Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Oisin Kearns and Version 4 by Jason Zhu.
Hyaluronic acid (HA) has been implemented for chemo and photothermal therapy to target tumour cells overexpressing the CD44+ receptor. HA-targeting hybrid systems allows carbon nanomaterial (CNM) carriers to efficiently deliver anticancer drugs, such as doxorubicin and gemcitabine, to the tumour sites.
- Hyaluronic Acid
- Carbon Nanomaterials
- tumour
- biomedical
- drug delivery
- Carbon Nanotubes
1. Hyaluronic Acid
Hyaluronic acid (HA) is a biocompatible, nonimmunogenic, and biodegradable anionic natural polysaccharide [1]. It consists of d-glucuronic acid and N-acetyl-d-glucosamine connected by alternating by β-(1→4) & β-(1→3) glycosidic linkages [2] (Figure 1).
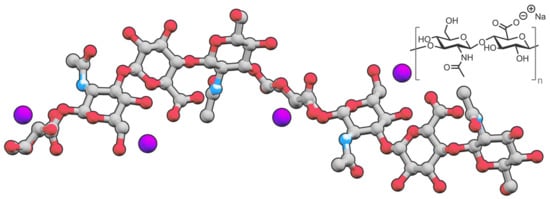
Figure 1. Structure of sodium hyaluronate, a salt derived from hyaluronic acid.
HA is naturally present in the human body and is a critical component of the extracellular matrix and body fluid, functioning as a regulator for normal structural integrity and development along with regulating tissue in response to injury, repair, and regeneration [3][4][3,4]. Its properties make HA a valuable candidate for biomedical applications. The high zeta potential, quantified in a paper by Cavalcanti et al. [5], confirms its highly hydrophilic properties, enabling HA to simultaneously confer water solubility to the overall hybrid delivery system in addition to its primary function as a targeting agent. In particular, Cavalcanti and co-workers showed how the presence of HA led to an increased zeta potential and thus water dispersibility in the HA/soy peptone synthetic mixture, showing its potential for hybrid drug delivery systems [5]. In addition, HA is susceptible to pH-induced structural changes. This finding concurs with studies into the use of HA under different pH conditions, where cumulative anticancer drug release was investigated by circular dichroism experiments. It was discovered that, at a pH of 3, structures such as double helices appeared, while random coils occurred under physiological conditions (pH 7) due to HA being a highly anionic polyelectrode [5]. HA is commonly used for the targeting of tumour cells because of its affinity to target particular over-expressed cell receptors—mainly to the cell surface receptors called hyaladherins, such as clusters of differentiation, commonly cluster of differentiation 44 (CD44+) as well as 36 (CD36), protein phosphatase 2 (PP2A), cyclin dependant kinase 9 (CDK9) [6], a receptor for hyaluronate-mediated mortality (RHAMM) [7], lymphatic vessel endothelial HA receptor (LYVE-1) [8], and tumour necrosis factor-stimulated gene-6 (TSG-6) [9][10][9,10].
Since CD44+ receptors are commonly overexpressed in tumour cells, HA becomes a necessary and powerful targeting component in a drug delivery nanocomposite. CD44+ receptors bind to hyaluronic acid and act as an adhesion regulator [11], where it operates in haematopoiesis and lymphocyte activation [12]. Since the purpose of HA is to act as a targeting agent, the practical targeting ability can be compared from one nanocomposite to the other. The most reliable approach for this would be the relative tumour volume reduction over a designated period. In this review, the comparison approach is used to extrapolate approximate results on the relative effectiveness of different nanocomposites bearing HA as a targeting agent and loaded with an anticancer drug for chemotherapy or with a photosensitizer for photothermal therapy.
Figure 2 provides a detailed but simplified visual representation of the HA receptor-mediated endocytosis [13], where HA-decorated graphene oxide nanosheets (HSG) are loaded with Doxorubicin (DOX). The HA is selectively targeted by tumour cell receptors such as CD44. The process proceeds with the accumulation of HSG-DOX within the tumour site followed by the receptor-mediated cellular internalisation. Then, hyaluronidase (HAAse)-mediated HA degradation breaks apart the endosome within the cell and a NIR irradiation allows for an endo/lysosomal escape, eventually leading to tumour inhibition. Cytotoxicity by this method is therefore directed toward the nucleus of the tumour cell as opposed to directing at non-malignant cells. This process of receptor-mediated endocytosis shows the necessity of the HA component in the hybrid drug delivery system.
