Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Reza Tahergorabi.
Marine sources are gaining popularity and attention as novel materials for manufacturing biopolymers such as proteins and polysaccharides. Due to their biocompatibility, biodegradability, and non-toxicity features, these biopolymers have been claimed to be beneficial in the development of food packaging materials. Several studies have thoroughly researched the extraction, isolation, and latent use of marine biopolymers in the fabrication of environmentally acceptable packaging.
- biodegradable food packaging
- marine sources
- marine biopolymers
- polysaccharides
- proteins
- films and coatings
1. Marine Biopolymers in Food Packaging
The significant development of marine biopolymers can be traced back to industrial processing where marine resources such as fish, crustaceans, and mollusks, etc. are processed to produce commercial products, leaving a considerable quantity of solid wastage (27–75%), depending on the type of product, towards final consumption [29][1]. For instance, studies reported that fish canning and lobster processing account for approximately 27% and 75% of wastage, respectively [29,30][1][2]. These by-products or wastages are the skins, bones, scales, tendons, and shells left after processing, which can be subjected for chemical and biological extractions to produce two crucial biopolymer groups—proteins and polysaccharides. Common marine proteins are divided into three major parts: muscle proteins, collagen, and gelatin, whereas common marine polysaccharides include chitin, chitosan, alginate, agar, and carrageenan. Afterward, these proteins and polysaccharides are employed in the development of biodegradable coatings and films with the required mechanical properties to protect the food inside from different spoilage factors. In the preparation of packaging materials from marine biopolymers, different solutions and solvents are then applied with different techniques, such as dipping, spraying, coating, wrapping, brushing, or panning [31,32,33][3][4][5]. After the final application in food packaging, the materials are usually discarded and subjected to decomposition for their biodegradable properties. The most important, and perhaps the most expected property of these packaging materials, is their ability to be decomposed in the environment. The decomposition of biodegradable polymers into the environment have been explained in a study and according to Figure 21; the initial step in the decomposition process usually starts with the involvement of ultraviolet (UV) radiation, mechanical force, and microorganisms, producing microplastic (fragmented small pieces) [34][6]. Then, the extracellular enzymes come into action, inaugurating the abiotic and biotic hydrolysis to break down the ester bond of the polymer, leading to the reduction of the molar mass and formulation of soluble intermediates. The soluble intermediates then go through the assimilation process by the action of intracellular enzymes. Intracellular enzymes use the degraded products as a source of carbon and energy to produce cell biomass and products such as carbon dioxide and water [34][6]. A complete projection of marine biopolymers, their chemical extraction, and how they can be biodegraded into the surrounding environment is presented in Figure 21. Some of the unique technological properties include water barrier ability, oxygen barrier ability, edibility, transparency, thickness, and elasticity, which are summarized in Figure 32 [35,36][7][8]. These properties aid in protecting the packaged food against oxidation and microbial contaminations [37][9]. Marine biopolymers have been reported to hold these unique properties and could be used in smart packaging, where the active monitoring of product condition and the interactive action between packages and internal ambiance of the package plays an important role in the extension of food shelf-life while maintaining the biochemical, sensorial, and microbial quality [38,39,40][10][11][12].
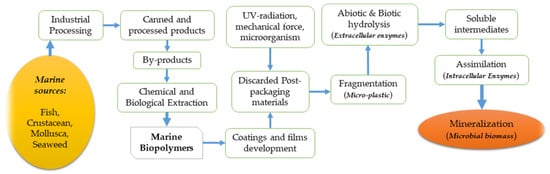
Figure 21. Schematic representation of the complete cycle of marine biopolymers—from production through to the biodegrading of the environment.

Figure 32. Technological properties of marine biopolymer-based packaging materials with specific functions.
