You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Jason Zhu and Version 2 by Jason Zhu.
Heavy metal stress (HMS) is one of the most destructive abiotic stresses which seriously affects the growth and development of plants. In general, three core signals are involved in plants’ responses to HMS; these are mitogen-activated protein kinase (MAPK), calcium, and hormonal (abscisic acid) signals. In addition to these signal components, other regulatory factors, such as microRNAs and membrane proteins, also play an important role in regulating HMS responses in plants.
- specific transmembrane proteins
- protein-lipid interactions
- plant metal homeostasis
- Heavy metal stress
1. Introduction
Heavy metal stress (HMS) negatively affects plant growth and reproduction, and thus, it can cause the loss of essential agronomic and other agroecological traits in plants [1]. Consequently, the yield and quality of crops are seriously affected, while food with excessive accumulation of heavy metals (HMs) is one of the major threats to human health and natural ecosystems [2]. Plants have specific mechanisms to cope with HMS, and they have evolved a series of strategies such as ion sensing, signal transduction, and segregation detoxification to ensure their optimum survival and reproduction under a certain level of HMS [3][4][5]. Recently, some advances have been made in the understanding of plant stress responses, especially downstream pathways, with the discovery of several functional membrane protein gene clusters that play important roles in regulating intracellular metal homeostasis [6][7][8][9]. However, these complex network cascades need to be studied with respect to the metal homeostasis regulation mechanisms in plants.
Recent studies have shown that the plant growth stage, from seedling to maturity, determines the formation of metal homeostasis mechanisms and tolerance in plants. Of course, the HM contents in fruits during the reproductive maturity stage of plants are significantly influenced by the metal homeostasis, though the systematic accumulation pathways and functional cascade relationships of HMs across different plant growth stages are still unclear [10][11]. Therefore, it is of great significance to explore the homeostasis mechanisms of HMs in plant cells. Currently, some studies have demonstrated that plants can regulate the balance of metal ions in the cell by increasing the rate of synthesis of various proteins and/or compounds [12][13][14][15]. This involves the molecular response signals of plants to HMS along with the regulation and transcription of some membrane proteins. In the plant cell, these metals cause the production of reactive oxygen species (ROS), which trigger the activation of various signal transduction pathways [16]. The key signal components involved in HMS are mitogen-activated protein kinase (MAPK), calcium, and hormonal signals. The MAPK signal has some important components and types, such as the MAP kinase kinases (MAPKKs) and mitogen-activated protein kinase kinases (MAPKS) [17][18]. The calcium signaling pathway uses a variety of calcium-sensitive proteins for Ca2+, such as calmodulins (CaMs), calmodulin-like proteins (CMLs), calcineurin-like proteins (CBLs), and calcium-dependent protein kinases (CDPKs). They activate and route different signal pathways along the circuit [19]. Regarding the role of hormone signal transduction, abscisic acid (ABA) [20], jasmonic acid (JA) [21], and citric acid (CA) [22] play important roles in metal tolerance in plants. In addition to the signal components, other regulatory factors, such as miRNAs and different types of transmembrane transporters, also play important roles in regulating HMS in plants [23][24]. Specific membrane proteins can bind different types of metal ions as substrates [25][26], thus making them capable of ion transport across the cell membrane, which is regulated by the interactions between membrane proteins, ions, and lipid types and contents. Some lipids bind to specific sites on membrane channels, and thus regulate the transport of metal ions inside and outside of the plant cell.
2. Interactions between Membrane Proteins and Lipids under HMS
All membranes, which are mainly composed of lipids and proteins, are highly dynamic and experience a large variety of interactions [27]. The complexity of these interactions can lead to the diversification of the structures and functions of the membrane proteins. Therefore, under HMS, the membrane proteins can promote intercellular communication, thus leading to the regulation of intracellular and extracellular homeostasis along with the detoxification of metal ions through energy transmission and signal transduction in plant cells. With the deepening of liposomics and structural analysis of membrane proteins, the specific sites and functions of lipid-binding membrane proteins can be visualized and studied. For example, applications of recent state-of-the-art analytical approaches such as X-ray crystallography, nuclear magnetic resonance (NMR) [28], electron paramagnetic resonance (EPR) [29], and native mass spectrometry (MS) combined with molecular dynamics (MDs) [30] have now enabled us to further study the properties of lipids that maintain the structure and functions of the membrane proteins. Thus, these approaches may provide new opportunities for an in-depth understanding of the processes underlying the interactions between lipids and membrane proteins and their role in HMS tolerance in plants.