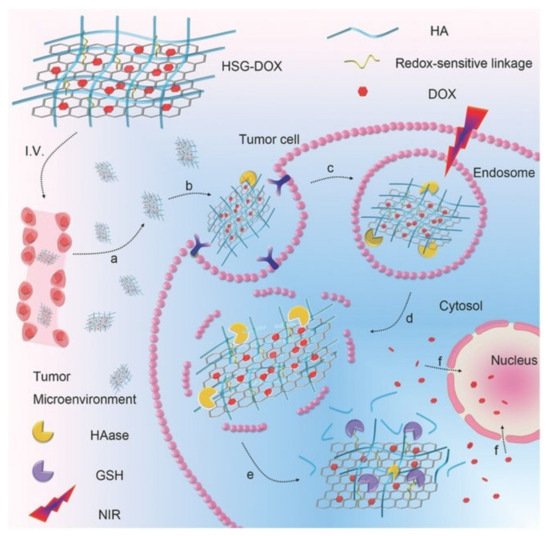
Figure 2. Targeted delivery of the nanocomposite to cancer cells via HA receptor-mediated endocytosis. Adapted with permission from Ref. [13]. Copyright 2017 John Wiley and Sons.
HA has surged in popularity within biological fields over the past two decades as the potential role of HA for the development of novel therapeutic strategies for many diseases has been discovered [14]. HA has become well known for many different biomedical applications and treatments. Most commonly, HA is known for its ability to moisturise skin and prevent skin ageing [14]. Most of the body’s hyaluronan is found within the skin, with the synthesis of HA increasing during tissue injury and wound healing [15]. It has been shown that HA in conjugation with drugs has excellent efficacy in vivo. The first reports of HA–drug conjugates were published by Akima et al. [16] in 1996. Their study details how HA can be used to enhance the delivery of antitumour drugs into regional lymph nodes and cancerous tissue via a hyaluronate receptor after intravenous (iv), intra-articular (ia), subcutaneous (sc), or intramuscular (im) administrations. Since then, the possibility of active targeting has been investigated by numerous researchers using HA due to its affinity for overexpressed tumorous cells. Biodegradability, biocompatibility, low toxicity, and selective targeting to focus sites enable HA to possess great potential for biomedical and pharmaceutical applications [13][17][13,17]. As this is a relatively new concept, most of the literature surrounding conjugated drug delivery has been published in the last ten years, with different drug delivery systems being developed.
Doxorubicin (DOX), one of the most used anticancer drugs, is a highly researched drug molecule that was first identified for its anticancer abilities in 1950 and was clinically approved in 1963, having been proven effective in vivo as an anticancer agent [18]. DOX doesn’t block existing cancerous growth but instead works by blocking an enzyme called topoisomerase 2, which is needed by cancer to divide and grow. To boost anticancer activity, DOX is often used in conjunction with other cancer drugs, such as the combination with Gamitrinib [19]. Gemcitabine (GEM) has also been recorded many times within the literature as a valuable anticancer drug [20]. GEM is a nucleoside metabolic inhibitor that works by slowing down the growth of cancer cells, which then kills them [21], and it has shown to be quite effective in treating several types of cancers such as cell lung cancers, pancreatic cancers, breast cancer, ovarian cancer, bladder cancer, head and neck cancer, cervical cancer, and renal cancer [20]. Several other types of anticancer drugs were used within the literature, such as epirubicin [22], mitoxantrone [23], quercetin [24][25][24,25], camptothecin [26], carboplatin [27], irinotecan [28], metformin [29], chlorin e6 [30], SNX-2112 [31], and salinomycin [32].
Near-infrared laser (NIR) photoablation is an important tool in the area of cancer therapy, as the NIR radiation efficiently penetrates throughout the tissues without harmful effects on healthy cells [33]. NIR lasers are typically used in conjunction with nanocomposites to actively target the tumorous cells, producing their selective death by photoablation, as schematically shown in Figure 3.
