Zea mays L. (maize, corn) is one of the world’s leading crops for food, feed, and fuel and as a raw material for different industrial products. Control of maize late wilt disease (LWD) has been at the forefront of research efforts since the discovery of the disease in the 1960s. The disease has become a major economic restraint in highly affected areas such as Egypt and Israel and is of constant concern in other counties. LWD causes dehydration and collapsing at a late stage of maize cultivation, starting from the male flowering phase. The disease causal agent, Magnaporthiopsis maydis, is a seed- and soil-borne phytoparasitic fungus, penetrating the roots at sprouting, colonizing the vascular system without aboveground symptoms, and spreading upwards in the xylem, eventually blocking the water supply to the plant’s upperparts. Nowadays, the disease’s control relies mostly on identifying and developing resistant maize cultivars. Still, host resistance can be limited because M. maydis undergoes pathogenic variations, and virulent strains can eventually overcome the host immunity. This alarming situation is driving researchers to continue to seek other control methods. The current entry will summarize the various strategies tested over the years to minimize the disease damage. These options include agricultural (crop rotation, cover crop, no-till, flooding the land before sowing, and balanced soil fertility), physical (solar heating), allelochemical, biological, and chemical interventions. Some of these methods have shown promising success, while others have contributed to our understanding of the disease development and the environmental and host-related factors that have shaped its outcome. The most updated global knowledge about LWD control will be presented, and knowledge gaps and future aims will be discussed.
- corn
- late wilt disease (LWD)
1. Introduction
Zea mays L. (maize, corn) is one of the world’s leading crops for food, feed, and fuel and as a raw material for different industrial products [1]. Worldwide annual maize production is expanding at a rate of 1.6%. It was forecast that this rate will not meet the global demand in 2050 [2]. Among many diseases threatening this cultivar [3][4], late wilt disease (LWD) has been reported so far in 10 countries and is considered a major concern in highly infected countries such as Egypt [5], Israel [6], India [7], Spain, and Portugal [8]. Economic losses due to LWD were up to 40% in Egypt [9], 50-100% in Israel [10][11], and 51% in India [12]. Incidences of the disease can reach 100% in Egypt and Israel, and 70% in India. Although the disease has not been reported in the United States, M. maydis is regarded as a potentially high-risk phytopathogen [13][14]. LWD harms yield production by erupting at the flowering growth phase, resulting in severe dehydration and plant death.
Since the discovery of LWD in Egypt in the early 1960s [15], worldwide scientific efforts have led to much progress in understanding the disease mode and the pathogen causing it, Magnaporthiopsis maydis [13]. Moreover, specific research tools for the study of LWD were developed and applied in the lab, in growth room experiments under controlled conditions, and in field trials. A significant part of these efforts was dedicated to creating diverse control methods to restrict the disease’s burst and spread and minimize its effect on commercial maize manufacture. Previously the techniques developed over the years to study LWD and monitor its causal agent were reviewed [16]. A follow-up review summarized the accumulated scientific knowledge and future perspectives. These aspects include the geographic disease distribution, the pathogenesis (including the environmental factors affecting it), the symptoms’ evolvement, and their outcome effect on commercial production [17]. All the updated information regarding the pathogen itself, M. maydis, was also summarized.
The current entry focuses on the vast efforts dedicated in the past 60 years to late wilt disease control. The inspected control methods produced different degrees of success and include agricultural options (flood fallowing and balanced soil fertility) [18][19], biofriendly approaches [20], physical (solar heating) [21], allelochemical [22], and chemical pesticide [6][23][24] practices. Recently, the tillage system’s impact, the cover crop, and the crop rotation have been shown to serve as bioprotective factors against M. maydis [25][26].
2. Late Wilt Disease
The late wilt causal agent, M. maydis, is a seed-borne and soil-borne vascular wilt fungal pathogen that penetrates the host roots and colonizes the xylem tissue [27][28]. The taxonomic tree of this fungus is: phylum: Ascomycota, subphylum: Pezizomycotina, class: Sordariomycetes, subclass: Sordariomycetidae, family: Magnaporthaceae, genus: Magnaporthiopsis, species: Magnaporthiopsismaydis. Former scientific names are Cephalosporium maydis (Samra, Sabet,& Hing, 1963) [29] and Harpophora maydis (Samra, Sabet,& Hing, 1963; Gams, 2000) [30].
maydis spread as sclerotia, spores, or hyphae on the plants’ residues [28]. The pathogen can persist in the stubble and maize debris; no-till systems may help maintain it [13]. M. maydis can survive in the ground for lengthy periods or by thriving inside diverse host plants, such as lupine (Lupinus termis L.) [31], cotton (Gossypium hirsutum L.) [32][33], watermelon (Citrullus lanatus), and green foxtail(Setaria viridis) [32][34].
LWD has been reported so far in 10 countries: Egypt (1961) [35], India (1970) [36], Hungary (1998) [37], Spain and Portugal (2011) [38], Israel (2013) [11], and possibly Nepal (2015) [39]. There are also unconfirmed reports (summarized by Johal et al., 2004 [13]) that LWD was discovered in Italy, Romania, and Kenya (Figure 1).