2.1. Membrane Proteins and Lipid Remodeling
HMS can cause the reorganization of plant endomembranes, wherein the redistribution of lipid-membrane protein binding is closely related to the regulation of metal homeostasis to a large extent [31]. Because of the position of membrane proteins in lipid bilayers, the structures and functions of the membrane proteins may be related to their positions in the lipids [32]. Different types of membrane proteins play different roles and can regulate ion homeostasis in different ways. Interestingly, membrane proteins can bind nonspecifically to a variety of lipids, which are usually used as protein adsorption solvents (cyclic lipids), while other small amounts of lipids can specifically bind to the membrane proteins as cofactors (non-cyclic lipids) to complete the process of remodeling [33]. Specific lipids are like buttons, while membrane proteins are like clothes, and thus these lipids precisely fix membrane proteins in their correct place. Meanwhile, the solvent lipids, which are also known as cyclic lipids, can form stable annular lipid shells around the membrane proteins and can further immobilize the membrane protein [34]. Therefore, they can jointly define the position of the membrane protein and ensure the stability of the membrane skeleton. In addition, these lipids can also interact with membrane proteins through configurational changes to regulate the HMS tolerance in plants.
During endomembrane reorganization, a small number of lipid-membrane protein interactions have also been reported in microorganisms (Figure 1), and their types are related to their structures and substrates. A common feature is that their locations and functions depend on the complexity of the membrane environment. As a cofactor, lipids (acyclic lipids) usually bind between the transmembrane helices of the membrane proteins, either within the membrane proteins and/or at the multi-subunit protein interface (Figure 1A–D). However, under HMS, a large number of metal ions are free, which may lead to the substitution and/or inactivation of these sites depending on their charge intensity [35][36] and the head groups of these lipids [37]. These conditions may trigger a series of membrane protein stress responses and signals, which could lead to alterations in the transmembrane domain of the membrane proteins [38].
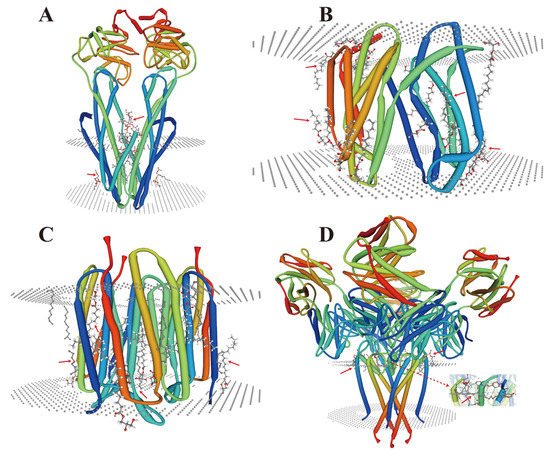

Figure 1. Crystal structure analysis of lipid-membrane protein interactions. (A) Escherichia coli (E. coli) MsbA in complex with LPS and inhibitor G907 (PDB ID: 4KSC) [39]; (B) Lipid II flippase MurJ, outward-facing conformation in Thermosipho africanus (PDB ID: 6NC9) [40]; (C) Steady-state-SMX dark state structure of the bacteriorhodopsin in Halobacterium salinarum (PDB ID: 6RQP) [41]; (D) Structure of the KcsA-G77A mutant or the 2,4-ion bound configuration of a K(+) channel selectivity filter in Streptomyces lividans (PDB ID: 6NFU) [42]. PDB, Protein Data Bank; MsbA, an essential ATP-binding cassette transporter in Gram-negative bacteria; LPS, lipopolysaccharide; MurJ, the lipid II flippase in E. coli. The red arrows indicate the positions where lipids and membrane proteins interact with each other.
According to the affinity between membrane proteins and lipids, the lipids that play a key role in intimal remodeling can be divided into bulk, annular and non-annular lipids. Bulk lipids (without direct contact with membrane proteins) expand rapidly in the bilayer. At the same time, annular lipids bind to the membrane proteins through hydrophobicity to form stable complexes and manipulate the membrane proteins to function and allow material exchange, which depends on the hydrophobicity of the membrane and the charge distribution of the lipid-membrane protein binding surface. In particular, non-annular lipids have multiple functions in regulating the membrane proteins because their different configurations can bind to membrane proteins to form very stable structures. Studies have found that they remain bound together even after proteins are washed, purified, and/or crystallized. This stable structure may play an important role in HMS in plants. For example, non-annular lipids act as cofactors to block ion conduction and regulate the activity of membrane proteins.
