The design and structural frameworks for targeted drug delivery of medicinal compounds and improved cell imaging have been developed with several advantages. However, metal-organic frameworks (MOFs) are supplemented tremendously for medical uses with efficient efficacy. These MOFs are considered as an absolutely new class of porous materials, extensively used in drug delivery systems, cell imaging, and detecting the analytes, especially for cancer biomarkers, due to their excellent biocompatibility, easy functionalization, high storage capacity, and excellent biodegradability. While Zn-metal centers in MOFs have been found by enhanced efficient detection and improved drug delivery, these Zn-based MOFs have appeared to be safe as elucidated by different cytotoxicity assays for targeted drug delivery. On the other hand, the MOF-based heterogeneous catalyst is durable and can regenerate multiple times without losing activity. Therefore, as functional carriers for drug delivery, cell imaging, and chemosensory, MOFs’ chemical composition and flexible porous structure allowed engineering to improve their medical formulation and functionality.
1. Introduction
Metal-organic frameworks (MOFs) are fascinating hybrid porous materials formed by a combination of metal and organic compounds
[1][2][1,2]. The coordinated organic ligand serves as linkers between varied metal interiors
[3]. Kinoshita and colleagues published the first report on metal-organic frameworks in 1959. Later, in the 1990s, Hoskin and Robson raised interest in this class of materials by adopting further logical methods to its “reticular” layout and synthesis
[4]. These hybrid MOFs are found in one-dimensional, two-dimensional, or three-dimensional structures
[5]. The primary objective for the development of MOFs was to achieve the desired characteristics by tuning their composition via changing either the metal or the organic component compared to their individual components
[6]. MOF cavities permit the encapsulation of different guest molecules to allow drug release and optimal imaging
[7][8][7,8]. If the volume of MOFs particles has been reduced to the nanoscale, nano-MOFs (NMOFs) could function as effective nano carriers to supply agents for imaging, chemotherapy, photo thermal therapy, photodynamic treatment, and rehabilitation
[9].
There are certain properties associated with MOFs, such as their versatile architecture, increased internal surface area, and ease of tuning their configuration by altering any of their components, i.e., either metal or organic ligand, that make them ideal candidates for applications in drug delivery systems, imaging, and sensors
[10]. Therefore, the selection of components in MOFs should be such that it is environmentally and biologically compatible
[11].
It is imperative to ensure the non-toxicity of MOFs for their successful application in biological domains such as intracellular imaging and drug carriers
[12][13][14][12,13,14]. In such instances, an important factor in designing MOFs is the likelihood to create mixtures of bioactive cations (Ca
2+, Zn
2+, Ag
+, and Fe
2+/
3+) with bioactive molecules as organic linkers that can medicinally transfer the component of interest through degradation of the framework
[15]. MOFs appeared to possess various unique characteristics over conventional nanocarriers such that they can be easily undergo modification (in situ synthetic or post-synthetic modification)
[16][17][16,17], which have made them ideal compared to conventional nanocarriers
[18].
2. Synthesis of Zn-Based MOFs
Mechano-chemical, hydrothermal, microwave-assisted, and ultrasound procedures have been adopted to develop MOFs with practical attributes and desired texture
[19][20][21][41,42,43]. The prime benefit of creating MOFs is the possibility of tuning their structure and functionality during their fabrication, where guest compounds bind and react with open sites. The selection of suitable building blocks during reticular synthesis ensures the creation of predefined shapes cavities. Using these approaches to make MOFs allows you to tune their structure and operation throughout the fabrication process, where visitor compounds can connect to vacant spaces and react with them
[22][23][44,45]. Various iso-reticular MOFs formulations have been prepared with zinc-based octahedral clusters (Zn
4O(CO)
2) serving the metallic centers surrounded by varied organic dicarboxylate nexus, thus constituting contrary 3D cubic structures
[24][25][46,47]. The free and fixed pores diameters range at 3.8–19.1 Å and 12.8–28.8 Å in isoreticular MOF-1 (IRMOF-1) and isoreticular MOF-6 (IRMOF-16), respectively
[26][27][48,49]. However, altering the organic linkers in MOFs variates the pore dimensions of the resulting framework and creates those with variable affinities for guest compounds
[28][50]. For example, as the organic linkers are altered in IRMOFs, selective hydrogen adsorption and diffusion in the porous structure are varied
[29][51].
