Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Jessie Wu and Version 2 by Jessie Wu.
Preterm birth (PTB) is a global health issue and one of the most challenging problems affecting 12.9 million births worldwide. PTB is a multi-etiological disease and remains incompletely understood.
- preterm birth
- microbiota
1. Vaginal Microbiome and Preterm Birth
During pregnancy, feto-maternal tolerance regulates the maternal immune response between anti-inflammatory and proinflammatory states. If this balance is disrupted by ascending microorganisms, the maternal immune system changes and leads to preterm labor [1]. It has been observed that the complexity and diversity of the vaginal microbiome increases with PTB, whereas the vaginal microbiome is less complex and less diverse in normal pregnancy. As mentioned before, the complement system plays an important role in normal pregnancy, therefore, complement activation leads to the chemotactic recruitment of immune cells including macrophage and dendritic cells involving PTB [2]. PTB is also related to proinflammatory cytokine profiles such as IL-1β, IL-6, macrophage inflammatory protein (MIP)-1β, and eotaxin [3]. Park et al. [4] investigated the roles of cytokines in the cervicovaginal fluid as predictive markers of PTB. MIP-1α, MIP-1β, IL-6, IL-7, and IL-17α in the cervicovaginal fluid were associated with PTB and IL-6, and IL-17α had a higher sensitivity than the fetal fibronectin test.
Previous studies showed that Lactobacillus iners was associated with an increased risk of PTB despite the differences depending on ethnicity, whereas Lactobacillus crispatus showed a protective effect against PTB in all ethnicities [5][6]. In addition, the dominant population of Lactobacillus iners around 16 gestational weeks was closely related to the increased risk of shortening of the cervix and PTB before 34 gestational weeks [7]. As well as Lactobacillus spp., bacterial vaginosis-associated bacteria including Gardnerella vaginalis, Atopobium vaginae, and Veillonellaceae bacterium were associated with an increased risk of PTB before 34 gestational weeks [3]. Son et al. [8] investigated the comparisons of obstetrical outcomes according to the vaginal microbiota grouped by trimester. Abnormal vaginal microbiota, especially the presence of Klebsiella pneumonia, in the 2nd trimester was associated with a significant increase in PTB before 28 weeks.
Results of recent studies between the vaginal microbiome and PTB are presented in Table 1. Molecular-based new technologies have been applied to take advantage of the new information about the role of the vaginal microbiota in spontaneous labor and PTB. However, the current evidence is still limited, and clinical data have poor quality and results are controversial. Recently, most of the studies have demonstrated an association between the composition of the vaginal microbiota and PTB (Table 1). The more recently published studies provide evidence of an association between a dysbiotic microbiota and PTB, especially the role of L. iners in vaginal eubiosis and dysbiosis.
Table 1. Recent studies between Vaginal Microbiome and Preterm Birth.
| Condition Studied | Summary | |
|---|---|---|
| 2019 Fettweis et al. [3] | 45 preterm and 90 term birth controls | Preterm-delivered women had significantly lower vaginal levels of Lactobacillus crispatus and higher levels of Sneathia amnii, and Prevotella species. |
| 2018 Freitas et al. [9] | 46 preterm and 170 term birth controls | The preterm-delivered women had increased richness and diversity and higher Mycoplasma or Ureaplasma prevalence. |
| 2017 Callahan et al. [10] | Low risk for PTB: predominantly Caucasian (n = 39) high-risk for PTB: predominantly African American (n = 96) | Lactobacillus crispatus was related to low risk of PTB, while Lactobacillus iners and Gardnerella vaginalis had association with PTB. |
| 2017 Stafford et al. [11] | No preterm labor group (n = 121), preterm labor group (n = 41) | The microbiome of women who experienced PTB showed 2-fold lower community state type (CST) I-dominated microbiota at 20–22 weeks. CST V was 2-fold higher in the preterm-delivered women compared to term-delivered women. |
| 2017 Stout et al. [12] | Nested case-control study, 24 cases and 53 controls | The vaginal microbiome demonstrated decreased vaginal richness and Shannon diversity in preterm delivery. |
| 2016 Nelson et al. [13] | Nulliparous African American women, 13 preterm and 27 term birth controls | Decreased bacterial diversity with lower abundance of Coriobacteriaceae, Sneathia, Prevotella, and Aerococcus were found in preterm delivery. |
| 2014 Romero et al. [14] | Nested case-control study, 18 cases and 72 controls | As pregnancy progressed, four Lactobacillus spp. were increased and anaerobic microbiomes were decreased. |
