Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 5 by Da Sun and Version 4 by Da Sun.
Bacterial ghosts (BGs) are empty bacterial envelopes of Gram-negative bacteria produced by controlled expressions of cloned gene E, forming a lysis tunnel structure within the envelope of the living bacteria.
- bacterial ghosts
- gene E
- vaccines
- immune
1. Introduction
Delivery systems for drugs, nucleic acids, and other biomolecules are recent developments in biotechnology [1][2]. There is a growing interest in the design and development of novel targeted delivery systems. Bacterial derivatives, including bacterial ghosts (BGs), extracellular vesicles, and alimental toxins, are popular biological nanomaterials that are potential vaccine and drug carriers [3]. These delivery platforms retain many of the advantages of bacteria, including the ability to colonize and target human tissues, enhance immunogenicity of vaccines, and have good loading capacities. Advances in genetic engineering and chemical biotechnology have facilitated the development of different types of BGs, which will be important in immobilized enzyme technology, agriculture and medicine [4]. Recently, BGs have received increased attention as potential candidates for targeted delivery of biomolecules [5][6].
Essentially, BGs are bacterial shells with pores. Genetic engineering or chemical methods can be used to induce the release of cellular contents; hence, they have no nucleic acids, ribosomes, or other components [7]. As such, the structural integrity of surface antigens on most BGs remains intact [8]. The preparation of inactivated vaccines using methods such as formaldehyde and heat treatments can destroy the surface structures of the bacteria [9][10]. Contrarily, BGs prepared by genetic engineering retain all the structural antigens expressed by pathogenic bacteria, which induces very strong, effective humoral and cellular immune responses [11]. The structure of BGs is shown in Figure 1. They contain pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), lipoprotein (LPP), peptidoglycan (PGN), and fimbriae, among others, which are highly conserved structures on the outer cell bacterial wall [12]. Once in the host, BGs are recognized by pattern recognition receptors (PRR) on immune cells, stimulating the production of several immune mediators that induce the maturation of antigen-presenting cells (APCs), such as dendritic cells (DCs) [13][14]. In general, pathogenic bacterial ghost serotypes are well preserved, and high concentrations of BGs can provide a high immunogenicity. Moreover, they can only be purified by washing, centrifugation and freeze-drying [15]. Based on these novel biological characteristics, BGs are potential vaccine delivery systems [16]. They have been progressively adopted in the delivery of nucleic acids, proteins, and chemical drugs [17].
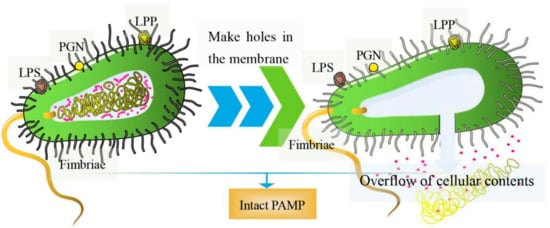
Figure 1. The diagram of BGs. (left) Pores are formed on the surface of bacteria; (right) overflow of cellular contents including nucleic acids, ribosomes, and other components.
2. Methods for BGs Preparation
2.1. Genetic Engineering
When ΦX174, a single-stranded DNA phage, infects Gram-negative bacteria, it inhibits the activities of the enzyme phospho-MurNAc-pentapeptide translocase (MraY) on the bacteria membrane. This is mediated by lysis protein E, a hydrophobic protein that inhibits the synthesis of PGN in bacterial cell walls [18]. First, the hydrophobic N-terminal binds the inner membrane of the bacterial cell wall. Then, the conformation of protein E changes, binding its hydrophobic C-terminal across the inner and periplasmic spaces to the outer membrane of the cell wall [19]. This disruption causes secondary effects, such as activation of phosphatase activities, and increased membrane mobilities. This increases internal osmotic pressures, inducing the release of cell contents [20]. Currently, the most commonly used method for the preparation of BGs involves cloning lysis gene E (276 bp, accession number: MF426914.1) into expression regulation systems of Gram-negative bacteria, which utilizes the switch function of the control element to control expression [21]. The preparation process for BGs is shown in Figure 2.
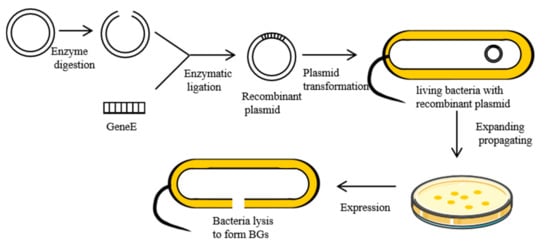

Figure 2. Preparation of the BGs using genetic engineering.
Constructed BGs are about 1–2 μm long and 0.5–2 μm wide. Lysis protein E connects the inner and outer membranes, minimizing the loss of enzymes in periplasmic spaces. During bacterial lysis, inner and outer membrane structures remain intact. Diameters of transmembrane pores are about 40–200 nm, but, under osmotic pressure, they can reach 500 nm [22]. Pore sizes are determined by sieve sizes of the PGN layer in the cell wall [23].
Transmembrane tunnels are formed at the bacterial division site or polar cap of bacteria, but mostly at the bacterial site, which is related to Z-ring formation mechanisms during bacterial division. The bacterial division protein FtsZ is necessary for Z-ring formation [24]. Witte, A. et al. found that protein E-mediated lysis occurs in the presence of GTPase activities of FtsZ, which is independent of Z-ring structure [25]. However, the mechanisms involved in conformational changes of protein E, which are caused by GTPase activities of FtsZ, should be further investigated.