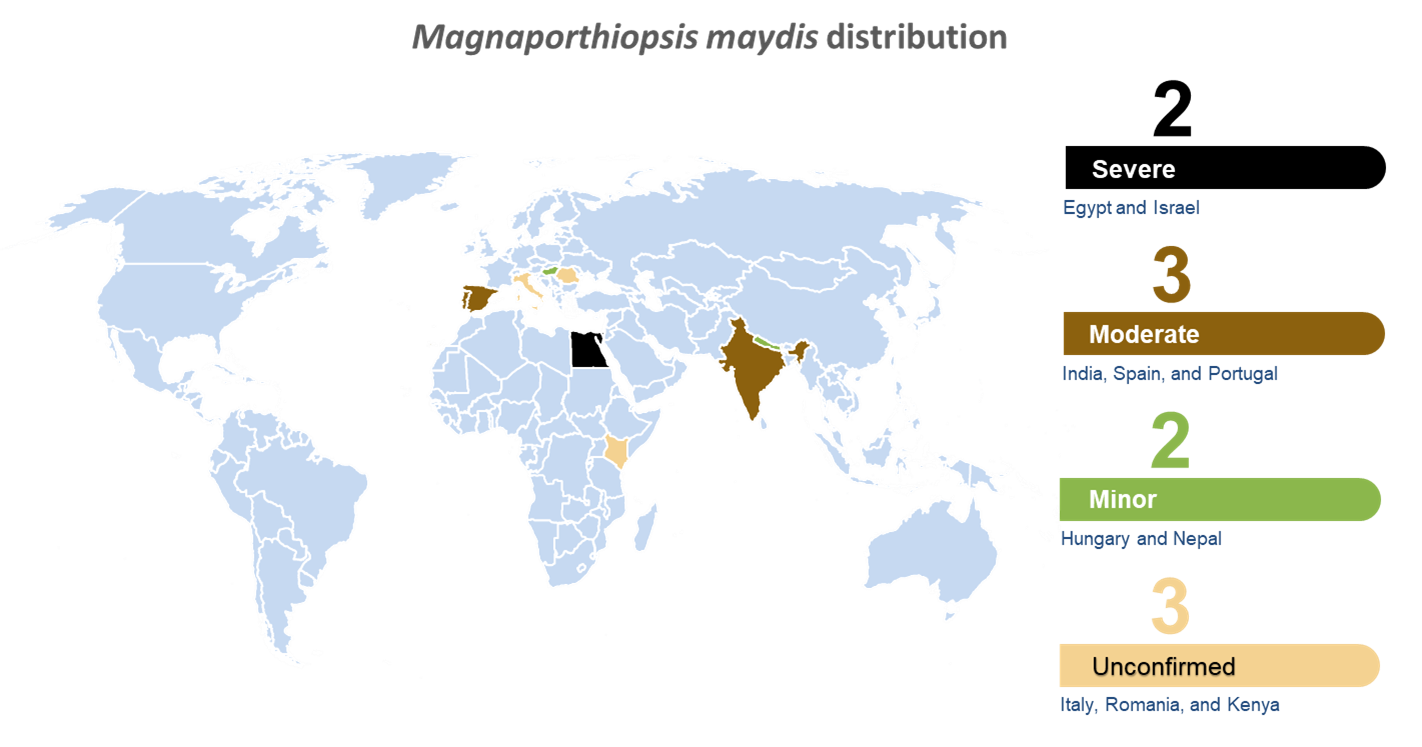
Figure 1. World distribution map for Magnaporthiopsismaydis. Disease severity is appraised according to the literature reports and is based on three categories: severe (4, Egypt and Israel); moderate (3, India, Spain, and Portugal); minor (2, Hungary and Nepal); and not certain/unconfirmed reports (1, Italy, Romania, and Kenya) [17].
The disease mode in LWD-sensitive maize cultivars is well detailed in the scientific literature (Figure 2). M. maydis infects maize seedlings during the first three weeks by sowing through their roots or mesocotyl (the seed-coleoptile connecting tissue). As the plants grow, they are less infected and become LWD-resistant about 50 days after sowing [28]. After root penetration, M. maydis colonizes xylem tissue (identified 21 days after sowing) and is rapidly transferred to the upper parts of the plant. M. maydis may occasionally cause seed rot or pre-emergence damping-off under high inoculum pressure [40].
The second critical infection phase starts when tassels first emerge (ca. day 55–65, R1 silking, silks visible outside the husks). At this stage, the fungus hyphae and conidia appear throughout the stalk [28], pathogen DNA levels reach their highest point in the stems [11], and the first aboveground symptoms are revealed. Later, when M. maydis colonizes the entire stalk, a vascular tissue occlusion by hyphae and gum-like secreted materials occurs, resulting in water supply suffocation, rapid dehydration, and death [13][28]. Although the disease appears as patches scattered in the field in many cases [16], LWD may result in total field infection and yield loss in heavily infected areas planted with susceptible maize cultivars [10][24]. A parallel asymptomatic infection mode, with some delay, occurs in resistant cultivars. This process can result in infected seeds that enhance the pathogen spread [10][11].
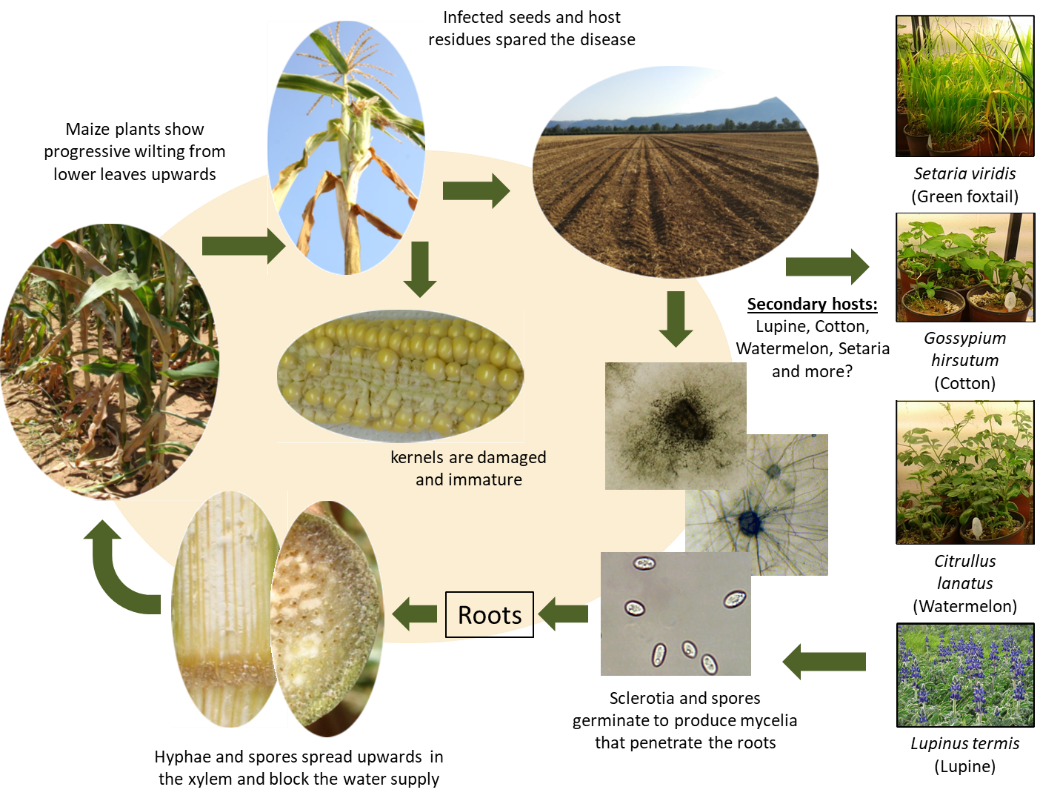
Figure 2. Disease cycle of maize late wilt caused by Magnaporthiopsismaydis [17] .