2.2. Metal Coordination of Lipids with Membrane Proteins
In recent years, great progress has been made in the structural analysis of membrane proteins. However, the interactions between lipid proteins and membrane proteins in complex cellular environments remain understudied. The recent emergence of X-ray free-electron laser (XFEL) femtosecond crystallography offers an innovative opportunity to explore the elusive membrane proteins and their complexes [43]. When combined with other techniques such as electron paramagnetic resonance (EPR), molecular dynamics (MDs), atomic force microscopy (AFM) [44], and fluorescence resonance energy transfer (FRET) [45], the metal ions and lipids that interact directly with membrane proteins, as well as the interaction sites, and even the covalent bond, can also be profiled and studied. Advances in instrumentation and technology provide unprecedented opportunities to investigate the synthesis of lipid-protein complexes. At present, the crystal structures of membrane protein complexes are mostly reported with the non-cyclic lipids [30]. It has been shown that lipids can protect membrane proteins by reducing deuterium absorption, while the sites protected by the lipid-binding can be studied [46][47]. Some membrane proteins exhibit metal coordination activity only in the presence of specific types of lipids (Figure 2). These protein complexes can be purified, and their crystal structures can be visualized mechanistically. Most of these lipids are non-annular lipids. However, studies of these types of protein-lipid interactions in plants are highly limited. It is anticipated that future research can rely on the existing advanced technology and jointly use it to analyze the structure of protein complexes more widely, thus providing the possibility of understanding the action mechanisms of the membrane lipids in complex cellular environments under HMS.
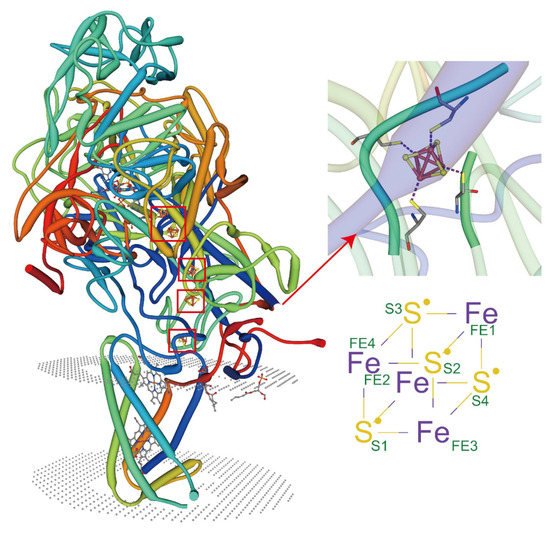

Figure 2. Lipids regulate the coordination of metal ions with membrane proteins. PDB ID: 3IR5, X-ray diffraction, 2.30 Å. Crystal structure of NarGHI mutant NarG-H49C in Escherichia coli. Tightly bound lipids exist between the transmembrane helices, while head groups bind in pockets composed of three subunits, and many positively charged residues participate in the binding.
2.3. Special Ion Channels as Lipid Sensors
The ion channel is a kind of membrane protein that promotes the passage of various chemicals through membranes. Channels open or close due to several factors, such as transmembrane potentials [48], compound stimulation [49], and membrane contraction forces. Under the influence of these factors, the channel undergoes a structural change from a closed to an open state, thus allowing the substrate to move along the electrochemical gradient. Since the selective permeation of cell membrane to sodium, potassium and chloride ions was discovered, the channels have been extensively studied. The ion channels of excitable cells respond to the synaptic stimuli and transmit action potentials through the establishment of resting membrane potentials [50]. In recent years, researchers have carried out a significant amount of work on the structures and functions of the membrane channels, particularly on using them as biosensors to monitor the development and applications of new molecules to study their role and activities in different cellular functions [51][52][53][54][55]. According to the methods of sensing external stimuli, ion channels are divided into three categories: (A) voltage-gated channels, (B) ligand-gated channels and (C) mechanosensitive channels. They respond to lipids in different ways (Figure 3).
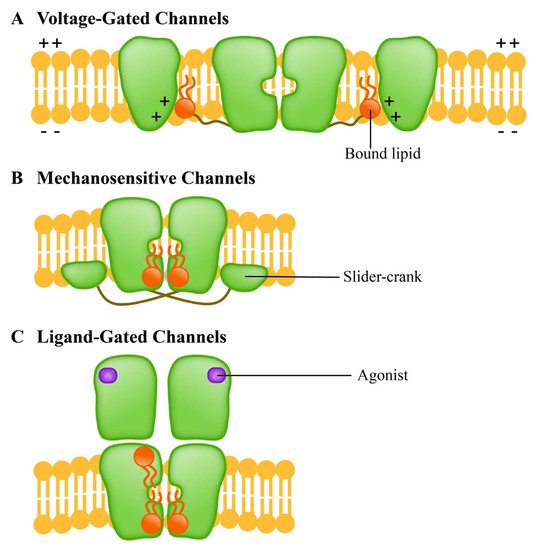

Figure 3. Different ion channel protein families have different lipid response patterns. (A) Voltage-gated channels (voltage-gated channel) is a tetramer; (B) Mechanosensitive channels (mechanically sensitive channel) uses the “crank slider” mechanism to convert increased double-layer tension into channel gating; (C) Ligand-gated channels (ligand gated channel) affects the function of TM helical stacking to change the channel function.