In most cases, the films and coatings act as a medium to preclude the passage of water, oxygen, and carbon dioxide [41][13]. Even though the technological properties of marine biopolymer-based packaging have been reported, the efficacy is still lower and underdeveloped compared to synthetic and plastic polymer materials [42][14]. Consequently, several researchers are making significant attempts to build high barrier and thermal properties food packaging systems from cheap, available, and renewable biopolymers, especially marine sources [15,36,43][15][8][16]. To this aim, several propositions already have been made, and a recent upgrade has been brought with the application of active agents with potent antimicrobial and anti-oxidative properties that target to suppress the growth of microorganisms and to diminish the oxidation of lipids and pigments in food and the surroundings in a packaging arrangement [44,45][17][18].
2. Films and Coatings from Marine Biopolymers in Food Packaging
Food preservation technologies are now confronted with many difficulties in extending the shelf-life of different kinds of food such as fruits, vegetables, fish, meat, refrigerated products, etc. [29,145][1][19]. In this regard, the use of edible films and coatings made from biopolymers, especially food-grade biopolymers, has progressed dramatically in recent years [145][19]. Food–grade biopolymers, such as proteins and polysaccharides from plants, marine life, animals, or food-processing by-products, are enormously utilized to formulate edible packaging systems [145,146][19][20]. Remarkably, biopolymers from marine resources are gaining significant attraction in developing bio-based packaging materials for enhanced food packaging. In most cases, the marine-derived biopolymers are used to prepare films and coatings and then coated, wrapped, or sprayed over the food through packaging, focusing on enhancing the packaged food’s shelf-life [35,36][7][8]. The films and coating create a barrier environment that stands against the transmission of gases, oxygen, vapors, etc., thereby enhancing shelf-life and quality attributes of the packaged food [36][8]. However, the functionality of the films and coatings as a packaging material developed from marine biopolymers requires different modifications before selecting in a packaging application [147][21]. For instance, a material characterization (such as mechanical property) of the packaging material is required to determine its suitability in specific applications [147][21]. The packaging material must serve its purpose to keep the product undamaged and undeteriorated [29,90][1][22]. The suitability and strength of the packaging materials developed from marine biopolymers may vary; however, in some cases, they have been found with low technological and functional properties [29,36][1][8]. Likewise, the low technological and functional properties may affect food rheological, sensorial, and microbial attributes when packaged [148][23]. In this regard, the application of active agents, plant extracts, biopolymers blends, nanoparticles, essential oils, and organic acids, etc., in line with marine biopolymers have been found effective in enhancing the functional and technological properties of packaging materials, leading to significant improvement in final packaging quality [149,150,151,152,153][24][25][26][27][28]. These biopolymer–active agents modification provides active properties to the packaging that extend the shelf-life and quality attributes.
2.1. Marine Protein Films and Coatings
Kaewprachu et al. [154][29] reported that fish myofibrillar protein films prepared in combination with catechin-Kradon extract (Careya sphaerica Roxb.) could reduce the growth of psychrophilic bacteria and retain sensory attributes up to 8 days in bluefin tuna during refrigerated storage. The researchers determined the total volatile base nitrogen, TBARS (thiobarbituric acid reactive substance assay), and peroxide value that indicates the primary and secondary metabolites of lipid oxidation during refrigerated storage. The study’s findings concluded that myofibrillar protein films prepared from fish incorporated with catechin-Kradon extract could control microbial growth and keep lipid oxidation inhibition during 10 days at refrigeration temperature (4 ± 1 °C), thereby prolonging shelf-life [154][29].
Collagen, another important marine protein biopolymer, is mainly converted into gelatin to produce films and coatings suitable for food packaging applications. Marine collagen is most often used in edible meat casings, where it can shrink and stretch to mimic the contraction and expansion of meat batter throughout continuous processing [155][30]. Despite its use as a food additive to increase rheological characteristics, marine collagen is currently underused in food packaging. The low heat stability and poor mechanical properties of collagen might be a problem. As a result, most of it is transformed into gelatin, which has been shown to have strong technical, rheological, and functional qualities, especially when paired with active agents [36][8].