3. Control Strategies
3.1. Host Resistance
The use of resistant genotypes is considered the best, most practical, eco-friendly, and cost-effective method of controlling the disease [41][42] (Figure 3). This method is preferred even though resistant hybrids to LWD are often low-yielding or have other undesirable agronomic characteristics [43].

Figure 3. Cultivars’ resistance test for late wilt disease (adapted from [44]). A semi-commercial examination of fodder corn genotypes was conducted in the southern coastal plain of Israel (Yavne field) in 2014. The experiment included 14 maize cultivars in 3 repetitions, with each plot containing 6 rows measuring about 200 m in length. The experimental area photo was taken close to harvesting (day 99 from sowing) by David Katsav. The brown lines are late wilt diseased cultivars with severe dehydration, while the green lines are healthy cultivars. The photos on the right: resistance cultivar—Pan 33–031 (Eden Seeds, Hatzav, Israel); reduced sensitivity cultivar—Colossus from HSR Seeds (CTS, Hod Hasharon, Israel); susceptible cultivar—Avgaro (Hazera Seeds Ltd., Berurim MP Shikmim, Israel).
Significant efforts were directed towards using specific genetic markers for LWD to identify resistant germplasm and subsequently develop genetically resistant maize inbred lines [45]. Despite these meaningful efforts, the reasons for LWD susceptibility differences among maize cultivars remain obscure. It was gradually revealed that the infection process’ outcome results from chemical and histological differences between cultivars [46]. Alongside these efforts, the development of new methods for tracking the pathogen [7][47][48], estimating its distribution and damage [8][14][49], and controlling it in various ways [22][25][41][50][51] remain major goals.
3.2. Chemical Control
3.2.1. From In Vitro Evaluation to a Field Assay of Selected Fungicides
It is important to locate LWD antagonists’ fungicides while evaluating their phytotoxicity and efficiency against M. maydis and other associated fungi (the stalk-rot complex) involved in LWD [52]. Together with this effort, developing rapid and efficient screening approaches to assess the potential of these fungicides is needed [10][53]. Preliminary tests on growth media plates aimed at screening many chemical preparations rapidly and indicating their efficiency can be conducted with minimal investment and can save time and effort. After eliminating inadequate potential fungicides, selected compounds will be verified in seeds, detached roots, and sprouts’ assays. Only in the final phase will a limited number of high-potential selected pesticides be tested in a field experiment throughout the growth season (Figure 4).
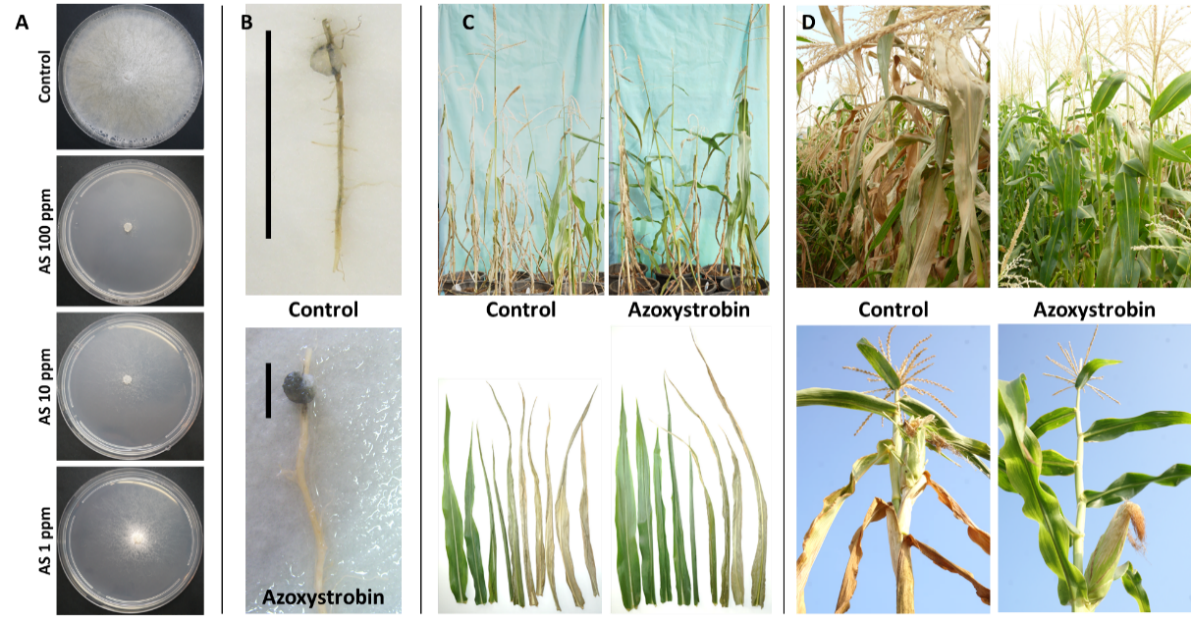
Figure 4. From in vitro evaluation to a field assay of Azoxystrobin. (A) Agar plate assay to evaluate the inhibition of Magnaporthiopsis maydis mycelial growth by Azoxystrobin (AS, CAS no. 131860-33-8, Amistar, Makhteshim Agan, Airport City, Israel) (adapted from [6]). Photos were taken five days after incubation at 28 °C in the dark. The fungicide was evaluated at a rate of 1, 10, and 100 mg/L active ingredients. Control—potato dextrose agar (PDA) plate without fungicide. (B) Detached root pathogenicity assay (adapted from [6]). The main (longest) inner feeder root was cut from a potted 3-week-old Jubilee cv. maize sprout. The detached root was inoculated by placing an M. maydis culture disk (6 mm diameter) taken from the margins of a 4–6-day-old colony, two cm away from its cut end. The inoculated roots were incubated in a moist atmosphere, in Petri dishes, at 28 °C in the dark for six days. The pathogen infection thread (a dark filament) within the root is marked in the photos by a black line near each root. Control—root inoculated with M. maydis without fungicides. (C) Effect of Azoxystrobin seed coating (0.002 cm3/seed) on plant development in a greenhouse (adapted from [54]). A photograph of all the plants’ aboveground parts (upper panel) and leaves (lower panel) in the treatment and the non-protected control groups 72 days after sowing (DAS), 13 days after fertilization (DAF). (D) Late wilt disease symptoms in a field experiment 71 DAS, 16 DAF (adapted from [55]). Upper panel—wilt symptoms of a non-protected plot (control) compared to an Azoxystrobin-treated plot. Lower panel—representative plants. The fungicide was applied in seed coating (0.002 cm3/seed, Azoxystrobin + Difenoconazole, “Ortiva-Top”, Syngenta, Basel, Switzerland, supplier Adama Makhteshim, Airport City, Israel) and at three intervals, 18, 31, and 45 DAS, 2.25 L/hectare. Disease symptoms include drying-out that progresses upwards in the plant, stem and leaf yellowing, and dehydration.