2.1. Hydro or Solvo/Thermal Technique
Previously, the hydrothermal technique was used for producing coordinated metal-organic composites
[30][31][52,53]. The problem encountered during the formation of these composites was the exclusion of remaining solvent from the matrix of MOFs caused by external heating. On the other hand, high temperatures could risk the composition of developed MOFs, which can be cut down by low-temperature solvent removal
[32][33][54,55]. However, Zn-based MOFs that have been synthesized with stable thermal and chemical properties were reported
[34][56]. In particular, hydrothermal, microwave-hydrothermal, and microwave-solvothermal methods are very low-temperature methods for processing nanophase materials of various sizes and shapes. These procedures conserve energy and are environmentally sustainable since the reactions take place in discrete, closed system environments. Using the above methods, the nanophase materials can be produced in either a batch or continuous process. This takes a long time (typically half to several days) and requires high energy (over a thousand watts) relative to the traditional hydro/solvothermal heating system
[35][57]. One set of MOFs was released in 2013. 1-dinebsional chains of tetrahedral ZnO
2(OH)
2 and octahedral ZnO
2(OH)
2 combine together by a 3-hydroxy bridging agent and produces Zn
2(BTC)(OH) (H
2O) 1.67H
2O. The carboxylate groups of BTC moieties also connect octahedral and tetrahedral entities. The [Zn
2O
6(OH) (H
2O)]n chains are linked in this manner to form an expanded three-dimensional structure
[36][58].
2.2. Electrochemical Method
For the first time, the mixed-ligand MOF [Zn(1,3-bdc)0.5(bzim)] was effectively synthesized electrochemically in this study. It discovered that the amount of current and reaction time were the important elements influencing quality and yield. The authors were able to synthesize pure desirable MOF (crystallite size of 32.3 nm) with a substantially greater products (87%) compared to previously reported methods such as hydrothermal, solvothermal or slow diffusion techniques. Furthermore, reaction time was reduced from at least 8 days (solvothermal approach) to only 2 h. According to early UV-Vis measurements, the material absorbed a large quantity of Ibuprofen (163.9 mg/g), which was comparable with the result found by
13C-NMR spectroscopy (160.7 mg/g)
[37][59].
2.3. Ultrasound Methods
Environmentally friendly and budget friendly nanoscale MOFs have been synthesized by developed ultrasound methods
[38][60]. The ultrasonic technique is more effective in fabricating Zn-based MOFs with fluorescent characteristics, mainly for the accurate sensing of ethylene amine. Similarly, as demonstrated in
Figure 12, varying the sonication time can result in the different morphologies of Zn-based MOFs, i.e., nano sheets, nano belts, and microcrystals
[39][61]. An example is [[Co
2(TATAB)(OH) (H
2O)2]·H
2O·0.6O]. In order to create nanostructures, the experiment was first constructed in an autoclave for 72 h at 180 °C and then irradiated for 15 min with ultrasound (80 W). Ultrasounds, on the other hand, have reduced the response time to 2 h
[40][62]. In comparison to previous approaches, ultrasonic irradiation reduces response time while simultaneously improving particle size and shape. [Zn
2(NH
2-BDC)2(4-bpdh)] is a Zn(II)-based MOF. 3,4-bis(4-pyridyl)-3,4-diaza-2,4-hexadiene, DMF=N,N dimethylformamide) is made (NH
2-BDC=amino-1,4benzenedicarboxylate,4-bpdh=2,5-bis(4-pyridyl)-3,4-diaza-2,4-hexadiene, DMF=N,N dimethylformamide). The direct pyrolysis of S-TMU-16 NH
2 MOF was used to produce ZnO octahedral nanostructures in a simple and easy manner
[41][63]. Two 3D porous Zn-based MOF prepared by the sonochemical process is [Zn(oba)(4-bpdh)0.5]n·(DMF)1.5 (TMU-5) and the other one is [Zn(oba)(4-bpmb)0.5]n (DMF)1.5 (TMU-6). Further calcination of these MOFs at 550 °C enhances RhB adsorption. The conclusion is that by changing the sonication time,
reswe
archers can achieve MOFs with different morphologies, sizes, and shapes via ultrasound-assisted irradiation methods
[42][43][64,65]. [Zn(NH
2-BDC)(4,4′-bpy) is a 1.7-micrometer-sized Zn-based MOF synthesized in 60 min by ultrasound method. Moreover, this combination of Al
3+ and NH
2BDC ligand induces an excellent potential as the fluorescent sensor for low limit detection of toxic Al
3+ and methanol in MOFs
[44][66].
Figure 12. The transmission electron microscopic (TEM) result of [Zn(BDC) (H
2O)]n following (
a) 10, (
b) 20, (
c) 30, (
d) 60, and (
e) 90 min sonication
[39][61]. Reprinted with permission from Ref.
[39][61]. Copyright 2008 Elsevier.