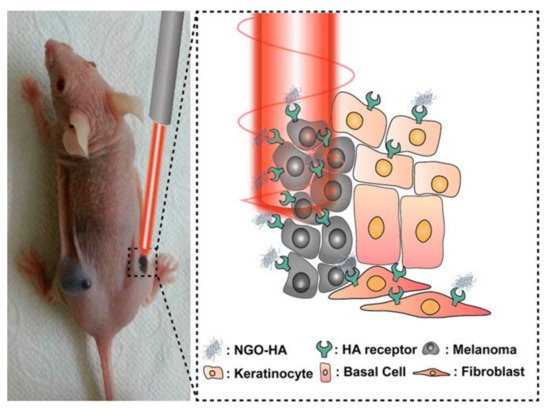
Figure 3. Use of NGO-HA to target HA receptors for photothermal ablation using a near-infrared (NIR) laser. Reprinted with permission from Ref. [33]. Copyright 2014 American Chemical Society.
In a typical photoablation experiment, a NIR laser is commonly focused on a particular point of interest, containing a high concentration of photosensitisers [33]. A photosensitiser is any non-toxic molecule that can be activated by light and generates molecular oxygen that can damage cellular structures [34][35][34,35]. The laser induces localised heating that eventually leads to cell death. The use of this technique allows for the elimination of tumorous cells by exploiting the affinity of HA to the HA receptor located on such tumorous cells. The HA, in conjunction with a specific carrier, can then be efficiently targeted by the laser, removing both HA and HA receptors. Photothermal therapy is a highly effective cancer treatment method that uses a photosensitizer to irradicate tumorous cells through targeted ablation, as shown in Figure 3 [33].
Photothermal therapy can be used alone as an efficient method of removing tumour volume and can also be used in conjunction with an anticancer drug in a process known as chemo-photothermal ablation. While photothermal ablation has been proven to be efficient on its own [33], there are limitations to the cancers that can be treated by this method due to the interference of skull thickness or adipose tissue thickness (ATT) on brain or muscle treatments [36]. Overall, the photothermal ablation method is a highly regarded method for tumour volume reduction and, used in conjunction with the targeting ability of HA, becomes one of the most effective techniques for removing tumour growth.
2. Carbon Nanomaterials and Interactions with Hyaluronic Acid
Carbon nanomaterials (CNMs)—a subclass of nanomaterials—have been utilised in several different fields, including biomedicine [37]. Within biomedicine, they have a number of specific applications, including acting as a carrier for traditional drugs to prevent the rise of drug resistance [37][38][37,38]. Drug resistance has always been a problem but has been reported to be mounting [38].
Several CNM-based drug carriers have been investigated in conjunction with HA, including carbon nanotubes (CNTs), graphene, graphene oxide (GO), graphene quantum dots (GQDs), and carbon nano-onions (CNOs) (Figure 4).
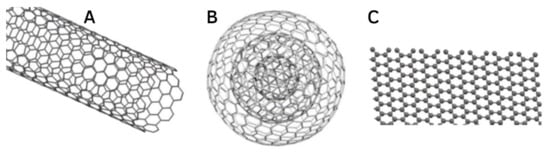
Figure 4.
Structures of (
A
) single-walled CNT, (
B
) CNO, and (
Aggregation in aqueous solutions, driven by intermolecular interactions, has been a challenge for many CNMs due to the hydrophobic nature of their pristine carbon surface. As with any carbon structure, CNMs are highly hydrophobic and have to be made hydrophilic for biological applications. While this presents its challenges, the benefits of utilising these biocompatible CNMs for drug delivery systems in vivo outweigh the key fundamental issues related to their hydrophobic nature.
In recent years, a number of efficient surface modification approaches has been developed to enhance the solubility of CNMs. Covalent strategies have the advantage to introduce onto the CNM surface polar groups such as carboxylic acid groups, leading to water-soluble and stable derivatives [40][41]. However, this strategy leads to the disruption of the regular sp2-hybridized network, thus potentially affecting the intrinsic properties of the materials [41][42][42,43]. Therefore, in order to preserve the pristine surface of CNMs and thus their native physicochemical properties, non-covalent approaches are typically preferred [43][44][44,45].
A standard non-covalent method for improving the dispersibility of CNMs is through π–π stacking [45][46]. This non-covalent approach is favoured over covalent interactions as it enables the CNMs to maintain their original pristine properties while promoting their dispersion in water. It has been proposed that, since both gemcitabine and single-walled carbon nanotubes (SWCNT) contain conjugated aromatic rings, π–π stacking interactions are expected to be established between the cystine ring of the gemcitabine and the SWCNT inner wall surface [46][47].