3.2.2. Seed Coating
Since the initial infection occurs during the seedling development period [28], the LWD can be managed efficiently with a fungicide seed treatment. Indeed, such attempts have been made in the past. In India, Begum et al. (1989, 1996) showed that seed treatments with captan, carboxin, carbendazim, and thiram significantly reduced late wilt severity and increased yield in the field [56][57]. Yield increased by 25.9% for carbendazim and 34.1% for captan (at 1 g/kg seed).
In contrast, seed treatments failed to prevent late wilt in Egyptian trials [23][58]. According to Johal et al. (2014) [13], such differences can result from variances in the virulence, chemical sensitivity of M. maydis isolates, or the consequences of the stalk-rot disease complex in Egyptian soils [52]. Nonetheless, non-chemical seed treatments were tested successfully in Egypt [50][59].
In Israel, in relatively resistant maize with only minor LWD symptoms, the Azoxystrobin seed coating (0.0025 mg active ingredient/seed) prevented fungal development and increased plant and cob weight [10]. Yet, this treatment could not defend a susceptible maize cultivar in heavily infested soil at the disease’s wilting burst (60 DAS) and later on. In follow-up work [54], the Azoxystrobin + Difenoconazole seed coating (AS + DC, “Ortiva-Top”, manufactured by Syngenta, Basel, Switzerland, supplied by Adama Makhteshim, Airport City, Israel) was applied. This treatment was efficient in the initial growth stages (up to 50 DAS). At the later growth stages, the AS-DC seed coating provided an additional layer of protection when combined with the injection of fungicides into the irrigation system, as elaborated in the following section (3.2.3).
3.2.3. Soil Treatments
Systemic fungicides and their fungi-toxic products translocate to maize leaves within two days and can last in maize roots for 90 days [23], so M. maydis may be repressed by these chemicals within the root, as well as in the soil. Thus, soil treatments with systemic fungicides, such as Benomyl (Benlate, methyl 1-(Butylcarbamoy l)-1H-1,3-benzimidazol-2-yl methylcarbamate, CAS no. 17804-35-2), were the preferred method of dealing with the disease.
The application method can be game-changing in this regard, as was proven in Egypt [23] and Israel. Abd-el-Rahim and colleagues (1982) found that the systemic fungicide Benylate applied at four 15-day intervals (2.5 kg/acre) after sowing resulted in the best LWD control [13]. In Israel, the application of fungicides into a dripline assigned for each row at 15-day time intervals from sowing inhibited wilt symptom development and recovered cob yield by 100%. More recently, an efficient and more economically applicable solution to LWD was suggested that could be applied on a large scale to shield-susceptible corn varieties in commercial fields [54]. This application is based on antifungal mixtures having a different mode of action to prevent resistance development. The method involved seed coating and injection of Azoxystrobin and Difenoconazole mixture (AS+DC) into the irrigation system at three 15-day intervals from sowing. Economic efficacy was reached using one dripline for two adjacent rows (a row spacing of 50 cm instead of 96 cm).
Nowadays, drip irrigation is considered one of the most effective chemical methods to restrict maize LWD but it is the most expensive of the present alternatives [54]. Moreover, growing resistance to these antifungals and the resulting control failure have become a significant problem [60]. Since fungicide treatment limitation exerts increasing pressure in many countries due to environmental and potential health risks, searching for alternatives to cope with LWD is a continuous effort.
3.3. Biological Control
3.3.1. Strengthening Beneficial Microorganism Communities in the Soil and Their
Secreted Metabolites
Biopesticides are environmentally friendly and occupy an increasingly central place in worldwide scientific research. Many studies were directed towards LWD biological control [20][61][62][63][64]. These methods include operating and strengthening beneficial microorganism communities in the soil (for example, by compost addition [61]) or direct intervention using antagonistic bacteria and fungi or their secreted metabolites. Plant-growth-promoting rhizobacteria and seed treatments with biocontrol formulations (B. subtilis, Bacillus pumilus, P. fluorescens, Epicoccum nigrum) were suggested for maize LWD control and tested in the field with encouraging results [51]. Another example is the use of a filtrate of mixed strains of the cyanobacteria Anabaena oryzae, Nostocmuscorum, and N. calcicolawere [61]. An alternative approach was to use marine algae and cyanobacteria A. oryzae extracts exhibiting antifungal activities to target the LWD pathogen [62].
3.3.2. Trichoderma spp. Maize Late Wilt Biocontrol
Late wilt disease can also be biologically controlled using Trichoderma spp. This genus’ species can form endophytic mutualistic relationships with various plant species [65]. Other Trichoderma species have been identified to possess biocontrol potential against plants’ fungal pathogens [66]. For instance, Trichoderma cutaneum reduced the incidence of LWD of maize under greenhouse conditions [63]. Likewise, Trichoderma harzianum treatment was efficient against M. maydis in the field [67]. The application of Trichoderma viride alone, or even better with chitosan NPs combined with the mycorrhizae Glomus mosseae, controlled late wilt and enhanced the plants’ growth indices [68]. To maximize the impact of Trichoderma-based treatments, Elshahawy and El-Sayed (2018) [20] showed that extracts of the microalgae, Chlorella vulgaris, with each of the Trichoderma species, T. virens and T. koningii, led to effective LWD control.
The potential for using Trichoderma-based treatment against Israeli M. maydis strains has only recently been tested [69]. Trichoderma longibrachiatum (T7407) and Trichoderma asperelloides (T203) isolates have solid antagonistic activity against the Israeli M maydis strain. These eco-friendly agents were tested in a series of experiments in the laboratory (Figure 5) until their final examination in pots under field conditions throughout an entire growing season (Figure 6).

Figure 5. In vitro estimation of Trichoderma asperelloides (T203)-secreted metabolites-based biological control against Magnaporthiopsis maydis (adapted from [69]). (A) T203-submerged cultures grown with shaking (150 rpm) for isolating secreted metabolites. (B) Static shallow media cultures of M. maydis on potato dextrose broth (PDB) medium containing T203-secreted metabolites filtrate. Control is PDB medium M. maydis cultures maintained under the same conditions. (C) Effect of growth media of T203 isolate on corn seed germination. The seeds were germinated in Petri dishes soaked in 4 mL of PDB (control) or PDB + secretion products (growth medium filtrate six days after T203 growth). All images are displayed after 5–6 days incubation at 28 ± 1 °C in the dark.