Gelatin, another important marine protein biopolymer, probably the best of other marine protein biopolymers, has already been declared effective in making films and coatings with active agents for food packaging application. Gelatin-based films and coatings have been applied in many food settings to improve the quality and shelf-life of packaged products. For instance, gelatin (0–6%) in combination with chitosan (0.5–1.5%) was applied to produce a film to preserve beef steak during 5 days of retail display [156][31]. The blend film was found effective in reducing weight loss, protecting lipid oxidation, and enhancing the color attributes of beef steak [156][31]. Another study developed a chitosan–gelatin film to preserve and improve the shelf-life of minced trout fillet during refrigerated storage over 11 days [157][32]. The findings concluded that fish spoilage was the lowest in the samples treated with film, with reduced bacterial growth [157][32]. In shrimp preservation, a gelatin and 2% orange leaf essential oil incorporated coating had a significant effect on the quality and shelf-life of the shrimp compared to the control group. The sensory evaluation found that a combination of gelatin film and orange leaf essential oil enhanced the shelf-life of the shrimp up to 14 days, whereas the control group only retained a shelf-life of 4 days [158][33]. Nowzari et al. [159][34] also reported the impact of gelatin–chitosan coatings and films on the quality of refrigerated rainbow trout. The chitosan–gelatin composite influenced the bacterial contamination of rainbow trout fillets, as evidenced by findings from TVB-N (total volatile basic nitrogen) and bacteriological studies [159][34]. A few findings related to the application of films and coatings made from marine biopolymers in food preservation, in line with other active agents, are presented in Table 1.
2.2. Marine Polysaccharide Films and Coatings
In the last few decades, the application of polysaccharide-based films and coatings in food packaging has increased dramatically. Noticeably, marine polysaccharides such as chitosan, alginate, agar, and carrageenan received significant attraction in the make-up of films and coatings in food packaging with the ability to protect from contamination and deterioration, thus improving the shelf-life and quality attributes [160][35].
Chitosan, one of the most ubiquitous marine biopolymers, has been applied in many food packaging applications as a film and coating material. Priyadarshi et al. [161][36] reported that a combination of chitosan and apricot kernel essential oil in the development of film significantly inhibited the fungal growth on packaged bread slices [161][36]. Besides, the modified films showed better water resistance and water vapor property. The result concluded that the developed film is filled with antimicrobial and antioxidant properties and can be an excellent approach to extend the shelf-life of bread for better preservation [161][36]. Alsaggaf et al. [162][37] reported that a chitosan–pomegranate peel extract could enhance the microbiological, chemical, and sensorial quality of Nile tilapia fillets during cold storage at 4 °C for 30 days. The coating application resulted in a strident decrease in the total microbial counts during storage. Furthermore, sensory assessments specified advanced preferences for the odor, texture, color, and overall quality of coated samples as compared to uncoated samples. Thus, the study recommends that a chitosan–pomegranate peel extract could be a natural approach to extend the shelf-life of Nile tilapia fillets during storage [162][37]. Halim et al. [163][38] also reported that a chitosan–tannic acid film could improve the physiochemical properties of cherry tomato and grapefruits during preservation. In another study, pork sausages in refrigerated storage were coated with a chitosan–clove oil blend, and the microbiological, physical, chemical, and sensory properties were monitored over 25 days during storage [164][39]. Although the psychrotrophic bacteria count, color value, peroxide value, and the thiobarbituric acid reactive substances were increasing, the changes were slowest in the samples with the chitosan–clove oil coating. Based on the sensory evaluation and microbiological quality, the chitosan–clove oil-treated samples retained a shelf-life of up to 20 days [164][39].