The voltage-gated channels mediate the transmembrane movement of K+, Na+, Ca2+ and Cl- in response to membrane potential changes [56]. This channel contains four homologous domains; each domain consists of six transmembrane helices (S1-S6). The S1-S4 is responsible for the induction of electrical signals, while the S5-S6 is responsible for the hole formation [57]. The ligand-gated channels, which are transmembrane proteins, are a common part of eukaryotic signal cascades. Plant cyclic nucleotide-gated channel (CNGC) is a cationic channel with a tetramer structure that allows the diffusion of univalent and divalent cations such as Ca2+ and K+ [58][59]. The CNGC family contributes to the absorption and transport of HM ions in plants [60]. Mechanosensitive channels, which are usually multimers, are the umbrella term for pore proteins that mainly respond to the mechanical stimuli. With the stimulation of mechanical force, the transport of substances (mainly ions) across the membrane is allowed, and the mechanical signals can be converted into electrical and/or chemical signals [61]. Mechanosensitive channels in plants may be activated by external mechanical stimuli such as touch, wind, salinity, and gravity [62][63]. Studies have shown that the homologue of bacterial mechanically-sensitive channels (MSCs) in plants can reduce the intracellular osmotic pressure by releasing small permeable substances in a process controlled by the membrane tension [62].
32.4. Role of Membrane Proteins in the Lipid Outflow
The plasma membrane is fundamental for cellular transport by using the lipid bilayer to form a tight barrier that allows the selective exchange of substances between the cell and external environmental factors. Membrane lipids modulate the functions of membrane transporters in two ways: one is to bind closely and specifically to the transporters, while the other is to regulate the functions of the transporters through the diversified lipid bilayer [63][64].
Members of the ABC family are involved in the transport and redistribution of various lipids and protein-lipid complexes [65]. The ABCs are mainly located in the plasma membrane, though some of them are also located in the microregions of cellular fluids (cholesterol and sphingomatidylin). These are also reported in other cellular organelles such as the Golgi apparatus and endoplasmic reticulum [66][67]. In general, apolipoprotein and albumin are receptors for most sphingolipids that are substrates for the ABC transporters in animals (Figure 4). Although studies on this aspect in plants are limited, future studies are expected to fill the gap.
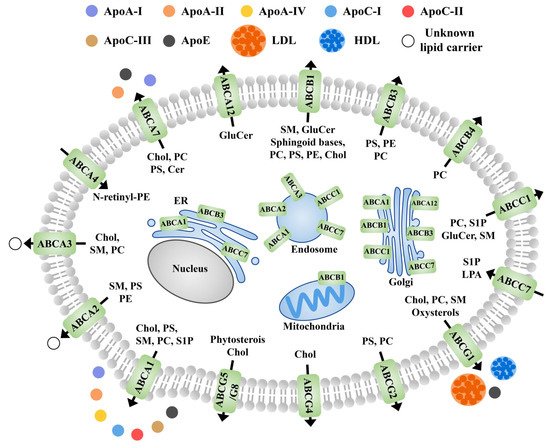

Figure 4. Schematic diagram of the subcellular localization, known receptors, and transport directions of ABC transporter proteins involved in lipid efflux in animals. Black arrows indicate the direction of transport across the plasma membrane. Apo, apolipoprotein; PS, phosphatidylserine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; Chol, cholesterol; HDL, high density lipoproteins; LDL, low density lipoproteins; GluCer, glucosylceramide; Cer, ceramide; SM, sphingomyelin; N-rentinyl phosphatidylethanolamine; LPA, lysophosphatidic acid; S1P, sphingosine-1-phosphate; N- retinyl-PE.
2.5. Synergistic Participation in Specific Binding of Metal Ions
Plant membranes are mainly comprised of phospholipids and contain a bewildering array of complex components such as sphingomyelins, sterols, carbohydrates linked by glycosylation, and membrane proteins [68]. The separation of cells from their external environment cannot be maintained solely by lipid barriers. In the case of organisms and cells exposed to HMs, the membrane proteins actively adjust the different conditions on both sides of the lipid barrier [69]. Membrane proteins may cross the phospholipid bilayer and/or interact with the polar heads of lipids, thus directly and indirectly affecting the structure of cell membranes and even the composition of membranes [70][71]. Notably, membrane proteins also need to allow controlled signals to occur through the structural barriers of the cell membrane (Figure 5). The structures and functions of membrane proteins are affected by the lipids, as well as the interactions between proteins and specific lipids [72].