Figure 6. Trichoderma longibrachiatum (T7407) biological control against Magnaporthiopsis maydis in the lab and the field (adapted from [69][70]). (A) Plate mycoparasitism assay to identify interactions between Magnaporthiopsis maydis and T7407 in a potato dextrose agar (PDA)-rich medium. The two fungi were placed opposite each other, T7407 on the left and M. maydis on the right. Photos were taken after 3 and 10 days of growth. (B) Field inoculation of 20-day-old seedlings by an M. maydis-infected toothpick. The toothpicks were used for stabbing each plant at the near-surface portion of the stem. (C) The lower stem (first aboveground internode) disease symptoms. (D) The cobs’ spathes disease symptoms. (E) The experiment’s plots. Representative images of the field plants were taken 82 days after sowing. Controls are unprotected diseased plants.
In solid and submerged media culture growth assays, these species secrete soluble metabolites that inhibit or kill the maize pathogen [69]. Such a metabolite was recently isolated and identified as pyrone 6-pentyl-2H-pyran-2-one (6-pentyl-α-pyrone or 6-PP) [71]. This potent M. maydis antifungal compound is secreted by Trichoderma asperellum (P1), an endophyte separated from maize seeds of a cultivar susceptible to LWD [72]. The 6-PP metabolite was previously identified as one of the key bioactive compounds of several Trichoderma species [73].
3.3.3. Manipulating the Plant Microbiome
The soil pathogen, M. maydis, interacts with the maize endophytes, which may provide the plant’s first defense line. Recently, such endophytes were isolated from six sweet and fodder maize hybrids with deferent sensitivity to LWD [72]. These include ten fungal species belonged to Chaetomium, Trichoderma, Penicillium, Rhizopus, Alternaria, and Fusarium genera, and one bacterial species, B. subtilis.
In cotton plants, interactions between M. maydis and Fusarium oxysporum (the cotton wilt agent) led to an interesting result—reduced symptoms of the cotton wilt disease [33]. Similar antagonistic relationships in cotton plants were found between M. maydis and Macrophomina phaseolina, the charcoal rot disease agent [32].
3.4. Agrotechnical Measures
3.4.1. Various Cultural Methods
Agrotechnical applications were also reported in varying degrees of maturity in field experiments. These methods, which had a beneficial impact on LWD suppression, include excessive irrigation (pot experiments conducted in an open-air enclosure) [74] and applying plant extracts (Lycium europaeum [22], aloe vera (Barbados Aloe) fleshy leaves, onion bulbs, garlic cloves, jimsonweed, and peppermint leaves). In addition, soil solarization to increase temperatures above 35 °C with transparent polyethylene film [21], balanced soil fertility [19][76], and avoiding drought stress[74][77] can reduce LWD severity and yield losses. Magnaporthiopsis maydis survival is restricted to the top 20 cm of soil [78], and survival depends mainly on infected crop residues. Thus, sanitation measures such as deep tillage and annual plowing may significantly impact LWD (Dr. J. Leslie, personal communication) [14]. Finally, it was shown that organic compounds [59] and non-traditional methods such as adding nanosilica and zinc oxide nanoparticles [51] have promising potential to reduce late wilt in the field.
3.4.2. Beneficial Mycorrhizal Communities
Preserving soil mycorrhizal fungi between growth periods has been essential in crop protection (summarized by [64]). Preserving the integrity and continuity of the soil mycorrhizal networks may provide the plant with higher resistance to soil diseases [79], including late wilt disease [25]. In Portugal, Patanita et al. (2020) [25] showed that M. maydis presence reduction and grain production were significantly improved when both minimum tillage and cover crop were applied. It was also found that arbuscular root colonization was higher following these practices.
In Israel, agricultural practice based on conserving soil microflora integrity (by avoiding tillage) and influencing its nature (by cultivating specific crops in a dual-season growth) was applied [26]. When maize was seeded on wheat soil, a significant improvement in growth parameters and LWD repression was measured. This achievement was not affected drastically by tillage. Indeed, crop plants acquired a mycorrhizal community closely related to that of the former host plant and different from that found when the soil was disturbed by tillage or not cropped before the growth [80].
References
- Gálvez Ranilla, L. The Application of Metabolomics for the Study of Cereal Corn (Zea mays L.). Metabolites 2020, 10, 300, doi:10.3390/metabo10080300.
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PloS one 2013, 8, e66428, doi:10.1371/journal.pone.0066428.
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Allen, T.W.; Bergstrom, G.C.; Bissonnette, K.M.; Bradley, C.A.; Byamukama, E.; Chilvers, M.I.; Collins, A.A. Corn yield loss estimates due to diseases in the united states and ontario, canada, from 2016 to 2019. Plant Health Progress 2020, 21, 238-247, doi:10.31274/cpn-20200922-1.
- Pratap, A.; Kumar, J. Alien Gene Transfer in Crop Plants, Volume 2: Achievements and Impacts; Springer: New York, NY, USA, 2014; Volume 2.
- El-Naggarr, A.A.A.; Sabryr, A.M.; Yassin, M.A. Impact of late wilt disease caused by Harpophora maydis on maize yield. J Biol Chem Environ Sc 2015, 10, 577-595.
- Degani, O.; Cernica, G. Diagnosis and Control of Harpophora maydis, the Cause of Late Wilt in Maize. Advances in Microbiology 2014, 04, 94-105, doi:10.4236/aim.2014.42014.
- Sunitha, N.; Gangappa, E.; Gowda, R.V.; Ramesh, S.; Biradar, S.; Swamy, D.; Hemareddy, H. Assessment of Impact of Late Wilt Caused by Harpophora maydis (Samra, Sabet and Hing) on Grain Yield and its Attributing Traits in Maize (Zea mays L.). Mysore Journal of Agricultural Sciences 2020, 54, 30-36.
- Ortiz-Bustos, C.M.; Testi, L.; García-Carneros, A.B.; Molinero-Ruiz, L. Geographic distribution and aggressiveness of Harpophora maydis in the Iberian peninsula, and thermal detection of maize late wilt. European Journal of Plant Pathology 2015, 144, 383-397, doi:10.1007/s10658-015-0775-8.