Alginate, another promising marine biopolymer, achieved significant attention due to its striking properties such as film-forming ability, non-toxicity, cheapness, and biocompatibility [165][40]. Albert et al. [166][41] reported that an alginate–salt film is able to protect microwaveable food during microwave operation. The alginate film successfully reduced the cooking time, lessened browning, and enhanced warming efficiency [166][41]. The application of a salty alginate edible film as a susceptor during microwave cooking seems to work well. The film layer that covers the substrate could warm up faster and distribute heat more evenly [166][41]. Alginate coating in combination with antimicrobials was effective in poached and deli turkey products preservation for 7 days storage at 22 °C [167][42]. The coating inhibited the growth of L. monocytogenes, with final counts of 4.3 log CFU (colony forming unit)/g (home-style poached turkey) and 6.5 log CFU/g (roasted deli turkey), respectively. In contrast, untreated equivalents had counts of 9.9 and 7.9 log CFU/g, respectively. As a result, this study shows that utilizing alginate-based antimicrobial coatings to improve the microbiological safety and quality of ready-to-eat poultry items during chilled storage is beneficial [167][42]. The effectiveness of a carrageenan–glycerol coating on the firmness and color components of papaya during storage was reported by Hamzah et al. [168][43]. Carrageenan and glycerol, at 0.78 percent (w/v) and 0.85 percent (w/v), respectively, were found to delay ripening and hence extend the shelf-life [168][43]. According to Seol et al. [169][44], a j-carrageenan-based film containing ovotransferrin and ethylene diamine tetra acetic acid (EDTA) had an antibacterial effect against E. coli, S. Typhimurium, S. aureus, and Candida albicans. Using a solvent casting process, antimicrobial films based on carrageenan were created by mixing grape seed extract in various quantities into the polymer. Because of the polyphenolic chemicals in the grape extract, the films were yellowish and showed strong antibacterial action against food-borne germs. These findings show that carrageenan films could be useful as antibacterial or active food packaging [170][45]. The essential oils of Zataria multiflora Boiss (ZEO) and Mentha pulegium (MEO) have good potential for incorporation with carrageenan to generate antioxidant and antibacterial films for food applications [171][46]. The antimicrobial properties of films created by integrating essential oils were improved, and S. aureus was found to be the most sensitive, followed by B. cereus and E. coli. Around the films that were mixed with 3 percent ZEO, the maximum inhibition area of 544.05 mm2 for S. aureus was recorded. For S. Typhimurium, the total inhibitory region of 3 percent MEO films was 20.43 mm2 and for P. aeruginosa it was 10.15 mm2. These findings demonstrated that ZEO and MEO are promising antioxidant and antibacterial films in food technology when combined with carrageenan [171][46]. Carrageenan is commonly utilized in the food sector due to its good physical and functional capabilities, which include antioxidant activity, stabilizing ability, gelling, emulsifying, and thickening agent [136,172,173][47][48][49]. They can also be found in water-based foods, animal products (as an oxygen barrier for delayed fat oxidation), and dairy products [174][50]. Importantly, both in vitro and in the cell system, oligosaccharides carrageenan and its derivatives were found to have antioxidant activity [175][51]. Sun et al. [176][52] also found that the sugar concentration limit, which corresponds to the removal of polymerization from kappa carrageenan, had a substantial impact on the antioxidant activity of carrageenan oligosaccharides. However, because carrageenan is typically utilized as a coating, there is not a lot of literature on how to make edible films with it [173][49]. Pork sausage containing carrageenan–soy protein showed a significant reduction in rancidity and discoloration during 16 weeks of frozen storage [177][53].
