Background: The impact o invesf sexually transmitted infections (STI) on male fertility is controversial.
Aims: To investigate the prevalence of urethritis-associated STIs (chlamydia, gonorrhoeae, Mycoplasma genitalium, trichomoniasis) among infertile males; to analyze the effect of STIs on semen parameters and blood PSA. Case-control study. Study group (n = 2000): males with fertility problems or desire for fertility check. Control group (n = 248): male partners of pregnant women.
Analyses: polymerase chain reaction for STI, seminal interleukin 6 (IL-6), semen and fractionated urine, blood analyses (PSA, reproductive hormones). The prevalence of M. genitalium and chlamydia in the study group was 1.1% and 1.2%, respectively.
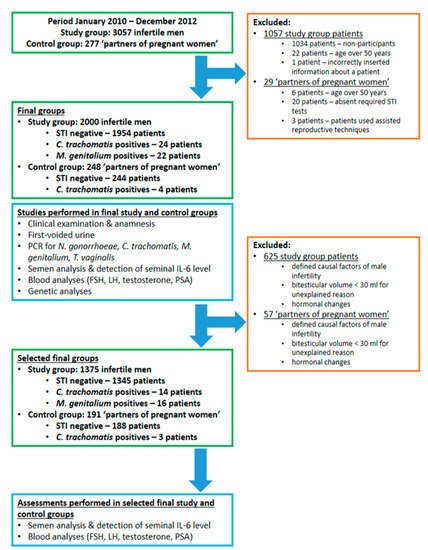
Results: The prevalence of chlamydia in the control group was 1.6%, while there were no M. genitalium cases. No cases with gonorrhoeae or trichomoniasis or combined infections were observed in neither group. There was a higher seminal concentration of neutrophils and IL-6 among M. genitalium positives compared with STI negatives. There was a trend toward a lower total count of spermatozoa and progressive motility among STI positives. No impact of STIs on PSA was found.
Conclusions: The prevalence of STIs among infertile males is low. M. genitalium is associated with seminal inflammation. The impact of STIs on semen parameters deserves further investigations.
- sexually transmitted infections
- sperm functions
- male
- infertility
- Mycoplasma genitalium
1. Introduction
2. Basic Clinical and Semen Parameters of Study Subjects
| Parameter | Study Group, Infertile Men (n = 2000) | Control Group, Fertile Men (n = 248) | p-Value |
|---|
4. Impact of STI on Inflammation in Semen
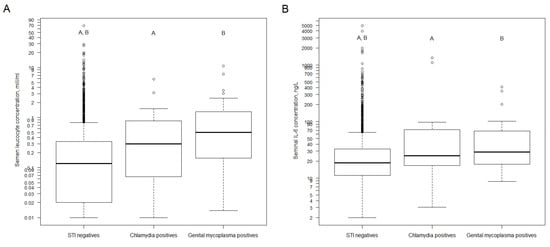
5. Impact of STI on Blood PSA and Semen Quality
| Parameter | STI Negative n = 1345 |
Either | C. trachomatis | or | M. genitalium | Positive n = 30 |
STI Negative vs. Either | M. genitalium | or | M. genitalium | Positive, p-Value | $$ | C. trachomatis | Positive n = 14 |
STI Negative vs. | C. trachomatis | Positive, p-Value | $$,① | M. genitalium | Positive n = 16 |
STI Negative vs. | M. genitalium | Positive, p-Value | $$,① |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (Range); 25th Centile; 75th Centile |
Median (Range); 25th Centile; 75th Centile |
|||||||||||||||||||||||
| Basic parameters | ||||||||||||||||||||||||
| Abstinence before semen analysis, in days | 4.0 (0.0–56.0); 3.0; 4.0 |
4.0 (1.0–7.0); 3.0; 5.0 |
0.920 | 3.5 (2.0–7.0); 2.0; 7.0 |
0.186 | 4.0 (1.0–7.0); 2.8; 5.0 |
0. 191 | |||||||||||||||||
| Age in years | 32.5 (18.2–49.8); 32.3 to 32.8 | $ | ||||||||||||||||||||||
| Volume of seminal fluid, in mL | 4.1 (0.8–40.0); 3.0; 5.2 | 31.8 (20.0–50.0); 31.1 to 32.6 | $ | 0.081 | ### | |||||||||||||||||||
| 4.1 (1.8–9.0); | 3.4; 5.3 | 0.812 | 3.7 (1.8–9.0); 3.6; 4.2 |
0.188 | 4.4 (1.8–6.9); 3.0; 5.4 |
0.159 | STI status | |||||||||||||||||