- Samra, A.; Sabet, K.; Kamel, M.; Abd El-Rahim, M. Further studies on the effect of field conditions and cultural practices on infection with stalk-rot complex of maize. Arab Republic of Egypt, Min. of Agriculture. Plant Protection Dept., Bull 1971.
- Degani, O.; Movshowitz, D.; Dor, S.; Meerson, A.; Goldblat, Y.; Rabinovitz, O. Evaluating Azoxystrobin Seed Coating Against Maize Late Wilt Disease Using a Sensitive qPCR-Based Method. Plant Dis 2019, 103, 238-248, doi:10.1094/PDIS-05-18-0759-RE.
- Drori, R.; Sharon, A.; Goldberg, D.; Rabinovitz, O.; Levy, M.; Degani, O. Molecular diagnosis for Harpophora maydis, the cause of maize late wilt in Israel. Phytopathologia Mediterranea 2013, 52, 16-29.
- Payak, M.; Sharma, R. Research on diseases of maize; Indian Council of Agricultural Research: New Delhi, India, 1978; p. 228.
- Johal, L.; Huber, D.M.; Martyn, R. Late wilt of corn (maize) pathway analysis: intentional introduction of Cephalosporium maydis. In: Pathways Analysis for the Introduction to the U.S. of Plant Pathogens of Economic Importance; 2004.
- Bergstrom, G.; Leslie, F.J.; Huber, D.; Lipps, P.; Warren, H.; Esker, P.; Grau, C.; Botratynski, T.; Bulluck, R.; Floyd, J.; et al. Recovery plan For late wilt of corn caused by Harpophora maydis syn. Cephalosporium maydis; National Plant Disease Recovery System (NPDRS): U.S.A, November 12, 2008 2008; p. 24.
- Samra, A.S.; Sabet, K.A.; Hingorani, M.K. A new wilt disease of maize in Egypt. Plant Dis. Rep. 1962, 46, 481-483.
- Degani, O.; Dor, S.; Movshovitz, D.; Rabinovitz, O. Methods for Studying Magnaporthiopsis maydis, the Maize Late Wilt Causal Agent. Agronomy 2019, 9, 181, doi:10.3390/agronomy9040181.
- Degani, O. A Review: Late Wilt of Maize—The Pathogen, the Disease, Current Status and Future Perspective. Journal of Fungi 2021, 7, 989, doi:10.3390/jof7110989.
- Samra, A.S.; Sabet, K.A.; Abdel-Rahim, M.F. Effect of soil conditions and cultural practices on infection with stalk rots; U.A.R. Ministry of Agric. Government Printing Offices: Cairo, Egypt, 1966; pp. 117-164.
- Singh, S.D.; Siradhana, B.S. Effect of macro and micronutrients on the development of late wilt of maize induced by Cephalosporium maydis. Summa Phytopath 1990, 16, 140-145.
- Elshahawy, I.E.; El-Sayed, A.E.-K.B. Maximizing the efficacy of Trichoderma to control Cephalosporium maydis, causing maize late wilt disease, using freshwater microalgae extracts. Egyptian Journal of Biological Pest Control 2018, 28, 48, doi:10.1186/s41938-018-0052-1.
- Fayzalla, E.; Sadik, E.; Elwakil, M.; Gomah, A. Soil solarization for controlling Cephalosporium maydis, the cause of late wilt disease of maize in Egypt. Egypt J. Phytopathology 1994, 22, 171-178.
- Tej, R.; Rodríguez-Mallol, C.; Rodríguez-Arcos, R.; Karray-Bouraoui, N.; Molinero-Ruiz, L. Inhibitory effect of Lycium europaeum extracts on phytopathogenic soil-borne fungi and the reduction of late wilt in maize. European Journal of Plant Pathology 2018, 152, 249-265, doi:10.1007/s10658-018-1469-9.
- Abd-el-Rahim, M.F.; Sabet, K.A.; El-Shafey, H.A.; El-Assiuty, E.M. Chemical control of the late-wilt disease of maize caused by Cephalosporium maydis. Agric. Res. Rev. 1982, 60, 31-49.
- Degani, O.; Weinberg, T.; Graph, S. Chemical control of maize late wilt in the field. Phytoparasitica 2014, 42, 559-570, doi:10.1007/s12600-014-0394-5.
- Patanita, M.; Campos, M.D.; Félix, M.d.R.; Carvalho, M.; Brito, I. Effect of Tillage System and Cover Crop on Maize Mycorrhization and Presence of Magnaporthiopsis maydis. Biology 2020, 9, 46, doi:10.3390/biology9030046.
- Degani, O.; Gordani, A.; Becher, P.; Dor, S. Crop Cycle and Tillage Role in the Outbreak of Late Wilt Disease of Maize Caused by Magnaporthiopsis maydis. Journal of Fungi 2021, 7, 706, doi:10.3390/jof7090706.
- Michail, S.H.; Abou-Elseoud, M.S.; Nour Eldin, M.S. Seed health testing of corn for Cephalosporium maydis. Acta Phytopathologica et Entomologica Hungarica 1999, 34, 35-42.
- Sabet, K.A.; Zaher, A.M.; Samra, A.S.; Mansour, I.M. Pathogenic behaviour of Cephalosporium maydis and C. acremonium. Ann. Appl. Biol. 1970, 66, 257-263, doi:10.1111/j.1744-7348.1970.tb06432.x.
- Samra, A.S.; Sabet, K.A.; Hingorani, M.K. Late wilt disease of maize caused by Cephalosporium maydis. Phytopathology 1963, 53, 402-406.
- Gams, W. Phialophora and some similar morphologically little-differentiated anamorphs of divergent ascomycetes. Studies in Mycology 2000, 45, 187-200.
- Sahab, A.F.; Osman, A.R.; Soleman, N.K.; Mikhail, M.S. Studies on root-rot of lupin in Egypt and its control. Egypt. J. Phytopathol. 1985, 17, 23-35.
- Degani, O.; Dor, S.; Abraham, D.; Cohen, R. Interactions between Magnaporthiopsis maydis and Macrophomina phaseolina, the Causes of Wilt Diseases in Maize and Cotton. Microorganisms 2020, 8, 249, doi:10.3390/microorganisms8020249.
- Sabet, K.; Samra, A.; Mansour, I. Interaction between Fusarium oxysporum f. vasinfectum and Cephalosporium maydis on cotton and maize. Annals of Applied Biology 1966, 58, 93-101, doi:10.1111/j.1744-7348.1966.tb05074.x.