Agar, a biopolymer derived mostly from red algae, is another potential biopolymer. Agar film is becoming a popular and sustainable alternative to plastic-based food packaging. Plain agar film, on the other hand, is brittle, has a high moisture permeability, and is thermally unstable. A lot of work has gone into refining the qualities of agar films so that they may be used in more places; for example, in nanomaterial reinforcement, merging with other biopolymers and integrating plasticizers, hydrophobic components, or antibacterial agents into the structure [178][54]. The impact of a bioactive film made of agar and containing green tea extract and probiotic bacteria on hake fillets were evaluated during 15 days of refrigerated storage. Throughout the storage period, the agar–green tea–probiotic strain film caused a drop in H2S-producing bacterium counts and total viable bacteria. The films influenced the fish quality indicators such as total volatile basic nitrogen (TVBN), trimethylamine nitrogen (TMA-N), and pH. For 15 days, the overall viable counts, H2S-producing bacteria, and TVB-N were below acceptable levels [179][55]. Agar containing alginate, collagen, silver nanoparticles, and grapefruit seed extract was discovered to be very transparent. The film had a superior water retention capacity and an outstanding UV-screening effect. Furthermore, composite films demonstrated excellent antibacterial action against Gram-positive (Listeria monocytogenes) and Gram-negative (Escherichia coli) food-borne pathogenic microorganisms. The microbiological, physical, and chemical characteristics of flounder fillets coated with agar–clove essential oil films were studied during 15 days at 5 °C. During storage, flounder fillet had low total viable bases, a low pH, and delayed development of H2S-producing bacteria [180][56]. According to Abdollahzadeh et al. [151][26], agar films infused with several essential oils (peppermint, chamomile, cumin, tarragon, dill, and cinnamon) can be effective against L. monocytogenes strains. Green grapefruit packed with the agar–zinc oxide nanoparticles film has great thermal stability and film thickness [181][57]. In ambient conditions, the packed green grape retained its fresh look for up to 14 days. The nanocomposite films’ thermal stability, elongation at break, and fruit preservation qualities were all improved. Green grapes wrapped in developed films kept their appearance over long periods under ambient storage. As a result, the nanocomposite film that was created might be employed as an effective packaging material for extending the shelf-life of green grapes. Current laboratory-scale agar film manufacturing has various issues, such as a long drying time, inability to manufacture continuous films, and imprecise thickness control, which need to be addressed before moving to an industrial scale. As a result, further research focusing on commercial sizes is needed to give more realistic information for commercializing agar-based food packaging materials [182][58].
Table 1. Application of protein and polysaccharide biopolymers derived from marine resources in combination with active agents in food packaging.
| Marine Biopolymer | Food Products | Matrix Constituent | Packaging | Outcomes | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| myofibrillar protein | bluefin tuna slices | myofibrillar protein–catechin–Kardon extract | film |
| [154] | [29] | ||||
| gelatin | beef steak | chitosan–gelatin | film |
| [156] | [31] | ||||
| gelatin | minced trout fillet | chitosan–gelatin–grape seed extract | film |
| [157] | [32] | ||||
| gelatin | pork sausage | gelatin–sodium alginate | film |
| [183] | [59] | ||||
| gelatin | refrigerated rainbow trout | chitosan–gelatin | coating and film |
| [159] | [34] | ||||
| gelatin | shrimp | gelatin–essential oil | coating |
| [158] | [33] | ||||
| chitosan | bread | chitosan–apricot kernel essential oil | film |
| [161] | [36] | ||||
| chitosan | Nile tilapia fillets | chitosan–pomegranate peel extract | coating |
| [162] | [37] | ||||
| chitosan | cherry tomato and grapes | chitosan–tannic acid | film |
| [163] | [38] | ||||
| chitosan | pork sausages | chitosan–clove oil | coating |
| [164] | [39] | ||||
| chitosan | pork fillets | chitosan– | Origanum vulgare | essential oil | coating |
| [184] | [60] | ||
| chitosan | chicken | chitosan–pink pepper extract–peanut skin extract | film |
| [185] | [61] | ||||
| chitosan | chicken breast | chitosan–pomegranate juice– | Zataria multiflora | essential oil | coating |
| [186] | [62] | ||
| alginate | microwave food | alginate–salt | film |
| [166] | [41] | ||||
| alginate | poached and deli turkey products | alginate–antimicrobials | coating |
| [167] | [42] | ||||
| alginate | shiitake mushroom | alginate–nano–Ag | coating |
| [187] | [63] | ||||
| alginate | fresh-cut pineapple | alginate–lemongrass essential oil | coating |
| [188] | [64] | ||||
| carrageenan | papaya | carrageenan–glycerol | coating |
| [168] | [43] | ||||
| carrageenan | pork sausage | carrageenan–soy protein | coating |
| [177] | [53] | ||||
| carrageenan | encapsulated aroma compound | carrageenan–glycerol | film |
| [189] | [65] | ||||
| carrageenan | fresh spinach | carrageenan–agar–konjac glucomannan | film |
| [41] | [13] | ||||
| carrageenan | chicken breast | carrageenan–chitosan–allyl isothiocyanate–mustard extract | coating |
| [190] | [66] | ||||
| agar | hake fillet | the agar–green tea–probiotic strain | film |
| [179] | [55] | ||||
| agar | fresh potato | agar–alginate, collagen blend–silver nanoparticles–grapefruit seed extract | film |
| [191] | [67] | ||||
| agar | flounder fillet | agar–fish protein hydrolysate–clove essential oil | film |
| [180] | [56] | ||||
| agar | minced fish | agar–essential oil | film |
| [151] | [26] | ||||
| agar | green grape | agar–zinc oxide nanoparticles | film |
| [181] | [57] | ||||
| agar | fish oil | agar–gelatin–titanium dioxide nanoparticles | film |
| [182] | [58] |
References
- Sustainable Food Packaging Technology, 1st ed.; Athanassiou, A. (Ed.) Wiley: Hoboken, NJ, USA, 2021.