About one-third of PTB are preceded by preterm premature rupture of membranes (PPROM) [15]. The cause of PPROM seems to be ascending microorganisms, and the rupture of membranes also can become the entrance of ascending microbes, so infection can be both a cause and a result of PPROM. Ascending pathogens trigger inflammation pathways, leading to the development of chorioamnionitis and funisitis [16]. A few studies have investigated the relationship between vaginal microbiota and the risk of PPROM [17][18]. One study showed that vaginal microorganisms collected from normal pregnant women were characterized by Lactobacillus spp. dominance and low diversity, whereas about half of the pregnant women who subsequently experienced PPROM had intermediate or low Lactobacillus spp. dominance and high diversity [18]. In another prospective cohort study, the authors reported that reduced Lactobacillus spp. abundance and high diversity were shown in about 25% of pregnant women prior to PPROM, but only 3% of women delivered the baby at term without the rupture of membranes [19]. PPROM was associated with changes in the microbiome during pregnancy and a shift toward higher diversity, predominantly occurring during the second trimester, although a vaginal microbiota dominated with any bacterial species rather than Lactobacillus was related to subsequent PPROM throughout all of the pregnancy period including during the first trimester [17]. This study also found that the first trimester miscarriage associated with a Lactobacillus spp-depleted vaginal microbiome and women who had the risk of miscarriage in the first trimester had a 2-fold increased risk of PTB and 3-fold increased risk of PPROM [20]. This study showed the potential relationship between miscarriage and PPROM and the first trimester microbiome.
2. Endometrial Microbiome in Preterm Birth
The endometrium is the important site where the blastocyst is implanted during pregnancy and is a crucial place, not only for supporting fetal growth by supplying oxygen and nutrients but also for preventing infections to protect the embryo and fetus [21]. During the implantation period, the endometrium undergoes significant morphologic and functional change, which is followed by decidualization, and many immune cells’ compositions are altered. The endometrium is not a sterile tissue, and microorganisms at the endometrium interact with the endometrial epithelium and modify immune cell expression and cytokines. This change can affect endometrial receptivity and may impair adequate implantation [22].
As well as implantation, modification of the endometrial immune system during pregnancy has been related to adverse pregnancy outcomes including miscarriage, preeclampsia (PE), FGR, and PTB [23]. A previous study reported a relationship between reduced levels of Lactobacillus species in the endometrial microbiota and adverse pregnancy outcome [24]. Interestingly, the endometrial bacterial population was different from the bacterial composition of the vagina but was similar with that of the cervix regarding bacterial load and composition. To date, the role of endometrial microbiota in pregnancy outcomes is not fully understood and much remains to be investigated.
3. Oral-Placental Microbiome in Preterm Birth
The tolerogenic maternal immune response is the most important factor for a healthy pregnancy. Interruption of this state leads to maternal anti-fetal rejection, placental damage, and obstetric complications such as FGR and PTB. The cause of this allograft rejection is either a cellular (T cell) or humoral (antibody) immune response, and severe rejection leads to fetal death akin to graft failure in organ transplantation. The fetal systemic inflammatory is similar to allograft rejection despite the absence of pathogens [25].
The possibility that the microbiome is present in the placental site was suggested [26]. The presence of fetal genital tract microbes colonization in the placenta or amniotic membranes has been thought to result in subclinical infection and a concomitant initiation of labor [27]. It is well known that ascending microbes from the vagina such as Ureaplasma, Mycoplasma, and GBS species have been related to placental colonization, chorioamnionitis, and PTB. Moreover, oral cavity microbes including Streptococcus and Fusobacterium spp. are known to contribute to the placental microbiome through hematogenous transfer [28]. Harboring bacteria were found in the placentas from term pregnant women who delivered by sterile cesarean section without infection sign and the amniotic fluid from women who had intact membranes [29]. A previous study reported that the placental microbiome from the vaginal and oral microbiomes was identified at the time of delivery using 16S ribosomal RNA gene sequencing analysis. An increased Fusobacterium nucleatum, Gemella asaccharolytica, and Ureaplasma spp. was found in the fetal membranes, and this is associated with shorter gestation and PTB [30]. A placental microbiome that is similar to the oral cavity, the tonsils and tongue, including Firmicutes, Tenericutes, and Fusobacteria, was found in placentas that were previously undetectable in the microbiome using 16S rRNA sequencing [27].