- Dor, S.; Degani, O. Uncovering the Host Range for Maize Pathogen Magnaporthiopsis maydis. Plants 2019, 8, doi:10.3390/plants8080259.
- Sabet, K.A.; Samra, A.S.; Hingorani, M.K.; Mansour, I.M. Stalk and root rots of maize in the United Arab Republic. FAO Plant Prot. Bull. 1961, 9, 121-125.
- Payak, M.M.; Lal, S.; Lilaramani, J.; Renfro, B.L. Cephalosporium maydis - A new threat to maize in India. Indian Phytopathol. 1970, 23, 562-569.
- Pecsi, S.; Nemeth, L. Appearance of Cephalosporium maydis Samra Sabet and Hingorani in Hungary. Mededelingen Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen, Universiteit Gent. 1998, 63, 873-877.
- Molinero-Ruiz, M.L.; Melero-Vara, J.M.; Mateos, A. Cephalosporium maydis, the cause of late wilt in maize, a pathogen new to Portugal and Spain. Plant Dis. 2011, 94, 379-379, doi:10.1094/pdis-94-3-0379a.
- Subedi, S. A review on important maize diseases and their management in Nepal. Journal of Maize Research and Development 2015, 1, 28-52, doi:10.3126/jmrd.v1i1.14242.
- Samra, A.; Sabet, K.; Abd-el-Rahim, M. Seed transmission of stalk-rot fungi and effect of seed treatment. Investigations on Stalk-Rot Diseases of Maize in UAR 1966, 94-116.
- Sunitha, N.; Gangappa, E.; GOWDA, R.V.; Ramesh, S.; SWAMY, S.D.; Hemareddy, H. Effectiveness of one cycle of phenotype-based backcross breeding for resistance to late wilt disease in maize (Zea mays L.). Mysore Journal of Agricultural Sciences 2020, 54, 47-50.
- GALAL, O.A.; ABOULILA, A.A.; MOTAWEI, A.; GALAL, A. Biochemical and molecular diversity and their relationship to late wilt disease resistance in yellow maize inbred lines. Egyptian Journal of Genetics Cytology 2019, 47.
- Kamara, M.M.; Ghazy, N.A.; Mansour, E.; Elsharkawy, M.M.; Kheir, A.M.S.; Ibrahim, K.M. Molecular Genetic Diversity and Line × Tester Analysis for Resistance to Late Wilt Disease and Grain Yield in Maize. Agronomy 2021, 11, 898, doi:10.3390/agronomy11050898.
- Amir, N.; Bosek, A.; Rabinovitz, O. Summary of cultivars resistance test for late wilt disease - Yavne (Israel central district) 2014 Nir Vatelem 2015, 58, 17-19.
- Zeller, K.A.; Jurgenson, J.E.; El-Assiuty, E.M.; Leslie, J.F. Isozyme and amplified fragment length polymorphisms from Cephalosporium maydis in Egypt Phytoparasitica 2000, 28, 121-130 doi:10.1007/BF02981741.
- Ghazy, N.; El-Gremi, S.; Belal, E.-S. Chemical and Histological Differences of Corn (Zea mays L.) Responsive to Harpophora maydis Infection. Environment, Biodiversity and Soil Security 2017, 1, 3-7, doi:10.21608/jenvbs.2017.2142.1017.
- Awad, A.M.; El-Abbasi, I.H.; Shoala, T.; Youssef, S.A.; Shaheen, D.; Amer, G.A.E.-A. PCR and Nanotechnology Unraveling Detection Problems of the Seed-borne Pathogen Cephalosporium maydis, the Causal Agent of Late Wilt Disease in Maize. International Journal of Nanotechnology Allied Sciences 2019, 3, 30-39.
- Campos, M.D.; Patanita, M.; Campos, C.; Materatski, P.; Varanda, C.M.; Brito, I.; Félix, M.d.R. Detection and Quantification of Fusarium spp. (F. oxysporum, F. verticillioides, F. graminearum) and Magnaporthiopsis maydis in Maize Using Real-Time PCR Targeting the ITS Region. Agronomy 2019, 9, 45, doi:10.3390/agronomy9020045.
- Magarey, R.D.; Borchert, D.M.; Engle, J.S.; Colunga-Garcia, M.; Koch, F.H.; Yemshanov, D. Risk maps for targeting exotic plant pest detection programs in the United States. EPPO Bulletin 2011, 41, 46-56, doi:10.1111/j.1365-2338.2011.02437.x.
- El-Shabrawy, E.-S.; Shehata, H. Controlling maize late-wilt and enhancing plant salinity tolerance by some rhizobacterial strains. Egyptian Journal of Phytopathology 2018, 46, 235-255, doi:10.21608/ejp.2018.87796.
- Hamza, A.M.; El-Kot, G.; El-Moghazy, S. Non-traditional methods for controlling maize late wilt disease caused by Cephalosporium maydis. Egyptian Journal of Biological Pest Control 2013, 23, 87-93.
- Khokhar, M.K.; Hooda, K.S.; Sharma, S.S.; Singh, V. Post flowering stalk rot complex of maize-Present status and future prospects. Maydica 2014, 59, 226-242.
- Degani, O.; Kalman, B. Assessment of Commercial Fungicides against Onion (Allium cepa) Basal Rot Disease Caused by Fusarium oxysporum f. sp. cepae and Fusarium acutatum. Journal of Fungi 2021, 7, 235, doi:10.3390/jof7030235.
- Degani, O.; Dor, S.; Movshowitz, D.; Fraidman, E.; Rabinovitz, O.; Graph, S. Effective chemical protection against the maize late wilt causal agent, Harpophora maydis, in the field. PloS one 2018, 13, e0208353, doi:10.1371/journal.pone.0208353.
- Degani, O.; Dor, S.; Chen, A.; Orlov-Levin, V.; Stolov-Yosef, A.; Regev, D.; Rabinovitz, O. Molecular Tracking and Remote Sensing to Evaluate New Chemical Treatments Against the Maize Late Wilt Disease Causal Agent, Magnaporthiopsis maydis. J Fungi (Basel) 2020, 6, 54, doi:10.3390/jof6020054.
- Begum, H.; Mohammad, S.; Rao, G.K.; Raj, R.B. Influence of seed dressing fungicides on the incidence of post flowering stalk rot (late wilt and charcoal rot), yield and profitability of maize. Crop Res. Hisar 1989, 2, 142-146.