Under ultrasonic irradiation, several chemical processes have been performed in high yield with reduced reaction durations
[45][67]. The interaction of Zn(OAc)
2 and H
2BDC in DMF during ultrasonic irradiation for 10 min yielded nanostructures with a breadth of 150–300 nm and a height of 2–5 µm. Whenever the response time was too long at 20 min, nanoparticles became larger in size. Having diameters ranging from 300 nm and length ranges 2 µm, such nanomaterials have regular quadrate morphologies. Improvements in reaction time were reflected in a 1.5% boost in nanostructures dimensions. However, when the reaction time was prolonged beyond 60 min, numerous microcrystals with size varying from hundred nanometers to tens of nanometers were obtained. Once reaction length increased to 90 min, massive microcrystals could not be identified, although nanoparticles of various shapes and sizes and those that were irregularly shaped were observed. This result indicated that throughout long-term ultrasonic irradiation, Zn(BDC) (H
2O) n microcrystals were degraded, and all these microcrystals were transformed into nanoparticles caused by weak covalent bonding among 2D conjugated polymers.
3. Medical Applications
Zn-Based MOF as Drug Carriers
Many medicinally active ingredients are confined to limited numbers in pharmaceutical applications due to poor stability in living systems, low solubility, and inability to cross natural barriers
[2]. Development in the pharmaceutical industry started in the 1970s by introducing drug Nano carriers to improve techniques to prevent the biodegradation of active ingredients, thus protecting the living system from their toxic effects. These techniques improved the effectiveness of the drug by increasing intracellular penetration. Furthermore, Nano techniques have also paved the way for targeting specific tissues, cells, and even cellular organelles
[30][52]. Zinc-based metal-organic frameworks are considered one of the best candidates for developing Nano encapsulates due to their versatility, lower toxicity, and being easily biodegradable.
Different MOFs based on Zn with their encapsulated drug, organic linker, loading degree, and release rate are presented in
Table 1. Likewise, a zinc-based MOF was established with bidentate carbene ligand as rigid support that makes it possible for Zn-MOF to assist in encapsulating and then discharge the drug (cisplatin). Cisplatin, an effective anticancer medicine, has been verified for its anticancer potential against ovarian cancer cells (A2780) through in vitro trials.
Table 1.
Details about recently developed Zn MOFs: their structure, drug loading and release rate.
| Zn-Based MOF |
bio-MOF-1 |
IFMC-1 |
Med-MOF-1 |
[Zn | 2 | (1,4-bdc) | 2 | (dabco)n] |
Zn-TBDA |
MOF-74 |
| Chemical/Empirical Formula |
Zn | 8 | (Ad) | 4 | (BPDC) | 6 | O | 2 | (NH | 2 | (CH | 3 | ) | 2 | ) + 8DMF, 11H | 2 | O |
- |
Zn | 3 | (curcumin) | 27 | (DMA) | 3 | (ethanol) |
[Zn | 2 | (1,4-bdc) | 2 | (dabco)n] |
[Zn (tbda)] | n |
Zn | 2 | DOT |
| Organic Linker |
Adenatite |
Triazole |
Curcumin |
1,4-diazabicyclo
[2.2.2] octane (DBCO) |
4′-(1H-tetrazol-5-yl)-
[1,1′-biphenyl]-3,5-dicarboxylic acid |
2,5-di hydroxyterephthalic acid |
| Drug |
Procainamide |
5-Fluorouracil |
Ibuprofen |
Ibuprofen |
Methotrexate |
Ibuprofen |
| Loading Degree |
0.22 | g | / | g |
30.48 wt% |
0.24 | g | / | g |
15 wt% |
12.59% |
313 k |
| Release Rate |
% |
20 |
89.8 |
97 |
80 |
61 |
- |
| Time |
72 h |
120 h |
80 h |
288 h |
48 h |
- |
| Reference |
[46] | [80] |
[47] | [81] |
[48] | [82] |
[49] | [83] |
[50] | [84] |
[51] | [85] |
Extremely water-stable (up to 3 weeks), microporous MOF, [Zn8(O)2(CDDB)6(DMF)4(H2O)] where CDDB = 4,4′-(9-H carbazole-3,6-diyl) dibenzoic, was synthesized by a solvothermal process based on an open N-H site, which demonstrated excellent loading potential (around 53.3 wt.%) and adequate release potential (64.9 and 81.9%) for 5-fluorouracil, and loading capacity is around 53.3 wt%.