Options other than π–π stacking are also available, but the non-covalent functionalization of the CNMs surface is preferable. The non-covalent attachment of HA to CNMs would favour stronger interactions over π–π stacking. Phospholipid structures have been used to preserve the pristine CNM carbon surface and enable non-covalent attachment. Several papers used this method, where structures such as 1,2-dimyristoyl-sn-glycerol-3-phosphoethanolamine (DMPE) [47][39], polyethyleneimine (PEI) [48], polyethylene glycol (PEG) [49][50][49,50], 2,2′-(ethylenedioxy)bis(ethyleneamine) (EDBE) [7], spiropyran [51], adipic acid dihydrazine (ADH) [52][53][52,53], poly(maleic anhydride-alt-1-octadecene) (PMAO) [54], carboxymethyl chitosan (CMC) [55], polyethylene oxide (PEO) [56], along with several other synthetic structures, proved to be relevant for the functionalisation of CNMs with HA.
HA can be linked to drugs or drug carriers and can improve retention times and the half-life of a drug, as was seen for Insulin by Chu et al. [57][58][57,58]. Effective interactions between HA and various CNMs have been hypothesised, and phospholipid structures have been used to overcome the highly hydrophilic and hydrophobic nature of HA and CNMs, respectively. Regarding graphene, computational calculations predicted that CH–π and OH–π interactions are formed primarily between HA and unmodified graphene [59]. On the other hand, there are a large number of OH–O and NH–O interactions between HA and GO, as HA is a hydrophilic molecule.
For biomedical applications where a moderate interaction strength could be required, tailoring interactions between biomolecules and graphene is the best option [60][61][60,61]. Wang et al. [59] detailed various possibilities and strengths of interactions and suggested that graphene functionalised with OH, COOH, O-containing, N-containing, or NO-containing groups would be appropriate for a moderate interaction strength. Such modifications could be implemented to improve the biomedical application of GO-conjugated HA. The use of N-doped GQDs in conjunction with HA is discussed by Campbell et al. [62]. The nanocomposite is covalently bound to ferrocene, which selectively targets cancer cells and causes the generation of reactive oxygen species [63] that are cytotoxic to cells.
3. Carbon Nanotubes
Carbon nanotubes (CNTs) are large cylindrical molecules consisting of a hexagonal arrangement of sp2-hybridised carbon atoms, which may be formed by rolling a single sheet of graphene, called single-walled carbon nanotubes (SWCNTs), or by rolling up multiple sheets of graphene, named multi-walled carbon nanotubes (MWCNTs) [64]. While these might be two completely different categories with respect to the chemistry of the CNTs, the experimental results did not appear to differ significantly in the relative tumour volume reduction or the cumulative drug release profiles of the respective anticancer drugs and therefore SWCNTs and MWCNTs are discussed under the same headings, with similar nanocomposites prepared for both. Various factors contribute to an effective drug delivery system using HA as a targeting agent. Primarily, the anticancer drug is the main component that affects tumour removal since this has the chemotherapy effect and is the active component. The effectiveness of the HA targeting ability is also important. Different Mw (6.4, 17, 51, 200 and 1500 kDa) HA were investigated by Arpicco et al. [65], using a non-covalently bound phospholipid structure 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (DMPE) to HA and SWCNTs, loaded with DOX (DOX/CNT/HA-DMPE). The hybrid nanocomposite with a HA Mw of 200 kDa resulted in a better targeting ability and drug release profiles than any of the other systems. In particular, the material showed a cumulative DOX release of ~7% at pH 7.4 and ~18% at pH 5.5 for 200 kDa, while the other HA Mw examined showed a DOX release of only ~4% at pH 7.4 and ~5 to 10% at pH 5.5 [65]. There are several targets for HA receptors similar to those found in tumorous cells, typically CD44+, CD36, PP2A, CDK9 [6], RHAMM [7], LYVE-1 [8], and TSG-6 [9][10][9,10]. Specificity for these overexpressed receptors on tumorous cells is imperative to prevent cytotoxic effects in other biological systems. Utilising a hybrid system consisting of chitosan/rhodamine B-hyaluronic acid-paclitaxel nanoparticles (CS/RB-HA-PTX NPs), Li et al. [66] were able to determine the specificity of the hybrid system targeting ability, with Rhodamine B (RB) being utilized for its intrinsic fluorescence spectrum. RB was also used alone as a control to demonstrate the background level of fluorescent intensity without the tumour targeting ability. The effectiveness of the targeted delivery of HA can be seen in Figure 5, showing the imaging of tumour-bearing mice and their respective organs and tumour when comparing an anticancer drug alone to the nanocomposite CS/RB-HA-PTX NPs. The scale along the y axis indicates the fluorescent intensity of the hybrid labelled with RB. From Figure 5, it can be seen that the highest fluorescent intensities are in fact seen within the tumour, implying that the specificity of the targeting agent, HA, is effective at delivering the hybrid system to the correct location due to the overexpressed receptor targets. However, specificity could be improved, as the intensities for the stomach and the intestine are both high after oral administration. In comparison to the RB alone, the liver, spleen, kidney, and heart all have greatly reduced intensities, while the tumour is vastly increased. From this perspective, the specificity is highly improved upon when compared to a non-targeted approach.