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Ensiling of fish industry waste for biogas production: A lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour. Technol. 2013, 127, 326–336.
- Garrido, T.; Uranga, J.; Guerrero, P.; de la Caba, K. The potential of vegetal and animal proteins to develop more sustainable food packaging. In Polymers for Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 25–59.
- Vázquez, J.A.; Meduíña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of valuable compounds and bioactive metabolites from by-products of fish discards using chemical processing, enzymatic hydrolysis, and bacterial fermentation. Mar. Drugs 2019, 17, E139.
- Murrieta-Martínez, C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Márquez Ríos, E. Edible protein films: Sources and behavior. Packag. Technol. Sci. 2018, 31, 113–122.
- Wang, G.; Huang, D.; Ji, J.; Völker, C.; Wurm, F.R. Seawater-degradable polymers: Seawater-degradable polymers—Fighting the marine plastic pollution. Adv. Sci. 2021, 8, 2170004.
- Wang, Z.; Hu, S.; Wang, H. Scale-Up Preparation and characterization of collagen/sodium alginate blend films. J. Food Qual. 2017, 2017, e4954259.
- Lionetto, F.; Esposito Corcione, C. Recent applications of biopolymers derived from fish industry waste in food packaging. Polymers 2021, 13, 2337.
- Kozłowicz, K.; Nazarewicz, S.; Góral, D.; Krawczuk, A.; Domin, M. Lyophilized protein structures as an alternative biodegradable material for food packaging. Sustainability 2019, 11, 7002.
- Goyal, N.; Rastogi, D.; Jassal, M.; Agrawal, A.K. Chitosan as a potential stabilizing agent for titania nanoparticle dispersions for preparation of multifunctional cotton fabric. Carbohydr. Polym. 2016, 154, 167–175.
- Demitri, C.; Moscatello, A.; Giuri, A.; Raucci, M.G.; Esposito Corcione, C. Preparation and characterization of EG-chitosan nanocomposites via direct exfoliation: A green methodology. Polymers 2015, 7, 2584–2594.
- Gokoglu, N. Innovations in seafood packaging technologies: A review. Food Rev. Int. 2020, 36, 340–366.
- Rhim, J.-W.; Wang, L.-F. Mechanical and water barrier properties of agar/κ-carrageenan/konjac glucomannan ternary blend biohydrogel films. Carbohydr Polym. 2013, 96, 71–81.
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625.
- Ruban, S. Biobased packaging—Application in meat industry. Vet. World 2009, 2, 79.
- Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, V.; Robin, J.-J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 11–33.
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019, 240, 191–198.
- Gómez-Estaca, J.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Montero, P. Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin–chitosan films. Food Hydrocoll. 2011, 25, 1461–1469.
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2021, 1–26.
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249.
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent PH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312.
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368.
- Armentano, I.F.; Yoon, K.; Ahn, J.; Kang, S.; Kenny, J.M. Bio-based PLA_PHB plasticized blend films: Processing and structural characterization. LWT Food Sci. Technol. 2015, 64, 980–988.