| Concentration of spermatozoa, M/mL | 56.0 (0.0–440.0); 30.0; 94.5 |
47.0 (1.3–210.0); 9.1; 69.8 |
0.034 | 49.0 (1.3–110.0); 17.1; 51.5 |
0.164 | 36.5 (1.3–210.0); 7.1; 95.0 |
0.396 |
| 1954 (97.7%) | 244 (98.4%) | ||||||||||||||
| Total count of spermatozoa, in M | 226.2 (0.0–2432.0); 110.4; 372.8 | 0.649 | ## | |||||||||||||||||||||
| 151.0 (2.3–1113.0); | 39.6; 303.1 | 0.028 | 169.5 (10.1–405.0); 55.9; 201.2 |
0.132 | 134.4 (2.3–1113.0); 42.0; 444.8 |
0.380 |
| 46 (2.3%) | 4 (1.6%) | 0.649 | ## | |||||||||||||
| 47 (0–83); | 35; 57 | A | 41 (0–71); 23; 57 |
0.187 | 48 (0–71); 39; 58 |
|||||||||||||||||||
| Spermatozoa with progressive motility, % | 0.167 | 34 (3–64); | 19; 57 | 0.088 |
| 24 (1.2%) | 4 (1.6%) | 0.541 | ## | |||||||||||||||
| Total count of spermatozoa with progressive motility, in M | 101.8 (0.0–1056.0); 44.3; 186.7 | A | 68.6 (0.0–489.7); 9.3; 156.6 |
0.034 | 75.9 (0.0–281.2); 22.8; 113.9 |
0.356 | 50.7 (0.3–489.7); 6.4; 202.5 |
0.194 |
| 22 (1.1%) | 0 (0.0%) | |||||||||||||
| Spermatozoa with normal morphology, % | 8 (0–53); 4; 12 | B | 0.162 | ## | ||||||||||||||||||||
| 5 (0–24); | 2; 24 | 0.242 | 8 (0–22); 3; 14 |
0.188 | 4 (0–24); 2; 10 |
0.184 | Bitesticular volume, in mL-s | 45.0 (0.0–100.0); | ||||||||||||||||
| Total count of spermatozoa with normal morphology, in M | 44.6 to 45.5 | $,A | 16.4 (0.0–389.1); 47.3 (23.0–100.0); 46.1 to 48.5 | $,A | <0.001 | ### | ||||||||||||||||||
| 5.3; 37.5 | B | 7.9 (0.0–189.2); 0.4; 35.5 |
0.105 | 14.3 (0.0–69.2); 1.7; 34.6 |
0.952 | 4.8 (0.0–189.2); 0.3; 33.3 |
0.236 |
| 22.0 (0.0–50.0); 21.8 to 22.3 | $,A | 22.9 (0.0–50.0); 22.2 to 23.6 | $,A | 0.016 | ### | ||||||||||
| Concentration of round cells in seminal fluid, in M/mL | 1.6 (0.0–75.0); 0.6; 3.0 |
1.8 (0.2–31.0); 0.8; 3.9 |
0.485 | 1.2 (0.2–7.2); 0.5; 2.0 |
0.746 | 3.0 (0.4–31.0); 1.0; 7.3 |
0.146 |
| 23.0 (0.0–50.0); 22.8 to 23.3 | $,A | 24.4 (6.0–50.0); 23.7 to 25.1 | |||||||||||||
| Percentage of neutrophils in seminal fluid, % | 8 (0–91); 3; 16 | $,A | 22 (0–63); 11; 39<0.001 | ### | ||||||||||||||||||||
| 7.379 × 10 | −6 | 20 (4–48); | 13; 30 | 0.004 | 25 (0–63); 10; 43 |
0.002 | STI in anamnesis | 459 (23.0%) | ||||||||||||||||
| Concentration of neutrophils in seminal fluid, in M/mL | 0.1 (0.0–68.3); | 51 (20.6%) | 0.422 | ## | ||||||||||||||||||||
| 0.0; 0.3 | 0.2 (0.0–10.70); 0.1; 0.9 |
0.001 | 0.2 (0.0–1.4); 0.1; 0.7 |
0.250 | 0.4 (0.0–10.7); 0.1; 2.6 |
0.004 |
| 95 (4.8%) | 7 (2.8%) | 0.197 | ## | |||||||||||||
| IL-6 in seminal plasma, in ng/L | 18.2 (2.0–4928.0); 11.1; 32.3 | C | 30.4 (3.0–1330.0); 17.9; 78.7 | D | 0.002 | 24.8 (3.0–1330.0); 20.8; 66.2 | D | 0.192 | 30.6 (9.2–406.0); 17.6; 84.5 |
0.016 |
| 259 (13.0%) | 27 (10.9%) | 0.419 | ## | |||||||||
| 85 (4.2%) | 9 (3.6%) | 0.739 | ## | ||||||||||||||||||||
| 15 (0.8%) | 1 (0.4%) | 1 | ## | ||||||||||||||||||||
| 47 (2.4%) | 12 (4.8%) | 0.032 | ## | ||||||||||||||||||||
| 68 (3.4%) | 11 (4.4%) | 0.364 | ## | ||||||||||||||||||||
| Patients with varicocele | 467 (23.4%) | 61 (24.6%) | 0.691 | ## | ||||||||||||||||||||