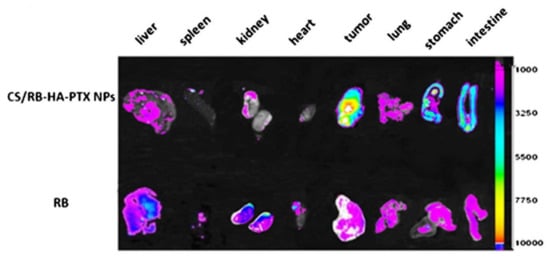

Figure 5. In vivo imaging of the tumour and various organs, showing the specificity of the targeted tumour delivery when using chitosan/rhodamine B-hyaluronic acid-paclitaxel nanoparticles (CS/RB-HA-PTX NPs) over rhodamine B (RB) taken orally. Adapted with permission from Ref. [66]. Copyright 2013 Springer Nature.
Tumour volume reduction is imperative to the success of HA-conjugated CNMs and their potential as hybrid drug delivery systems. In particular, Bhirde et al. [67] showed an exceptional use of chemo-photothermal therapy using a cholinic acid-derivatized hyaluronic acid and semiconducting single-walled carbon nanotube loaded with Doxorubicin (CAHA-sSWCNT-DOX). The CAHA component of the hybrid provided extra stability in vivo and could undergo versatile chemical modification and eradicate the tumour growth after only two days. The cell viability of the ovarian cancer cell line (OVCAR8) and the ovarian cancer cell line/Adriamycin resistant cell line (OVCAR8/ADR) cells dropped to almost 0% after photothermal therapy, making this paper highly effective at treating tumour growth. On the other side, in the absence of a NIR irradiation, the hybrid showed a cell viability of <10% and 60% in OVCAR8 and OVCAR8/ADR, respectively. The strong optical absorption of CNTs in the near-infrared biological widow and their drug delivery abilities enabled the eradication of multi-drug resistance tumours in vivo with a single dose of drug in combination with PTT.
There are a number of factors that make this hybrid so effective. First, the CAHA-sSWCNTs composite is quite stable without any aggregation over time; second, the ssWNCTs are able to act not only as the drug carrier but their strong optical absorption can also be utilized for the PTT; finally, the combination of DOX in conjunction with PTT is an effective combination, as it prevents not only the growth of the tumour but also eradicates the existing tumour cells [67].
Several combinations of nanocomposites and anticancer drugs can be investigated to augment the observed anticancer effects [19]. In a paper reported by Yao et al. [68], Epirubicin was selected as the drug in conjunction with a carrier consisting in SWCNTs functionalized with disteraroylphosphatidylethanolamine-hyaluronic acid (EPI-SWCNTs-DSPE-HA). In another work reported by Datir et al. [7], the multi-walled counterparts were utilized and functionalized with a HA-2,2′-(ethylene dioxy)bis(ethylene amine) derivative and then loaded with Doxorubicin (HA-EDBE-MWCNT-DOX). In both cases, the engineered nanocomposites were successful to target the cancer cells overexpressing the HA receptors. In particular, the HA-EDBE-MWCNT-DOX hybrid showed no evidence of increased tumour volume after 6 days post-injection and was comparable to the control group. After 10 days of treatment, it was evident that cardiotoxicity levels were increased significantly in animal groups treated with free DOX. Conversely, the cardiotoxicity of the CNT-treated groups showed insignificant differences from the control group, essentially eliminating the risk DOX poses to cardiotoxicity on biological systems [7]. Overall, this is an effective combination to enhance the tumour targeting ability and reduce the cardiotoxicity seen from the anticancer drug DOX alone.