An odd 4,8-connected 3D net with (46)2(412•612•84) topology was shown by the coordination polymer. The medication 5-fluorouracil (5-FU) was connected to the desolated one at around 22.5% wt. per gram of the dehydrated one. With 92% of the prescription released after 120 h, 5-Fu is discharged in a closely regulated and regular manner. This analysis provides a new method for MOF to be used as possible drug delivery. Compared to zeolites and activated carbons, MOF showed both hydrophilic/hydrophobic entities, and their versatile nature allows porosity to be tailored to physicochemical characteristics. In comparison, structural instability in water has been observed in specific Zn-based metal-organic frameworks. However, to overcome this issue, a biofriendly and water-stable anionic Zn-MOF has been prepared with a dicarboxylic acid as the organic linker.
This medication had a 25% drug loading weight and 95.6% removal efficiency after 100 h. The non-toxicity of the synthesized drug has been testified by conducting in vitro MTT assay and in vivo toxicity tests. Drug loading in water (53.3% by weight and release rate of 81.9%) and 64.9% in PBS was achieved for 5-fluorouracil with negligible cellular toxicity.
4. Applications of Zn-Based MOFs for Gases Adsorption, Imaging and Sensors
Zn-based MOFs have added beneficial aspects in drug delivery as efficient medicinal carriers; they also have other applications. Here,
rwe
searchers will summarize the applications of MOFs in various disciplines
[52][107].
4.1. Zn-Based MOFs for Active Gases Adsorption
Zn-based MOFs are capable of capturing, separating, storage, and effective delivery of bio medically essential gases. Nitric oxide is an exceptional biomolecule with antibacterial, anti-thrombosis properties and an excellent vasodilating agent
[53][108]. In progressive studies, the capability of Zn
2+ exchanged zeolite: polytetrafluoroethylene (50:50) for nitric oxide loading, release, and antibacterial properties was examined. They found better antibacterial activity for nitric oxide-releasing zeolites, whereas nitric oxide-free Zn
2+-exchanged zeolite provided excellent potential for fabricating extremely operative dual functionality-bacterial materials, while the application of such nitric oxide donors might include experienced confinement due to the production of certain pro-inflammatory by-products
[54][109].
However, metal-organic frameworks acquire good potential because of their improved storage and control over reaction ability between gas molecules and components of MOF by making appropriate alterations in components. Thus, a Zn-based MOF has been developed by incorporating tetracyanoquindimethane as a linker to create a porous structure for selective absorption of nitric oxide and other gas CO
2, N
2, C
2H
2, and molecules
[55][110]. CPO-27 metal-organic frameworks exist in the form of iso-structures formed by the combination of 2-5-dihydroxyterephthalic acid with different metallic parts, including Mg, Zn, and Ni
[56][111]. These clusters have pore sizes ranging 11–12 Å and could be employed to store and release nitric oxide. Furthermore, Ni
2+ doped CPO-27 (Zn) has been used to strengthen CPO-27 (Zn) in order to enhance future nitrous oxide release in nitric oxide retention. This composite possessed good thermal stability and the efficient ability to synchronize the solvent molecules, freely subjected to heat to create open sites to capture the nitric oxide
[57][112].
Hydrogen sulfide (H
2S) is also an extremely toxic gas; it could be biologically significant if delivered in a required amount at the desired rate. It has been discovered that metal-organic frameworks with the CPO-27 structure can be used to improve H
2S adsorption and preservation. Correspondingly, H
2S binding and releasing capacity of Ni-CPO and Zn-CPO has been testified, demonstrating better nickel delivery (i.e., 1.8 mmol·g
−1 from nickel and 0.5 mmol·g
−1 from Zn in 30 min) as compared to Zn-based material. However, the ability to deliver less H
2S from Zn-based material could be linked with difficulties, such as the incomplete activation of the sample, as earlier reported, or the irreversible and substantial binding potential of zinc to sulfur
[58][113].
4.2. Zn-MOF as Contrast Agents in MRI
MOFs have recently been used to replace contrast chemicals in biomedical imaging modalities such as magnetic resonance imaging (MRI). Magnetic resonance imaging (MRI) is widely acknowledged as one of the most prospective imaging methods that do not require ionizing radiation or a radioactive nucleus. Magnetic nanoparticles, on the other hand, can change the MRI signal by speeding up the relaxation phase of protons in nearby H
2O molecules under an outside magnetic field
[59][114]. Research studies on the fabrication of MOFs as MRI contrast agents have been ongoing since the mid 2000s
[60][115]. The recent developments on Gd III-based nanoscale with good paramagnetic properties have enabled their application as MRI contrast agents.