Even though common oral pathogens were identified in the placenta of women with periodontal disease, which is related to increased risk of PTB, it is not clear if the management of periodontal disease during pregnancy decreased PTB [31]. There is insufficient information to determine whether periodontal management can prevent preterm birth. Many studies have been performed to reveal the relationship between the existence of an oral-placental microbiome and adverse pregnancy outcomes, but this research area is still controversial.
4. Microbiomes. The Prevention of the PTB
There is evidence to show the relationship between maternal microbiome profiles and increased risk of PTB, therefore, a large number of studies have investigated the effectiveness of antibiotics for the treatment and prevention of PTB. The maternal microbiome including the vaginal, oral-placenta, and gut microbiomes can play important roles in causing preterm birth (Figure 1).
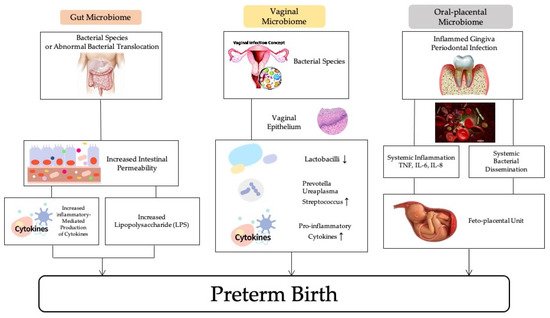

Figure 1. The association of maternal microbiomes and preterm birth.
The target of treatment for pregnant women was usually BV and the results were inconsistent [32][33]. The double blinded PREMEVA trial was conducted to evaluate the effect of oral clindamycin in early pregnancy to prevent late miscarriage (16–21 weeks of gestation) or spontaneous early PTB (22–32 weeks of gestation). There was no difference between the treatment and the placebo group [34]. However, another study showed that screening and treating BV in pregnant women with previous history of PTB is still effective in preventing PTB [32]. The use of certain antibiotics such as metronidazole may cause bacterial lysis and the release of endotoxins [35], and these are strong stimulators of inflammation and may enhance the inflammatory phenotype [36]. In addition, some antibiotics may be effective against Lactobacillus but not against microorganisms associated with BV, which was commonly found in antibiotic resistance genes [37].
Antibiotic treatment to ameliorate PTB could fail for women with abnormal vaginal microbes, positive fetal fibronectin, or previous PTB history, and it has raised interest in the positive regulation of vaginal microbiomes using probiotics or live bio-therapeutic products. Several studies have been conducted to reveal the effectiveness of probiotics to prevent PTB. Probiotics may be taken orally or, less commonly, vaginally. One study found that oral probiotic use in pregnancy did not decrease the risk of PTB [38], but an observational study revealed that probiotic milk intake in early pregnancy, not mid to late pregnancy, was related to reduce the risk of PTB [39]. Recent randomized controlled studies have reported that oral probiotics do not affect the vaginal microbiome during pregnancy [40][41].
Increased concentration of folic acid has been found in the placenta of PTB women without excess gestational weight gain [42]. One study showed that folic acid consumption started after the first and second trimester is related to an increase in the risk of PTB [43], but another study found folic acid supplementation slightly reduces the risk of PTB [44].
References
- Integrative HMP Research network Consortium The Integrative Human Microbiome Project. Nature 2019, 569, 641–648.
- Dunn, A.B.; Dunlop, A.L.; Hogue, C.J.; Miller, A.; Corwin, E.J. The Microbiome and Complement Activation: A Mechanistic Model for Preterm Birth. Biol. Res. Nurs. 2017, 19, 295–307.
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021.
- Park, S.; You, Y.A.; Yun, H.; Choi, S.J.; Hwang, H.S.; Choi, S.K.; Lee, S.M.; Kim, Y.J. Cervicovaginal fluid cytokines as predictive markers of preterm birth in symptomatic women. Obstet. Gynecol. Sci. 2020, 63, 455–463.
- Petricevic, L.; Domig, K.J.; Nierscher, F.J.; Sandhofer, M.J.; Fidesser, M.; Krondorfer, I.; Husslein, P.; Kneifel, W.; Kiss, H. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 2014, 4, 5136.
- Tabatabaei, N.; Eren, A.M.; Barreiro, L.B.; Yotova, V.; Dumaine, A.; Allard, C.; Fraser, W.D. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: A case-control study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 349–358.
- Gerson, K.D.; McCarthy, C.; Elovitz, M.A.; Ravel, J.; Sammel, M.D.; Burris, H.H. Cervicovaginal microbial communities deficient in Lactobacillus species are associated with second trimester short cervix. Am. J. Obstet. Gynecol. 2020, 222, 491.e1.
