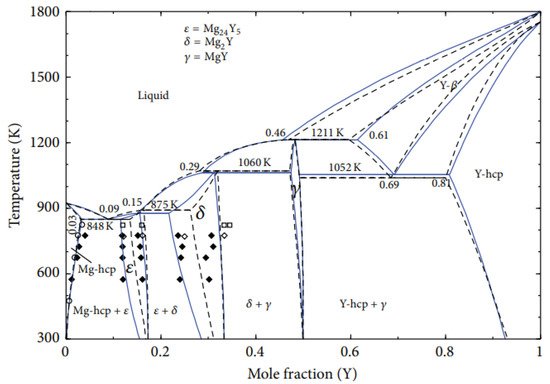
4. Magnesium-Zinc-Yttrium Ternary Phase Diagram
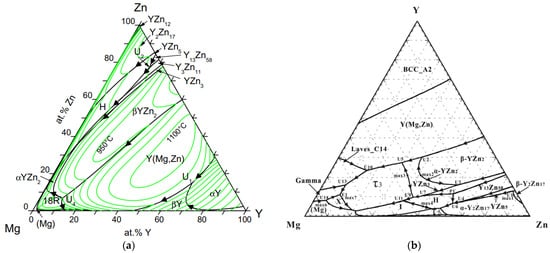
5. Mechanical Properties of Mg-Zn-{Ce, Y} Alloys
| Nominal Composition (wt.%) |
(wt.%)Yield Strength (MPa) |
Ultimate Tensile Strength | Yield Strength (MPa) (MPa) |
Ultimate Tensile Strength Ductility |
Process Conditions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (MPa) | Ductility | Process Conditions | |||||||||||
| Mg-2Zn+0.4Y | 160 | 240 | 30% | Samples were cast at 690 °C and then extruded at 310 °C | |||||||||
| Mg-2Zn+0.4Ce | 190 | [ | 51 | ] | Samples were cast at 690 °C and then extruded at 310 °C [154] | ||||||||
| 255 | 18% | Samples were cast at 690 °C and then extruded at 310 °C | [ | 55] | Samples were cast at 690 °C and then extruded at 310 °C [48] | Mg-14.4Zn-3.3Y | 365 ± 3.5 | 380 | 8% | Samples were cast and solutionized at 480 °C for 24 h followed by extrusion at 430 °C, then aged at 150 °C [52] | Samples were cast and solutionized at 480 °C for 24 h followed by extrusion at 430 °C, then aged at 150 °C [155] | ||
| Mg-2Zn-0.2Ce (ZE20) |
69 | 170 | 31% | Samples were cast at 700 °C and then extruded at 400 °C [55] | Samples were cast at 700 °C and then extruded at 400 °C [48] | Mg-14.4Zn-3.3Y | 171 | 320 | 12 | Samples were cast and solutionized at 480 °C for 24 h followed by extrusion at 430 °C [52] | Samples were cast and solutionized at 480 °C for 24 h followed by extrusion at 430 °C [155] | ||
| Mg-5Zn-0.2Ce (ZE50) |
135 | 247 | 15% | Mg-1.5Zn-0.2Y | 17% | The ingots were homogenized at 450 °C for 12 h, then rolled at 400 °C, and after that sheet annealed at 350 °C for 1 h [53] | The ingots were homogenized at 450 °C for 12 h, then rolled at 400 °C, and after that sheet annealed at 350 °C for 1 h [156] | ||||||
| Mg-6.0Zn-1.0Y | |||||||||||||
| Mg-8Zn-0.2Ce | 135 | (ZE80)268.3 | 12.9% | Mg-1.5Zn-0.2Ce | Alloys were solutionized at 480 °C and then extruded at 390 °C [54] | Alloys were solutionized at 480 °C and then extruded at 390 °C [157] | |||||||
| 136 | 238 | 289 | 16% | 140 | 240 | 19% | The ingots were homogenized at 450 °C for 12h, then rolled at 400 °C and after that sheet annealed at 350° C for 1 h [53] | The ingots were homogenized at 450 °C for 12h, then rolled at 400 °C and after that sheet annealed at 350° C for 1 h [156] | Mg-6.0Zn-1.0Y | 288.7 | 17.3% | Alloys were solutionized at 480 °C and then extruded at 390 °C and aged at 150 °C for 48h [54] | |
| Mg-6Zn-0.2Ce | 225 | 270 | 30% | Alloys were solutionized at 480 °C and then extruded at 390 °C and aged at 150 °C for 48h [157] | |||||||||
| Alloys were cast at 750 °C and homogenized at 350 °C for 12 h. After extrusion, alloys aged at 175 °C from 0.5 to 80 h | [ | 54 | ] | Alloys were cast at 750 °C and homogenized at 350 °C for 12 h. After extrusion, alloys aged at 175 °C from 0.5 to 80 h [157] | Mg-3.0Zn-0.5Y | 262 | 18.3% | Alloys were solutionized at 480 °C and then extruded at 350 °C [54] | Alloys were solutionized at 480 °C and then extruded at 350 °C [157] |