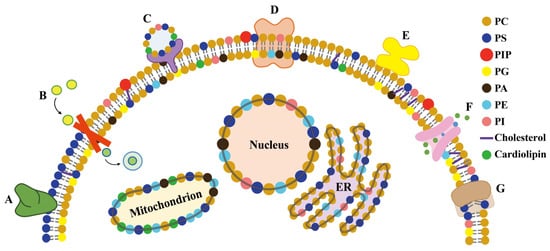

Figure 5. Proteins bind to lipids in different ways. (A) The peripheral protein binds to the hydrophobically anchored PS suppository with lipid specificity. (B) The integrin receptors involved in signal perception and transmission across cell membranes. (C) Proteins that induce vesicle fusion. (D) Integrins with local curvature induced by hydrophobic mismatch. (E) The peripheral proteins are attached to the membrane lipids, and their globular domains interact with the membrane interface regions without being embedded in the membrane. (F) The ion channels interact with cholesterol to control ion transport across the membrane. (G) The phospholipid-scrambling enzymes transport lipids through the membrane.
Plants are inevitably exposed to environmental stresses, such as metal contamination, while membrane proteins can induce mechanisms underlying plant tolerance to different stresses [73]. The plants’ interactions with HMs produce several cellular, physiological, and biochemical responses. Of course, these reactions are influenced by a complex transduction network of multiple signal components in plant cells, which eventually help plants counter HMS by synthesizing metalloproteins [74].
3. Analysis of Membrane Protein-Metal Binding Domains and Interaction Based on the Novel Metallomics Database
Trace metals are inorganic elements; all organisms need them for their growth, development, and other cellular functions. Cells need metal ions as cofactors to assemble and activate metalloproteins. Most metals bind directly to specific sites on membrane proteins, while a few metals must form metal-containing cofactors or complexes to insert into the target protein [75]. Metalloproteins are types of protein with large quantities and varieties in the cellular proteome. They not only catalyze an array of important biochemical reactions but also demonstrate unique structures and regulatory functions [76]. It is speculated that about a third of proteins must bind with metals for their biological functions, and almost half of the enzymes must be combined with one or more metal ions for their functioning. The number of metalloproteins in the family depends on the number and types of metals they bind in the cell. There are estimated to be hundreds of zinc-related protein families, while the nickel-dependent type protein families number fewer than 10 [77]. The existence of metalloproteins requires strict regulation of metal metabolism and homeostasis in order to maintain the appropriate metal concentrations in the cells while avoiding toxic effects during transport, storage, detoxification, and excretion processes in cells [78].
In recent years, with the development of genome sequencing technology, the amount of genomic data has increased exponentially, so there is an urgent need to develop bioinformatics algorithms to predict new metalloproteins or even search for a whole set of metalloproteins. Many computational tools and methods have been developed to predict metalloprotein genes and metal-binding sites, especially related to Zn and Fe [74].
References
- Jalmi, S.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the Links between Heavy Metal Stress and Plant Signaling. Front. Plant Sci. 2018, 9, 12.
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12.
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.-Y.; Li, J.; Wang, P.-Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674.
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86.
- Takahashi, F.; Shinozaki, K. Long-distance signaling in plant stress response. Curr. Opin. Plant Biol. 2019, 47, 106–111.
- Deason-Towne, F.; Perraud, A.-L.; Schmitz, C. Identification of Ser/Thr phosphorylation sites in the C2-domain of phospholipase C γ2 (PLCγ2) using TRPM7-kinase. Cell. Signal. 2012, 24, 2070–2075.
- Qi, Z.; Ci, X.; Huang, J.; Liu, Q.; Yu, Q.; Zhou, J.; Deng, X. Asiatic acid enhances Nrf2 signaling to protect HepG2 cells from oxidative damage through Akt and ERK activation. Biomed. Pharmacother. 2017, 88, 252–259.
- Li, Q.; Xu, F.; Chen, Z.; Teng, Z.; Sun, K.; Li, X.; Yu, J.; Zhang, G.; Liang, Y.; Huang, X.; et al. Synergistic interplay of ABA and BR signal in regulating plant growth and adaptation. Nat. Plants 2021, 7, 1108–1118.
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609.
- Rankenberg, T.; Geldhof, B.; van Veen, H.; Holsteens, K.; Van de Poel, B.; Sasidharan, R. Age-Dependent Abiotic Stress Resilience in Plants. Trends Plant Sci. 2021, 26, 692–705.
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Ziegler, J.; Nobori, T.; Nair, A.; Krüler, V.; Winkelmüller, T.M.; Wang, Y.; Mine, A.; et al. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373.