| 173 (8.7%) | 25 (10.1%) | 0.668 | ## | ||||||||||||||||||||
| 211 (10.6%) | 27 (10.9%) | 0.777 | ## | ||||||||||||||||||||
| 32 (1.6%) | 4 (1.6%) | 1 | ## | ||||||||||||||||||||
| 5 (0.3%) | 0 (0.0%) | 1 | ## | ||||||||||||||||||||
| 24 (1.2%) | 3 (1.2%) | 1 | ## | ||||||||||||||||||||
| 22 (1.1%) | 2 (0.8%) | 1 | ## | ||||||||||||||||||||
| Smokers | 667 (33.4%) | 74 (31.1%) | 0.513 | ## | ||||||||||||||||||||
| Concentration of FSH in serum, in U/L | 3.8 (0.1–74.1); 2.6; 5.6 | B | 3.6 (0.6–15.2); 2.6; 5.1 |
0.065 | # | |||||||||||||||||||
| Concentration of LH in serum, in U/L | 3.9 (0.1–25.9); 2.8; 5.4 | C | 3.6 (0.6–11.7); 2.4; 4.6 | C | <0.001 | # | ||||||||||||||||||
| Concentration of total testosterone in serum, in nmol/L | 16.0 (0.7–47.8); 12.4; 20.4 | D | 16.6 (6.0–49.3); 12.9; 20.2 |
0.489 | # | |||||||||||||||||||
| PSA in serum, in µg/L | 0.71 (0.11–8.34); 0.52; 0.99 | E | 0.69 (0.14–7.58); 0.47; 0.96 | E | 0.053 | # | ||||||||||||||||||
| Height, cm | 181.1 (153.0–209.0); 180.8 to 181.4 | $, F | 180.8 (167.0–198.0); 180.0 to 181.5 | $ | 0.468 | ### | ||||||||||||||||||
| Weight, kg | 85.1 (47.0–189.0); 75.7; 95.5 | G | 82.0 (52.0–139.0); 74.5; 90.9 |
0.005 | # | |||||||||||||||||||
| BMI | 26.0 (14.9–76.2); 23.6; 28.7 | H | 24.9 (18.0–42.4); 22.9; 27.8 |
0.003 | # | |||||||||||||||||||
| 812 (42.3%) | H | 85 (34.3%) | 0.016 | ## | |||||||||||||||||||
| 340 (17.7%) | H | 34 (13.7%) | 0.129 | ## | |||||||||||||||||||
| Waist circumference, cm | 93.0 (62.0–152.0); 85.0; 101.0 | I | 90.0 (68.5–124.0); 83.5; 97.1 | I | <0.001 | # | ||||||||||||||||||
| Semen parameters | ||||||||||||||||||||||||
| Abstinence before semen analysis, in days | 4.0 (0.0–56.0); 3.0; 4.0 | J | 3.0 (1.0–60.0); 3.0; 5.0 |
0.238 | # | |||||||||||||||||||
| Volume of seminal fluid, in mL-s | 4.0 (0.0–40.0); 2.9; 5.1 | J | 3.8 (1.2–9.5); 2.9; 5.1 |
0.361 | # | |||||||||||||||||||
| Concentration of spermatozoa, in M/mL | 50.0 (0.0–487.0); 21.9; 88.0 | J | 79.0 (6.0–355.0); 46.8; 125.0 |
<0.001 | # | |||||||||||||||||||
| Total count of spermatozoa, in M | 189.0 (0.0–2432.0); 79.2; 341.3 | J | 302.5 (14.4–1657.6); 164.8; 499.3 |
<0.001 | # | |||||||||||||||||||
| Spermatozoa with progressive motility, % | 45 (0–83); 34; 56 | K | 53 (11–84); 44; 60 |
<0.001 | # | |||||||||||||||||||
| Spermatozoa with normal morphology, % | 7 (0–53); 4; 11 | L | 10 (0–27); 7; 14 |
<0.001 | # | |||||||||||||||||||
| Concentration of round cells in seminal fluid, in M/mL | 1.5 (0.0–75.0); 0.6; 3.0 | M | 1.5 (0.0–45.0); 0.5; 3.0 |
0.231 | # | |||||||||||||||||||
| Percentage of neutrophils in seminal fluid, % | 9.0 (0.0–92.0); 3.0; 18.0 | M | 4.0 (0.0–69.0); 0.0; 12 |
<0.001 | # | |||||||||||||||||||
| Concentration of neutrophils in seminal fluid, in M/mL | 0.1 (0.0–68.3); 0.0; 0.4 | M | 0.0 (0.0–27.5); 0.0; 0.3 |
<0.001 | # | |||||||||||||||||||
| IL-6 in seminal plasma, in ng/L | 18.6 (2.0–4928.0); 11.2; 32.9 | N | 19.3 (2.0–486.0); 12.0; 35.0 |
0.676 | # | |||||||||||||||||||