- Son, K.A.; Kim, M.; Kim, Y.M.; Kim, S.H.; Choi, S.J.; Oh, S.Y.; Roh, C.R.; Kim, J.H. Prevalence of vaginal microorganisms among pregnant women according to trimester and association with preterm birth. Obstet. Gynecol. Sci. 2018, 61, 38–47.
- Freitas, A.C.; Bocking, A.; Hill, J.E.; Money, D.M. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome 2018, 6, 117.
- Callahan, B.J.; DiGiulio, D.B.; Goltsman, D.S.A.; Sun, C.L.; Costello, E.K.; Jeganathan, P.; Biggio, J.R.; Wong, R.J.; Druzin, M.L.; Shaw, G.M.; et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc. Natl. Acad. Sci. USA 2017, 114, 9966–9971.
- Stafford, G.P.; Parker, J.L.; Amabebe, E.; Kistler, J.; Reynolds, S.; Stern, V.; Paley, M.; Anumba, D.O.C. Spontaneous Preterm Birth Is Associated with Differential Expression of Vaginal Metabolites by Lactobacilli-Dominated Microflora. Front. Physiol. 2017, 8, 615.
- Stout, M.J.; Zhou, Y.; Wylie, K.M.; Tarr, P.I.; Macones, G.A.; Tuuli, M.G. Early pregnancy vaginal microbiome trends and preterm birth. Am. J. Obstet. Gynecol. 2017, 217, 356.e1.
- Nelson, D.B.; Shin, H.; Wu, J.; Dominguez-Bello, M.G. The Gestational Vaginal Microbiome and Spontaneous Preterm Birth among Nulliparous African American Women. Am. J. Perinatol. 2016, 33, 887–893.
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Bieda, J.; Chaemsaithong, P.; Miranda, J.; Chaiworapongsa, T.; Ravel, J. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2014, 2, 18.
- Parry, S.; Strauss, J.F. 3rd. Premature rupture of the fetal membranes. N. Engl. J. Med. 1998, 338, 663–670.
- Puri, K.; Taft, D.H.; Ambalavanan, N.; Schibler, K.R.; Morrow, A.L.; Kallapur, S.G. Association of Chorioamnionitis with Aberrant Neonatal Gut Colonization and Adverse Clinical Outcomes. PLoS ONE 2016, 11, e0162734.
- Brown, R.G.; Chan, D.; Terzidou, V.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; MacIntyre, D.A.; Bennett, P.R. Prospective observational study of vaginal microbiota pre- and post-rescue cervical cerclage. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 916–925.
- Brown, R.G.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Lehne, B.; Kindinger, L.M.; Terzidou, V.; Holmes, E.; Nicholson, J.K.; Bennett, P.R.; et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018, 16, 9.
- Brown, R.G.; Al-Memar, M.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Chan, D.; Lewis, H.; Kindinger, L.; Terzidou, V.; Bourne, T.; et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl. Res. 2019, 207, 30–43.
- Al-Memar, M.; Bobdiwala, S.; Fourie, H.; Mannino, R.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Timmerman, D.; Bourne, T.; Bennett, P.R.; et al. The association between vaginal bacterial composition and miscarriage: A nested case-control study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 264–274.
- Kitaya, K.; Yasuo, T.; Tada, Y.; Hayashi, T.; Iwaki, Y.; Karita, M.; Funabiki, M.; Taguchi, S.; Spillers, D.; Nakamura, Y.; et al. Unusual inflammation in gynecologic pathology associated with defective endometrial receptivity. Histol. Histopathol. 2014, 29, 1113–1127.
- Di Simone, N.; Santamaria Ortiz, A.; Specchia, M.; Tersigni, C.; Villa, P.; Gasbarrini, A.; Scambia, G.; D’Ippolito, S. Recent Insights on the Maternal Microbiota: Impact on Pregnancy Outcomes. Front. Immunol. 2020, 11, 528202.
- Liu, S.; Diao, L.; Huang, C.; Li, Y.; Zeng, Y.; Kwak-Kim, J.Y.H. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol. 2017, 124, 44–53.
- Moreno, I.; Franasiak, J.M. Endometrial microbiota-new player in town. Fertil. Steril. 2017, 108, 32–39.
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Kim, J.S. Chronic inflammation of the placenta: Definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S53–S69.