A versatile framework for magnetized derived medication delivery, MRI agents, and biomedical imaging has also been established using an iron-based MOF nanoscale; two major applications of MOFs include the following: drug carriers for controlled release and the potent contrast agent in MRI image augmentation. ZIF-8 is one of the most extensively studied Nano carriers, emphasizing its application in cancer imaging and treatment. Progressively, glutathione and pH-dependent ZIF-8 were employed to serve as platforms for assembling small Fe
3O
4 nanoparticles as T1 contrast agents in the assembly of Fe
3O
4–ZIF-8 acting as a T2 contrasting agent
[61][116]. In vivo investigations used MRI contrast agents with Fe
3O
4-based T2–T1 switching for cancer diagnostics. To replace 2-methylimidazolate in ZIF-8
[62][117], a pH-sensitive 19F MRI Nano probe was created. With a low background and excellent penetration depth, this probe was well suited for stimuli-responsive detection. However, due to their toxicity, these materials are not suited for biotechnology. Nanoparticles have indeed been created for effective delivery systems by combining biosynthetic pathways and scanning features with MOFs. A strong MR-active saturation magnetization micro-MOF linked with folic acid as a fluorescent marker has been efficiently produced that delivers aqueous anticancer medication paclitaxel (
Figure 24). These Fe
3O
4@IRMOF-3/FA NPs are a multipurpose spongy framework that could be used for medication administration, bio imaging, and MRI contrast
[63][118].
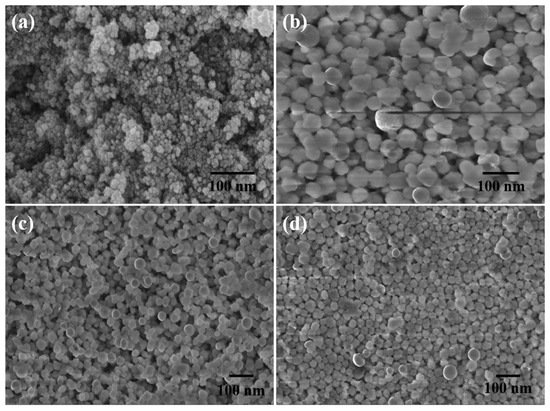
Figure 24. FESEM scans of (
a) Fe
3O
4 nanoparticles, (
b) IRMOF-3, (
c) Fe3O4@IRMOF-3, and (
d) Fe
3O
4@IRMOF-3/FA
[63][118]. Reprinted with permission from Ref.
[63][118]. Copyright 2016 Royal Society of Chemistry.
However, due to their toxicity, these materials are not suited for biotechnology. Nanoparticles theranostics have indeed been created for effective delivery systems by combining biosynthetic pathways and scanning features with MOFs. A strong MR-active saturation magnetization micro-MOF linked with folic acid as a fluorescent marker has been efficiently produced that can deliver the aqueous anticancer medication paclitaxel. These Fe
3O
4@IRMOF-3/FA NPs are a spongy multipurpose framework that could be used for medication administration, bio imaging, and MRI contrast. Regarding cancerous cells, concatenated folic acid demonstrated a greater T2-weighted MRI distinction. ZIF-8, a more investigated nanoparticles that has been employed in clinical therapy and diagnosis, is a much more investigated nanoparticle. Small Fe
3O
4 NPs (T1 distinction agent) have indeed been produced together into T2 contrast material Fe
3O
4–ZIF-8 architecture that used a pH and glutathione-responsive ZIF-8 as both structures. For in vivo diagnostic procedures, an Fe
3O
4-based precise T2–T1 switching image reconstruction approach is particularly successful. Since 1H MRI has a low exposure, the number of protons in human tissue causes strong background signals.
4.3. Nanoscale Metal-Organic Framework for Ultrasonographic Scanning
Nanoscale MOFs have been investigated for their possible use in a variety of applications such as X-ray computed tomography imaging (CT imaging) as contrast agents with the addition of high atomic number elements as their building blocks
[64][119]. Meanwhile, Cu
2+ and Zn
2+ were employed as metal parts along with 2,3,4,5,6-tetraiodo-1,4-benzenedicarboxylate (I4-BDC) as bridging ligands for the fabrication of iodinated nanoscale metal-organic frameworks [Cu(I4-BDC) (H
2O)2] ·2H
2O and [Zn(I4-BDC)- (EtOH)2] ·2EtOH, respectively. These fabricated MOFs contained a high level of 63% of iodine weight. In contrast, phantom studies verified that X-ray attenuation coefficients of these MOFs are analogous to the conventionally used molecular contrast agent such as iodixanol. Therefore, it can be concluded that these frameworks provided a unique platform for devising efficient contrast agents that could be used in CTI by incorporating iodinated compounds as bridging ligands
[65][120].