- Satyanarayana, E.; Begum, H. Relative efficacy of fungicides (seed dressers) and irrigation schedule for the control of late wilt of maize. Curr. Res. Univ. Agric. Sci. Bangalore 1996, 25, 59-60.
- Sabet, K.A.; Samra, A.S.; Abdel-Rahim, M.F. Systemic action of benomyl against late-wilt disease of maize. Ann. Appl. Biol. 1972, 71, 211-218, doi:10.1111/j.1744-7348.1972.tb05084.x.
- Ashour, A.; Sabet, K.; El-Shabrawy, E.-S.; Alhanshoul, A. Control of maize late wilt and enhancing plant growth parameters using rhizobacteria and organic compounds. Egyptian Journal of Phytopathology 2013, 41, 187-207, doi:10.21608/ejp.2013.100361.
- Avila-Adame, C.; Koller, W. Characterization of spontaneous mutants of Magnaporthe grisea expressing stable resistance to the Qo-inhibiting fungicide azoxystrobin. Current genetics 2003, 42, 332-338, doi:10.1007/s00294-002-0356-1.
- El-Moghazy, S.; Shalaby, M.E.; Mehesen, A.A.; Elbagory, M. Fungicidal effect of some promising agents in controlling maize late wilt disease and their potentials in developing yield productivity. Environment, Biodiversity and Soil Security 2017, 1, 129-143, doi:10.21608/jenvbs.2017.1849.1013.
- Doleib, N.M.; Farfour, S.A.; Al-shakankery, F.M.; Ammar, M.; Hamouda, R.A. Antifungal activates of cyanobacteria and some marine algae against Cephalosporium maydis, the cause of Maize Late Wilt disease In Vitro. Bioscience research 2021, 18, 536-543.
- El-Mehalowy, A.A.; Hassanein, N.M.; Khater, H.M.; Daram El-Din, E.A.; Youssef, Y.A. Influence of maize root colonization by rhizosphere actinomycetes and yeast fungi on plant growth and on the biological control of late wilt disease. Inter. J. Agric. Biol. 2004, 6, 599-605.
- Ghazy, N.; El-Nahrawy, S. Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Archives of microbiology 2020, 1-15, doi:10.1007/s00203-020-02113-5.
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species--opportunistic, avirulent plant symbionts. Nature reviews. Microbiology 2004, 2, 43-56, doi:10.1038/nrmicro797.
- Harman, G.E. Overview of Mechanisms and Uses of Trichoderma spp. Phytopathology 2006, 96, 190-194, doi:10.1094/PHYTO-96-0190.
- Shalaby, M.; El-Moghazy, S.; Mehesen, A.A. Biological control of maize late wilt disease caused by Cephalosporium maydis. J. Agric. Res. Kafrelsheikh Uni 2009, 35, 1-19.
- El-Gazzar, N.; El-Bakery, A.M.; Ata, A.A. Influence of some bioagents and chitosan nanoparticles on controlling maize late wilt and improving plants characteristics. Egyptian Journal of Phytopathology 2018, 46, 243-264, doi:10.21608/ejp.2018.115896.
- Degani, O.; Dor, S. Trichoderma Biological Control to Protect Sensitive Maize Hybrids Against Late Wilt Disease in the Field. Journal of Fungi 2021, 7, 315, doi:10.3390/jof7040315.
- Degani, O.; Rabinovitz, O.; Becher, P.; Gordani, A.; Chen, A. Trichoderma longibrachiatum and Trichoderma asperellum Confer Growth Promotion and Protection against Late Wilt Disease in the Field. Journal of Fungi 2021, 7, 444, doi:10.3390/jof7060444.
- Degani, O.; Khatib, S.; Becher, P.; Gordani, A.; Harris, R. Trichoderma asperellum Secreted 6-Pentyl-α-Pyrone to Control Magnaporthiopsis maydis, the Maize Late Wilt Disease Agent. Biology 2021, 10, 897, doi:10.3390/biology10090897.
- Degani, O.; Danielle, R.; Dor, S. The microflora of maize grains as a biological barrier against the late wilt causal agent, Magnaporthiopsis maydis. Agronomy 2021, 11, 965, doi:10.3390/agronomy11050965.
- Hamrouni, R.; Molinet, J.; Dupuy, N.; Taieb, N.; Carboue, Q.; Masmoudi, A.; Roussos, S. The Effect of Aeration for 6-Pentyl-alpha-pyrone, Conidia and Lytic Enzymes Production by Trichoderma asperellum Strains Grown in Solid-State Fermentation. Waste and Biomass Valorization 2020, 11, 5711-5720, doi:10.1007/s12649-019-00809-4.
- Ortiz‐Bustos, C.; López‐Bernal, A.; Testi, L.; Molinero‐Ruiz, L. Environmental and irrigation conditions can mask the effect of Magnaporthiopsis maydis on growth and productivity of maize. Plant Pathology 2019, doi:10.1111/ppa.13070.
- El-Moghazy, S.; Shalaby, M. Effects of some aqueous plant extracts and sulphur compounds on the control of maize late wilt disease caused by Cephalosporium maydis. J. Agric. Res. Tanta Univ 2006, 32, 758-775.
- Mosa, H.; Motawei, A.; El-Aal, A.A. Nitrogen fertilization influence on combining ability for grain yield and resistance to late wilt disease in maize. J. Agric. Res. Kafrelsheikh Univ. 2010, 36, 278-291.
- Abd El‐Rahim, M.F.; Fahmy, G.M.; Fahmy, Z.M. Alterations in transpiration and stem vascular tissues of two maize cultivars under conditions of water stress and late wilt disease. Plant Pathology 1998, 47, 216-223, doi:10.1046/j.1365-3059.1998.00211.x.
- Sabet, K.A.; Samra, A.S.; Mansour, I.M. Saprophytic behaviour of Cephalosporium maydis and C. acremonium. Annals of Applied Biology 1970, 66, 265-271, doi:10.1111/j.1744-7348.1970.tb06433.x.
- Sharma, M.; Sudheer, S.; Usmani, Z.; Rani, R.; Gupta, P. Deciphering the omics of plant-microbe interaction: perspectives and new insights. Current Genomics 2020, 21, 343-362, doi:10.2174/1389202921999200515140420.
- Brígido, C.; van Tuinen, D.; Brito, I.; Alho, L.; Goss, M.J.; Carvalho, M. Management of the biological diversity of AM fungi by combination of host plant succession and integrity of extraradical mycelium. Soil Biology and Biochemistry 2017, 112, 237-247, doi:10.1016/j.soilbio.2017.05.018.