- Mor, G.; Kwon, J.Y. Trophoblast-microbiome interaction: A new paradigm on immune regulation. Am. J. Obstet. Gynecol. 2015, 213, S131–S137.
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65.
- Vander Haar, E.L.; So, J.; Gyamfi-Bannerman, C.; Han, Y.W. Fusobacterium nucleatum and adverse pregnancy outcomes: Epidemiological and mechanistic evidence. Anaerobe 2018, 50, 55–59.
- Parris, K.M.; Amabebe, E.; Cohen, M.C.; Anumba, D.O. Placental microbial-metabolite profiles and inflammatory mechanisms associated with preterm birth. J. Clin. Pathol. 2021, 74, 10–18.
- Doyle, R.M.; Harris, K.; Kamiza, S.; Harjunmaa, U.; Ashorn, U.; Nkhoma, M.; Dewey, K.G.; Maleta, K.; Ashorn, P.; Klein, N. Bacterial communities found in placental tissues are associated with severe chorioamnionitis and adverse birth outcomes. PLoS ONE 2017, 12, e0180167.
- Puertas, A.; Magan-Fernandez, A.; Blanc, V.; Revelles, L.; O’Valle, F.; Pozo, E.; León, R.; Mesa, F. Association of periodontitis with preterm birth and low birth weight: A comprehensive review. J. Matern. Fetal. Neonatal. Med. 2018, 31, 597–602.
- Brocklehurst, P.; Gordon, A.; Heatley, E.; Milan, S.J. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst. Rev. 2013, 1, CD000262.
- Lamont, R.F.; Nhan-Chang, C.L.; Sobel, J.D.; Workowski, K.; Conde-Agudelo, A.; Romero, R. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2011, 205, 177–190.
- Subtil, D.; Brabant, G.; Tilloy, E.; Devos, P.; Canis, F.; Fruchart, A.; Bissinger, M.C.; Dugimont, J.C.; Nolf, C.; Hacot, C.; et al. Early clindamycin for bacterial vaginosis in pregnancy (PREMEVA): A multicentre, double-blind, randomised controlled trial. Lancet 2018, 392, 2171–2179.
- Morency, A.M.; Bujold, E. The effect of second-trimester antibiotic therapy on the rate of preterm birth. J. Obstet. Gynaecol. Can. 2007, 29, 35–44.
- Migale, R.; Herbert, B.R.; Lee, Y.S.; Sykes, L.; Waddington, S.N.; Peebles, D.; Hagberg, H.; Johnson, M.R.; Bennett, P.R.; MacIntyre, D.A. Specific Lipopolysaccharide Serotypes Induce Differential Maternal and Neonatal Inflammatory Responses in a Murine Model of Preterm Labor. Am. J. Pathol. 2015, 185, 2390–2401.
- Bostwick, D.G.; Woody, J.; Hunt, C.; Budd, W. Antimicrobial resistance genes and modelling of treatment failure in bacterial vaginosis: Clinical study of 289 symptomatic women. J. Med. Microbiol. 2016, 65, 377–386.
- Grev, J.; Berg, M.; Soll, R. Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2018, 12, Cd012519.
- Nordqvist, M.; Jacobsson, B.; Brantsæter, A.L.; Myhre, R.; Nilsson, S.; Sengpiel, V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: A prospective observational cohort study in Norway. BMJ Open 2018, 8, e018021.
- Yang, S.; Reid, G.; Challis, J.R.G.; Gloor, G.B.; Asztalos, E.; Money, D.; Seney, S.; Bocking, A.D. Effect of Oral Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the Vaginal Microbiota, Cytokines and Chemokines in Pregnant Women. Nutrients 2020, 12, 368.
- Husain, S.; Allotey, J.; Drymoussi, Z.; Wilks, M.; Fernandez-Felix, B.M.; Whiley, A.; Dodds, J.; Thangaratinam, S.; McCourt, C.; Prosdocimi, E.M.; et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: A randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 275–284.
- Antony, K.M.; Ma, J.; Mitchell, K.B.; Racusin, D.A.; Versalovic, J.; Aagaard, K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am. J. Obstet. Gynecol. 2015, 212.
- Alwan, N.A.; Greenwood, D.C.; Simpson, N.A.; McArdle, H.J.; Cade, J.E. The relationship between dietary supplement use in late pregnancy and birth outcomes: A cohort study in British women. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 821–829.
- Mantovani, E.; Filippini, F.; Bortolus, R.; Franchi, M. Folic acid supplementation and preterm birth: Results from observational studies. BioMed Res. Int. 2014, 2014, 481914.
More