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340.
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446.
- Sako, K.; Nguyen, H.M.; Seki, M. Advances in Chemical Priming to Enhance Abiotic Stress Tolerance in Plants. Plant Cell Physiol. 2021, 61, 1995–2003.
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F. The role of salicylic acid and gibberellin signaling in plant responses to abiotic stress with an emphasis on heavy metals. Plant Signal. Behav. 2020, 15, 1777372.
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208.
- Kaur, R.; Das, S.; Bansal, S.; Singh, G.; Sardar, S.; Dhar, H.; Ram, H. Heavy metal stress in rice: Uptake, transport, signaling, and tolerance mechanisms. Physiol. Plant. 2021, 173, 430–448.
- Sinha, A.K.; Jaggi, M.; Raghuram, B.; Tuteja, N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal. Behav. 2011, 6, 196–203.
- Luan, S.; Kudla, J.; Rodriguez-Concepcion, M.; Yalovsky, S.; Gruissem, W. Calmodulins and Calcineurin B–like Proteins: Calcium sensors for specific signal response coupling in plants. Plant Cell 2002, 14, S389–S400.
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161.
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic acid: A key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2021, 40, 1513–1541.
- Mallhi, Z.I.; Rizwan, M.; Mansha, A.; Ali, Q.; Asim, S.; Ali, S.; Hussain, A.; Alrokayan, S.H.; Khan, H.A.; Alam, P.; et al. Citric Acid Enhances Plant Growth, Photosynthesis, and Phytoextraction of Lead by Alleviating the Oxidative Stress in Castor Beans. Plants 2019, 8, 525.
- Ding, Y.; Ding, L.; Xia, Y.; Wang, F.; Zhu, C. Emerging Roles of microRNAs in Plant Heavy Metal Tolerance and Homeostasis. J. Agric. Food Chem. 2020, 68, 1958–1965.
- Vishwakarma, K.; Mishra, M.; Patil, G.; Mulkey, S.; Ramawat, N.; Pratap Singh, V.; Deshmukh, R.; Kumar Tripathi, D.; Nguyen, H.T.; Sharma, S. Avenues of the membrane transport system in adaptation of plants to abiotic stresses. Crit. Rev. Biotechnol. 2019, 39, 861–883.
- Laganowsky, A.; Reading, E.; Allison, T.M.; Ulmschneider, M.B.; Degiacomi, M.; Baldwin, A.J.; Robinson, C.V. Membrane proteins bind lipids selectively to modulate their structure and function. Nat. Cell Biol. 2014, 510, 172–175.
- Hilgemann, D.W.; Dai, G.; Collins, A.; Larricia, V.; Magi, S.; Deisl, C.; Fine, M. Lipid signaling to membrane proteins: From second messengers to membrane domains and adapter-free endocytosis. J. Gen. Physiol. 2018, 150, 211–224.
- Nicolson, G.L. The Fluid—Mosaic Model of Membrane Structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40years. Biochim. et Biophys. Acta (BBA)-Biomembr. 2014, 1838, 1451–1466.
- Tycko, R. BIOMOLECULAR SOLID STATE NMR: Advances in Structural Methodology and Applications to Peptide and Protein Fibrils. Annu. Rev. Phys. Chem. 2001, 52, 575–606.
- Sahu, I.D.; Lorigan, G.A. Electron Paramagnetic Resonance as a Tool for Studying Membrane Proteins. Biomolecules 2020, 10, 763.
- Bolla, J.R.; Corey, R.; Sahin, C.; Gault, J.; Hummer, A.; Hopper, J.T.S.; Lane, D.P.; Drew, D.; Allison, T.M.; Stansfeld, P.J.; et al. A Mass-Spectrometry-Based Approach to Distinguish Annular and Specific Lipid Binding to Membrane Proteins. Angew. Chem. Int. Ed. 2020, 59, 3523–3528.
- Putta, P.; Creque, E.; Piontkivska, H.; Kooijman, E.E. Lipid−protein interactions for ECA1 an N-ANTH domain protein involved in stress signaling in plants. Chem. Phys. Lipids 2020, 231, 104919.
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124.
- Lee, A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta Biomembr. 2004, 1666, 62–87.
- Lee, A.G. Lipid–protein interactions. Biochem. Soc. Trans. 2011, 39, 761–766.
- Rapoport, T.A. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nat. Cell Biol. 2007, 450, 663–669.
- Von Heijne, G. Membrane-protein topology. Nat. Rev. Mol. Cell Biol. 2006, 7, 909–918.
- Lange, C.; Nett, J.H.; Trumpower, B.L.; Hunte, C. Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. EMBO J. 2001, 20, 6591–6600.