In another paper reported by Liu et al. [69], the authors compared the effectiveness at reducing the human breast tumour volume (MDA-MB-231) of free DOX to that of a system composed of DOX-loaded SWCNTs functionalized with Hyaluronic acid (SWCNT-DOX-HA). After five days, the MDA-MB-231 spherical tumour volume was greatly reduced for the free DOX, SWCNTs-DOX, and the SWCNTs-DOX-HA, meaning that even though the nanocomposite could be used for a more targeted approach, it was almost as effective when applied directly to the tumour. The migration index, which indicates how cells move to change and reach their proper position to execute their function [70], showed that free DOX was less than half that of SWCNT-DOX-HA and the apoptosis rate was approximately half for SWCNT-DOX-HA (<40%) as it was for DOX (~75%); however, this is far superior to the SWCNT-DOX nanocomposite (~10%) with a background control value of 2%. The in vitro tumorous spheroids became irregular and smaller, indicating that the SWCNTs-DOX-HA could penetrate deep into the centre of the cell to induce cell apoptosis [69].
3.1. Cumulative Release Profiles of HA-Conjugated CNTs Loaded with Anticancer Drugs
Several papers used varying methods for the cumulative release profiles of the anticancer drug, with some being effective at all pH conditions, some being pH specific, and some having a general mix for both fast and slow-release profiles.
An example of a pH-triggered drug release profile is seen in a paper by Mo et al. [8], where single-walled carbon nanotubes were functionalized with chitosan and a 6 kDa HA and then loaded with Doxorubicin (SWNTs-CHI-HA-DOX). The nanocomposite showed to be stable at a pH of 7.4, with virtually no drug release seen after 72 h (Figure 6A). However, this nanocomposite is exceptionally efficient when introduced to conditions imitating that of lysosomal conditions (i.e., at pH 5.5), where a cumulative release of 85% was seen after the same period (Figure 6B). It is accepted that there is a lower release profile when considering conditions at a physiological pH of 7.4 compared to a more acidic pH such as a pH of 5.5. Chitosan is sensitive to pH, and this could be the influencing factor as to why there is such a noticeable increase in its effectiveness. Similarly, to keep drug release profiles high at pH conditions of 7.4, one paper utilised α-tocopheryl succinate and >1000 kDa HA functionalized MWCNTs loaded with DOX (α-TOS-HA-MWCNTs/DOX) to improve the cumulative release of DOX at all pH conditions [71]. The release across all nanocomposites was quite high, as it showed a steady increase in cumulative release, nearly reaching 20% in comparison to the 5% release seen for a very similar formulation in the paper by Mo et al. [8]. Variations in the cumulative drug release profiles were seen in several papers. Based on the SWNT-CHI-HA-DOX release profile seen in Figure 6, it would be expected that single-walled nanotubes loaded with chitosan-hyaluronic acid and paclitaxel (SWNT-CHI-HA-PTX) would have a similar release profile.
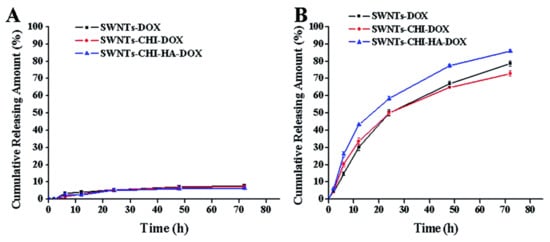

Figure 6. DOX release at 37 °C in (A) pH 7.4 and (B) pH 5.5 PBS. Reprinted with permission from Ref. [8]. Copyright 2015 Royal Society of Chemistry.