- López de Lacey, A.M.; López-Caballero, M.E.; Montero, P. Agar films containing green tea extract and probiotic bacteria for extending fish shelf-life. LWT Food Sci. Technol. 2014, 55, 559–564.
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carb. Polym. 2018, 184, 453–464.
- Abdollahzadeh, E.; Mahmoodzadeh Hosseini, H.; Imani Fooladi, A.A. Antibacterial activity of agar-based films containing nisin, cinnamon EO, and ZnO nanoparticles. J. Food Saf. 2018, 38, e12440.
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.; Martin-Belloso, O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT Food Sci. Technol. 2013, 50, 240–246.
- Rojas-Graü, M.A.; Avena-Bustillos, R.J.; Olsen, C.; Friedman, M.; Henika, P.R.; Martín-Belloso, O.; Pan, Z.; McHugh, T.H. Effects of plant essential oils and oil compounds on mechanical, barrier and antimicrobial properties of alginate-apple puree edible films. J. Food Eng. 2007, 81, 634–641.
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Suthiluk, P.; Rawdkuen, S. Shelf life extension for bluefin tuna slices (Thunnus thynnus) wrapped with myofibrillar protein film incorporated with catechin-kradon extract. Food Control 2017, 79, 333–343.
- Ahmad, M.; Nirmal, N.P.; Chuprom, J. Molecular characteristics of collagen extracted from the starry triggerfish skin and its potential in the development of biodegradable packaging film. RSC Adv. 2016, 6, 33868–33879.
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.D.; de Miranda Gomide, L.A.; Ramos, E.M. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94.
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and ziziphora clinopodioides essential oil on survival of listeria monocytogenes and chemical, microbial and sensory properties of minced trout Fillet. LWT Food Sci. Technol. 2016, 72, 432–438.
- Alparslan, Y.; Yapıcı, H.H.; Metin, C.; Baygar, T.; Günlü, A.; Baygar, T. Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT Food Sci. Technol. 2016, 72, 457–466.
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan–gelatin composite and bilayer coating and film Effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672.
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Natural polymer based cling films for food packaging. Int. J. Pharm. Pharm. Sci. 2015, 7, 10–18.
- Priyadarshi, R.; Sauraj, K.B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166.
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505.
- Halim, A.L.A.; Kamari, A.; Phillip, E. Chitosan, gelatin and methylcellulose films incorporated with tannic acid for food packaging. Int. J. Biol. Macromol. 2018, 120, 1119–1126.
- Lekjing, S. A Chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016, 111, 192–197.
- Vu, C.H.T.; Won, K. Novel water-resistant UV-activated oxygen indicator for intelligent food packaging. Food Chem. 2013, 140, 52–56.
- Albert, A.; Salvador, A.; Fiszman, S.M. A film of alginate plus salt as an edible susceptor in microwaveable food. Food Hydrocoll. 2012, 27, 421–426.
- Juck, G.; Neetoo, H.; Chen, H. Application of an active alginate coating to control the growth of listeria monocytogenes on poached and deli turkey products. Intl J. Food Microbiol. 2010, 142, 302–308.
- Hamzah, H.M.; Osman, A.; Tan, C.P.; Mohamad Ghazali, F. Carrageenan as an alternative coating for papaya (Carica papaya L. Cv. Eksotika). Postharvest Biol. Technol. 2013, 75, 142–146.
- Seol, K.-H.; Lim, D.-G.; Jang, A.; Jo, C.; Lee, M. Antimicrobial effect of κ-carrageenan-based edible film containing ovotransferrin in fresh chicken breast stored at 5 °C. Meat Sci. 2009, 83, 479–483.
- Kanmani, P.; Rhim, J.-W. Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266.
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Khaksar, R. Characterization of κ-carrageenan films incorporated plant essential oils with improved antimicrobial activity. Carb. Polym. 2014, 101, 582–591.