- Eghiaian, F. Lipid Chirality Revisited: A Change in Lipid Configuration Transforms Membrane-Bound Protein Domains. Biophys. J. 2015, 108, 2757–2758.
- Ho, H.; Miu, A.; Alexander, M.K.; Garcia, N.K.; Oh, A.; Zilberleyb, I.; Reichelt, M.; Austin, C.D.; Tam, C.; Shriver, S.; et al. Structural basis for dual-mode inhibition of the ABC transporter MsbA. Nat. Cell Biol. 2018, 557, 196–201.
- Kuk, A.C.Y.; Hao, A.; Guan, Z.; Lee, S.-Y. Visualizing conformation transitions of the Lipid II flippase MurJ. Nat. Commun. 2019, 10, 1736.
- Weinert, T.; Skopintsev, P.; James, D.; Dworkowski, F.; Panepucci, E.; Kekilli, D.; Furrer, A.; Brünle, S.; Mous, S.; Ozerov, D.; et al. Proton uptake mechanism in bacteriorhodopsin captured by serial synchrotron crystallography. Science 2019, 365, 61–65.
- Tilegenova, C.; Cortes, D.M.; Jahovic, N.; Hardy, E.; Hariharan, P.; Guan, L.; Cuello, L.G. Structure, function, and ion-binding properties of a K+ channel stabilized in the 2,4-ion–bound configuration. Proc. Natl. Acad. Sci. USA 2019, 116, 16829–16834.
- Neutze, R.; Brändén, G.; Schertler, G.F. Membrane protein structural biology using X-ray free electron lasers. Curr. Opin. Struct. Biol. 2015, 33, 115–125.
- Fotiadis, D. Atomic force microscopy for the study of membrane proteins. Curr. Opin. Biotechnol. 2012, 23, 510–515.
- Liao, X.; Zhang, B.; Blatt, M.R.; Jenkins, G.I. A FRET method for investigating dimer/monomer status and conformation of the UVR8 photoreceptor. Photochem. Photobiol. Sci. 2019, 18, 367–374.
- Gupta, K.; Donlan, J.; Hopper, J.T.S.; Uzdavinys, P.; Landreh, M.; Struwe, W.; Drew, D.; Baldwin, A.J.; Stansfeld, P.; Robinson, C. The role of interfacial lipids in stabilizing membrane protein oligomers. Nat. Cell Biol. 2017, 541, 421–424.
- Muallem, S.; Chung, W.Y.; Jha, A.; Ahuja, M. Lipids at membrane contact sites: Cell signaling and ion transport. EMBO Rep. 2017, 18, 1893–1904.
- Tronin, A.Y.; Maciunas, L.J.; Grasty, K.C.; Loll, P.J.; Ambaye, H.A.; Parizzi, A.A.; Lauter, V.; Geragotelis, A.D.; Freites, J.A.; Tobias, D.J.; et al. Voltage-Dependent Profile Structures of a Kv-Channel via Time-Resolved Neutron Interferometry. Biophys. J. 2019, 117, 751–766.
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177.
- Zhang, X.C.; Liu, Z.; Li, J. From membrane tension to channel gating: A principal energy transfer mechanism for mechanosensitive channels. Protein Sci. 2016, 25, 1954–1964.
- Lewis, R.S. Store-operated calcium channels: From function to structure and back again. Cold Spring Harb. Perspect. Biol. 2020, 12, a035055.
- Uehara, C.; Takeda, K.; Ibuki, T.; Furuta, T.; Hoshi, N.; Tanudjaja, E.; Uozumi, N. Analysis of Arabidopsis TPK2 and KCO3 reveals structural properties required for K+ channel function. Channels 2020, 14, 336–346.
- Singh, R.K.; Deshmukh, R.; Muthamilarasan, M.; Rani, R.; Prasad, M. Versatile roles of aquaporin in physiological processes and stress tolerance in plants. Plant Physiol. Biochem. 2020, 149, 178–189.
- García-Rubio, D.L.; de la Mora, M.; Cerecedo, D.; Blesa, J.M.S.; Villagrán-Muniz, M. An optical-based biosensor of the epithelial sodium channel as a tool for diagnosing hypertension. Biosens. Bioelectron. 2020, 157, 112151.
- Thompson, M.J.; Baenziger, J.E. Ion channels as lipid sensors: From structures to mechanisms. Nat. Chem. Biol. 2020, 16, 1331–1342.
- Jegla, T.; Busey, G.; Assmann, S.M. Evolution and Structural Characteristics of Plant Voltage-Gated K+ Channels. Plant Cell 2018, 30, 2898–2909.