| Nominal Composition | |||||
|---|---|---|---|---|---|
| Mg-2%Zn-0.5%Ce (ZE20) | |||||
| 199.2 | |||||
| ~245 | 6% | Samples were prepared by continuous casting, then homogenized at 823 K for 8 h. Sheets were rolled by conventional rolling at 673 K | [ | 56] | Samples were prepared by continuous casting, then homogenized at 823 K for 8 h. Sheets were rolled by conventional rolling at 673 K [158] |
| Mg-2%Zn-0.5%Ce (ZE20) | 125 | ~235 | 13.8% | Samples were prepared by continuous casting, then homogenized at 823 K for 8 h. Sheets were rolled by conventional rolling at 673 K. Sheets were annealed at 673 K [56] | Samples were prepared by continuous casting, then homogenized at 823 K for 8 h. Sheets were rolled by conventional rolling at 673 K. Sheets were annealed at 673 K [158] |
| Mg-2%Zn-0.5%Ce (ZE20) | ~170 | ~240 | 28.23 | Samples were prepared by continuous casting, then homogenized at 823 K for 8 h. Sheets were rolled by packed rolling at 673 K. Sheets were annealed at 673 K [56] | Samples were prepared by continuous casting, then homogenized at 823 K for 8 h. Sheets were rolled by packed rolling at 673 K. Sheets were annealed at 673 K [158] |
| Mg-2%Zn-0.5%Ce (ZE20) | ~165 | ~236 | 33.4% | Samples were prepared by continuous casting, then homogenized at 823 K for 8 h. Sheets were rolled by packed rolling at 723 K. Sheets were annealed at 723 K [56] | Samples were prepared by continuous casting, then homogenized at 823 K for 8 h. Sheets were rolled by packed rolling at 723 K. Sheets were annealed at 723 K [158] |
| Mg-0.5Zn-0.2Ce | 133 | 213 | 25% | Samples were heated at 723 K for 20 min and sheets were rolled by unidirectional rolling at 353 K. Then, sheets were annealed at 623 K for 90 min [22] | Samples were heated at 723 K for 20 min and sheets were rolled by unidirectional rolling at 353 K. Then, sheets were annealed at 623 K for 90 min [81] |
| Mg-1.0Zn-0.2Ce | 110 | 202 | 23% | ||
| Mg-1.5Zn-0.2Ce | 116 | 206 | 29% | ||
| Mg-2.0Zn-0.2Ce | 118 | 222 | 25% | ||
| Mg-2.5Zn-0.2Ce | 131 | 228 | 16% | ||
| Mg-1.5Zn-0.2Ce | 153 | 231 | 26% | Samples were extruded at 703 K and then annealed at 623K for 90 min [26] | Samples were extruded at 703 K and then annealed at 623K for 90 min [85] |
| Mg-1.5Zn-0.2Ce | 194 | 248 | 20% | Samples were extruded at 573K and then annealed at 623K for 90 min [26] | Samples were extruded at 573K and then annealed at 623K for 90 min [85] |
| Mg-1.0Zn-1.0Ce | 95 | 191 | 22% | Samples were annealed at 350 °C and then annealed at 450 °C for 1 h [27] | Samples were annealed at 350 °C and then annealed at 450 °C for 1 h [71] |
| Mg-2.0Zn-1.0Ce | 101 | 197 | 26.2% | ||
| Mg-4Zn-1.0Ce | 109 | 220 | 18% | ||
| Mg-1.0Zn-0.5Ce | 95 | 191 | 30% |