However, a paper by Yu et al. [72] used single-walled carbon nanotubes functionalized with chitosan and hyaluronic acid and loaded with Paclitaxel (SWNTs-CHI-HA-PTX) and they produced a much shorter release profile for the majority of the cumulative release of PTX. The quick cumulative release profile, releasing 60% out of 70% within the first 2–3 h, could be attributed to the 6 kDa HA that has been shown to have slower release profiles for higher Mw. However, since chitosan has been previously used for pH-sensitive drug release, it would not have been expected that the pH 7.4 would release 40% of PTX. This result contradicts the pH-dependance of chitosan as seen in the paper by Mo et al. [8], where chitosan has a much more significant effect on implementing pH-dependent drug release, and further investigations could be explored. The correlation of higher Mw HA having slow-release profiles also continues the trend between the papers by Mo et al. and Singhai et al. [71], as the profile by Mo et al. extended over >5 h before levelling off at a pH of 7.4 while the study by Singhai et al. [71] extended over 125 h and appeared as if it had not levelled off.
A paper presented by Prajapati et al. [49] using gemcitabine loaded onto HA-multi-walled carbon nanotubes (GEM/HA-MWCNTs) has an exceptional release profile at pH 7.4 (Figure 7), with a cumulative release of >80% seen after the 144-h study.
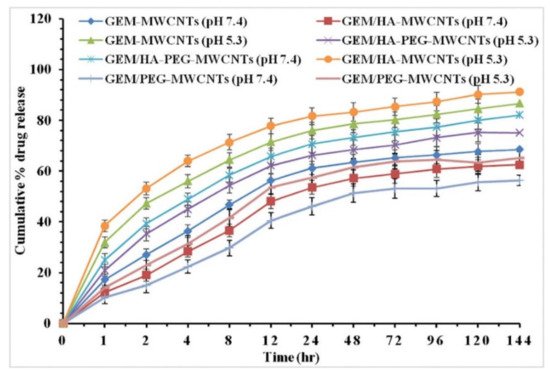

Figure 7. In vitro drug release profiles of GEM-MWCNTs, GEM/HA-MWCNTs, GEM/PEG-MWCNTs, and GEM/HA-PEG-MWCNTs at phosphate buffer (pH 7.4) and lysosomal conditions (pH 5.3). Reprinted with permission from Ref. [49]. Copyright 2019 Elsevier.
Figure 6 and Figure 7 are complete reversals of each other from a pH-controlled drug release perspective, as, in Figure 6, the drug release is completely controlled by the pH and the cumulative drug release is only really seen at a pH 5.5, with an 18 fold increase from the 5% release at a pH of 7.4. Figure 7 is not highly specific to the pH for either measurements, with >5% change between the cumulative release seen at a pH of 5.3 and the release seen at a pH of 7.4. Under certain circumstances, it is preferable if the nanocomposite would not be affected by pH, as this could be more inclusive for various cancer treatments. However, pH targeted drug delivery can be preferential under specific conditions; the possibility of cumulative release being higher for pH 5.3 or pH 7.4, dependent on constituents of the hybrid drug delivery system, leaves universal possibilities for nanomaterial hybrid chemotherapy treatment rather than only pH specific treatments.
3.2. Relative Tumour Volume Reduction
The binding of HA to the CNMs is essential to effectively carry the anticancer drugs to the tumour sites. A few papers showed a lower relative tumour reduction than expected, and this could be due to the inefficient binding of the HA to the CNM surface. A study by Hou et al. [73] compared the effects of the functionalization of a 46 kDa HA to different CNMs, namely, SWNTs, GO, and a C60 fullerene for photothermal ablation applications. While the control sample showed a relative tumour volume increase of 700% after 10 days, the nanohybrid formulations were all virtually ineffective, with the greatest inhibition being the hyaluronic acid-conjugated single-walled carbon nanotube in conjunction with an 808 nm laser. In particular, it showed a 450% increase in tumour volume after the same time period. The antitumor effect of all the nanohybrid systems for photothermal therapy was essentially inexistent, suggesting there could have been a common issue across all nanohybrid systems. The results of this paper provide evidence of the hypothesis that HA may have not coordinated properly with the CNMs and that the hybrid structures cannot be used efficiently as a targeting system for drug or photosensitizer delivery.