- Choveau, F.S.; Abderemane-Ali, F.; Coyan, F.C.; Es-Salah-Lamoureux, Z.; Baró, I.; Loussouarn, G. Opposite Effects of the S4–S5 Linker and PIP2 on Voltage-Gated Channel Function: KCNQ1/KCNE1 and Other Channels. Front. Pharmacol. 2012, 3, 125.
- Urquhart, W.; Chin, K.; Ung, H.; Moeder, W.; Yoshioka, K. The cyclic nucleotide-gated channels AtCNGC11 and 12 are involved in multiple Ca2+-dependent physiological responses and act in a synergistic manner. J. Exp. Bot. 2011, 62, 3671–3682.
- Jarratt-Barnham, E.; Wang, L.; Ning, Y.; Davies, J. The Complex Story of Plant Cyclic Nucleotide-Gated Channels. Int. J. Mol. Sci. 2021, 22, 874.
- Moon, J.Y.; Belloeil, C.; Ianna, M.L.; Shin, R. Arabidopsis CNGC Family Members Contribute to Heavy Metal Ion Uptake in Plants. Int. J. Mol. Sci. 2019, 20, 413.
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nat. Cell Biol. 2020, 587, 567–576.
- Iida, H.; Furuichi, T.; Nakano, M.; Toyota, M.; Sokabe, M.; Tatsumi, H. New candidates for mechano-sensitive channels potentially involved in gravity sensing in Arabidopsis thaliana. Plant Biol. 2013, 16, 39–42.
- Shepherd, V.; Beilby, M.; Shimmen, T. Mechanosensory ion channels in charophyte cells: The response to touch and salinity stress. Eur. Biophys. J. 2002, 31, 341–355.
- Stieger, B.; Steiger, J.; Locher, K.P. Membrane lipids and transporter function. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166079.
- Neumann, J.; Rose-Sperling, D.; Hellmich, U.A. Diverse relations between ABC transporters and lipids: An overview. Biochim. et Biophys. Acta (BBA)-Biomembr. 2017, 1859, 605–618.
- Ogasawara, F.; Kodan, A.; Ueda, K. ABC proteins in evolution. FEBS Lett. 2020, 594, 3876–3881.
- Aye, I.L.; Singh, A.T.; Keelan, J.A. Transport of lipids by ABC proteins: Interactions and implications for cellular toxicity, viability and function. Chem. Interactions 2009, 180, 327–339.
- Spector, A.; Yorek, M. Membrane lipid composition and cellular function. J. Lipid Res. 1985, 26, 1015–1035.
- Sytar, O.; Ghosh, S.; Malinska, H.; Zivcak, M.; Brestic, M. Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol. Plant. 2020, 173, 148–166.
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167.
- Muller, M.P.; Jiang, T.; Sun, C.; Lihan, M.; Pant, S.; Mahinthichaichan, P.; Trifan, A.; Tajkhorshid, E. Characterization of Lipid–Protein Interactions and Lipid-Mediated Modulation of Membrane Protein Function through Molecular Simulation. Chem. Rev. 2019, 119, 6086–6161.
- Li, H.; Yan, C.; Guo, J.; Xu, C. Chapter Three-Ionic protein-lipid interactions at the plasma membrane regulate the structure and function of immunoreceptors. In Advances in Immunology; Dong, C., Jiang, Z., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 144, pp. 65–85.
- Wang, P.; Chao, D. Phytoremediation of heavy metal contamination and related molecular mechanisms in plants. Sheng Wu Gong Cheng Xue Bao 2020, 36, 426–435.
- Zhang, Y.; Zheng, J. Bioinformatics of Metalloproteins and Metalloproteomes. Molecules 2020, 25, 3366.
- Röth, S.; Fulcher, L.J.; Sapkota, G.P. Advances in targeted degradation of endogenous proteins. Cell. Mol. Life Sci. 2019, 76, 2761–2777.
- Moreno, L.A.; Omidi, M.; Wurlitzer, M.; Luthringer, B.; Helmholz, H.; Schluter, H.; Willumeit, R.; Fügenschuh, A. Understanding Protein Networks using Vester’s Sensitivity Model. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 17, 1440–1450.
- Herzberg, M.; Schüttau, M.; Reimers, M.; Große, C.; Hans-Günther-Schlegel, H.-G.-S.; Nies, D.H. Synthesis of nickel–iron hydrogenase in Cupriavidus metallidurans is controlled by metal-dependent silencing and un-silencing of genomic islands. Metallomics 2015, 7, 632–649.
- Barwinska-Sendra, A.; Waldron, K.J. The Role of Intermetal Competition and Mis-Metalation in Metal Toxicity. Adv. Microb. Physiol. 2017, 70, 315–379.
More