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Tye, Y.Y.; Lai, T.K.; Easa, A.M.; Rosamah, E.; Fazita, M.R.N.; Syakir, M.I.; Adnan, A.S.; Fizree, H.M.; et al. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renew. Sustain. Energy Rev. 2017, 77, 353–362.
- Rhim, J.W. Physical-mechanical properties of agar/κ-carrageenan blend film and derived clay nanocomposite film. J. Food Sci. 2012, 77, N66–N73.
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374.
- Varela, P.; Fiszman, S.M. Hydrocolloids in fried foods. A Review. Food Hydrocoll. 2011, 25, 1801–1812.
- Yuan, H.; Song, J.; Zhang, W.; Li, X.; Li, N.; Gao, X. Antioxidant activity and cytoprotective effect of κ-carrageenan oligosaccharides and their different derivatives. Bioorganic Med. Chem. Lett. 2006, 16, 1329–1334.
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318.
- Ho, C.P.; Huffman, D.L.; Bradford, D.D.; Egbert, W.R.; Mikel, W.B.; Jones, W.R. Storage stability of vacuum packaged frozen pork sausage containing soy protein concentrate, carrageenan or antioxidants. J. Food Sci. 1995, 60, 257–261.
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176.
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carb. Polym. 2020, 238, 116178.
- Da Rocha, M.; Alemán, A.; Romani, V.P.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Effects of agar films incorporated with fish protein hydrolysate or clove essential oil on flounder (Paralichthys orbignyanus) fillets shelf-life. Food Hydrocoll. 2018, 81, 351–363.
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867.
- Vejdan, A.; Ojagh, S.M.; Abdollahi, M. Effect of gelatin/agar bilayer film incorporated with TiO2 nanoparticles as a UV absorbent on fish oil photooxidation. Int. J. Food Sci. Amp. Technol. 2017, 52, 1862–1868.
- Liu, L.; Kerry, J.F.; Kerry, J.P. Application and assessment of extruded edible casings manufactured from pectin and gelatin/sodium alginate blends for use with breakfast pork sausage. Meat Sci. 2007, 75, 196–202.
- Papparella, A.; Mazzarrino, G.; Chaves-López, C.; Rossi, C.; Sacchetti, G.; Guerrieri, O.; Serio, A. Chitosan boosts the antimicrobial activity of origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol. 2016, 59, 23–31.
- Serrano-León, J.S.; Bergamaschi, K.B.; Yoshida, C.M.P.; Saldaña, E.; Selani, M.M.; Rios-Mera, J.D.; Alencar, S.M.; Contreras-Castillo, C.J. Chitosan active films containing agro-industrial residue extracts for shelf life extension of chicken restructured product. Food Res. Int. 2018, 108, 93–100.
- Bazargani-Gilani, B.; Aliakbarlu, J.; Tajik, H. Effect of pomegranate juice dipping and chitosan coating enriched with zataria multiflora boiss essential oil on the shelf-life of chicken meat during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015, 29, 280–287.
- Jiang, T.; Feng, L.; Wang, Y. Effect of alginate/nano-Ag coating on microbial and physicochemical characteristics of shiitake mushroom (Lentinus edodes) during cold storage. Food Chem. 2013, 141, 954–960.
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Mohd Adzahan, N. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol. Technol. 2014, 88, 1–7.
- Hambleton, A.; Fabra, M.-J.; Debeaufort, F.; Dury-Brun, C.; Voilley, A. Interface and aroma barrier properties of iota-carrageenan emulsion–based films used for encapsulation of active food compounds. J. Food Eng. 2009, 93, 80–88.
- Olaimat, A.N.; Fang, Y.; Holley, R.A. Inhibition of campylobacter jejuni on fresh chicken breasts by κ-carrageenan/chitosan-based coatings containing Allyl isothiocyanate or deodorized oriental mustard extract. Int. J. Food Microbiol. 2014, 187, 77–82.
- Wang, L.-F.; Rhim, J.-W. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int. J. Biol Macromol. 2015, 80, 460–468.
More