Photothermal ablation using a laser is a faster and effective technique for eradicating the tumour. When photosensitizers are present in significant quantity around the overexpressed HA-receptor tumour cells, the NIR radiation can be applied for a full ablation effect. This has the ability to induce tumour inhibition not only to a level where tumour growth would be prevented but to a level where the tumour would also be reduced in size. Phototherapy is a proven technique in many different papers, with efficient relative tumour reduction [28][74][28,74]. However, the paper by Hou et al. [73] showed virtually no noticeable difference in tumour volume when using photothermal ablation therapy compared to the blank control. The most likely issue is the covalent bonding, which would be predicted to occur between HA and the CNM. The attachment of HA to the pristine surface of these CNMs (SWNT, GO, and C60) involved covalent attachment rather than the preferable non-covalent approach. The use of a phospholipid structure to act as a bridging material between the highly hydrophobic CNM surface and the hydrophilic HA would be beneficial from this point of view, with π–π stacking being available for surface attachment without modifying the intrinsic properties of the CNMs.
Two possibilities could be used in conjunction with a phospholipid to improve the efficiency of the nanocomposites. The first method could be to use more than one wavelength for the laser [30]. The second is to use a combined chemo-photothermal therapy approach that has proven to be quite effective in several papers at removing tumour growth, and the preposition of DOX-loaded CNMs could be quite effective, as DOX is known for preventing the further growth of cancerous cells by blocking topoisomerase 2, preventing cancerous cells from dividing and growing [18].
The current challenge in nanomedicine is defining which combination of CNMs and anticancer drugs is most effective, as there are so many effective combinations seen in this review. An interesting example is a paper by Yao et al. [32], where Salinomycin-loaded Single-walled nanotubes functionalized with chitosan and hyaluronic acid (SAL-SWNTs-CHI-HA) was utilized. Notably, this paper used Salinomycin (SAL), not as commonly seen in conjunction with the other CNM nanocomposites within the literature, showing the opportunities that SAL offers as an anticancer drug. The results showed it to be quite effective in reducing tumour volume. From a chemotherapy point of view, when the possibility of treating tumours for photothermal ablation isn’t available, the composite described by Yao et al. [32] is exceptionally effective, reaching an 18.2 ± 1.2% relative tumour volume after day 6 for the SAL-SWNTs-CHI-HA, while the control reached 433.3 ± 6% in the same time period. There is a strong possibility that the mammospheres (mammary epithelial stem cell aggregates, derived from breast tumours) were penetrated to the centre by the drug delivery system because of their granular and irregular shape on the outside and finally broke into pieces, similar to what was seen by Liu et al. [69].
In the area of Carbon Nano Onions (CNOs), there have only been two papers directly relating to hyaluronic acid [47][75][39,75]. While these papers show good promise for HA-conjugated CNO as a potential drug carrier for targeted drug delivery, the lack of literature proved challenging to make any direct comparisons. Concerning the results, which could be compared to other CNMs, the cell viability is relatively high, and the confocal imaging shows that the HA is quite effective at localising around the receptors. Both papers demonstrate the ability of the CNOs to disperse in aqueous media. The paper by Zhang et al. [75] even stated that all the nanomaterial dispersions were stable, with no evidence of precipitation over several weeks. Prospects would be high in the area of CNOs in conjunction with HA based on previous biocompatibility testing. However, the lack of data surrounding CNOs’ relationship to HA leaves such bio-applications inconclusive.
Opposed to the results seen from photothermal therapy, which will be seen in the following section for graphene, a paper by Shi et al. [76] utilizing hematoporphyrin monomethyl ether and hyaluronic acid functionalized carbon nanotubes with 532 and 808 nm lasers (HMME-HA-CNTs) noted no reduction in tumour volume after 9 days. This result comes as a surprise, as HMME is a promising photosensitizer [77]. Similar results are also seen in a paper by Hou et al. [78], where Gadolinium doped Single-walled carbon nanotubes functionalized with HA (Mw of 12 kDa) linked by a disulfide bond to Doxorubicin (GD/SWCNTs-HA-ss-DOX) is used for a combined chemo-phototherapy. In general, chemo-photothermal papers are regarded as the most efficient at reducing tumour volume either entirely or nearly entirely. However, the tumour volume either remained constant or slightly below the original tumour volume on the 9th day in this specific case. Primarily, the main question that would be asked would be surrounding the targeting ability of the HA. In vivo imaging shows high fluorescence surrounding the tumour but also high fluorescence in other areas showing high but not total specificity, possibly causing a lack of hybrids surrounding the tumour. Since there was no tumour growth, it is probable that the anticancer drug DOX was successful in inhibiting the tumorous cells’ further division and growth.