Expressions of temperature-sensitive λ pL/pR-cI857 or chemically induced expression systems (efficient expression systems of all kinds of hybrids based on Lac promoter) are generally used as markers for selection of successful transformation [22]. For λ pL/pR-cI857 expression, at appropriate temperatures, bacteria are cultured to the logarithmic growth phase, before raising the temperature to 42 °C to induce the expressions of gene E [26][27][28]. For chemically induced expression systems, IPTG or arabinose is added to the original culture medium to obtain BGs [20]. Obtained BGs are lyophilized. Lyophilized BGs can be stored at room temperature for many years [29]. Barisani Asenbauer et al. cultured E. coli in a fermentor until OD600 = 0.9, which took about 90 min. Under temperature-sensitive expression systems, a temperature of 42 °C induces the expressions of protein E. After 120 min of expression, lytic efficiency reached 99.9% [30]. Langemann et al. prepared BGs by heating a mixed culture media of bacteria to 42 °C. After 2 h, bacterial lytic efficiency reached 99.99% [20]. Even with the excellent lysis, residual pathogenic bacteria are potentially harmful. Therefore, secondary inactivation is required to achieve complete lysis. Since UV radiation or formaldehyde inactivation can damage the structures of BG surface antigens, the commonly used method involves the addition of β-acetone (BPL) for inactivation, freeze drying, and storage at −20 °C [10][31]. Gentamicin and streptomycin are also used to inactivate non-lysed bacteria [32]. Double gene inactivation has also been used for secondary inactivation. Zhu et al. enhanced the lytic efficiency of bacteria by incorporating the staphylococcal nuclease A(SNUC) gene into a lysis plasmid (mE-L-SNA) expressing the E fusion gene. The lytic efficiency of E. coli at the logarithmic growth stage reached 99.99995%, thus improving the safety of BGs [33]. Tian et al. constructed Streptococcus pullorum ghosts by fusing the antimicrobial peptide gene, SMAP29, with lysis gene E. Twenty-four hours after induced lysis, they did not find any viable bacteria [34]. Hjelm et al. constructed a mutant strain of E. coli (MC4100) by deleting the ASD gene, which encodes aspartate semialdehyde dehydrogenase involved in diaminopimelic acid (DAP) synthesis. The growth of mutant strains is entirely dependent on the amount of DAP added to the culture medium. After gene E was induced, the target bacteria/BGs were incubated in LB medium without DAP for 12 h, centrifuged, collected, and freeze-dried to completely inactivate E. coli [35]. Chemical and genetic engineering methods can inactivate residual pathogenic bacteria.
Currently, regulation of the expression of the gene E lysis system has been successfully applied against several gram-negative bacteria strains, Salmonella typhimurium [36], Salmonella enteritidis [8], Vibrio cholerae [37], Pectobacterium cypripedii [38], Helicobacter pylori [39], Actinobacillus-pleuropneumoniae [40], Haemophilus influenzae [41], Pasteurella multocida [42], Brucella [43], and Aeromonas hydrophila [44], among others. Given the wide spectrum of bacteria, these findings imply that any suitable gene E carrier, perhaps all Gram-negative bacteria, can produce lysis-based BGs. However, the preparation methods for BGs are associated with several challenges. (I) Plasmids are not applicable to all Gram-negative bacteria, which should be investigated and modified for each strain. Uneven distributions of plasmids during bacterial division leads to plasmid loss; therefore, there is a need to determine whether gene E can be cloned into the bacterial genome. (II) During the preparation of BGs, the antibiotic resistance gene is introduced alongside lysis gene E. The antibiotic resistance gene can be laterally transferred in the environment [45]. (III) There are many lytic resistant mutants, especially E. coli, which requires double gene inactivation, such as combining gene E and gene SNUC [46].
2.2. The Chemical Method
The “sponge-like” method is a common chemical process for preparing BGs. In this method, pores are made through bacterial cell walls using chemical reagents. Then, cellular contents are removed by centrifugation (Figure 3A). Amara et al. used less than the minimum inhibitory concentration (MIC) of chemicals such as NaOH, SDS, H2O2, and CaCO3 to prepare BGs. Then, they used the Plackett–Burman experimental design to optimize the preparation conditions for “sponge-like” E. coli ghosts [47]. Sheweita et al. prepared “sponge” BGs by incubating Acinetobacter baumannii Ali190 in a mixture of NaOH, Na2CO3 and a solution of H2O2 [11]. Under these methods, cell wall integrity remains intact. Sameh et al. designed a novel chemical method of preparing BGs by culturing Salmonella for 24 h in a culture media supplemented with 7% Tween 80 [48]. The pH of the medium was reduced to 3.6 using lactic acid. Tween 80 causes the dissolution of hydrophobic components in the outer membrane of the bacteria, thus forming weak areas. These areas facilitate puncture formation caused by the sudden decrease in pH. In another biochemical method, BGs were developed by incubating bacteria in an artificial synthetic model amphiphilic peptide (MAP) dissolved in Na2HPO4 solution (Figure 3B) [49][50]. The chemical process can be performed at any stage of bacterial growth and only requires dilution to control OD600 = 0.1. The efficiency of gene E-mediated lysis was best in the logarithmic growth phase, and lysis in the stationary phase resulted in the survival of live bacteria [51]. Furthermore, the chemical method is not limited to Gram-negative bacteria as it is also effective for Gram-positive bacteria and yeasts [52].
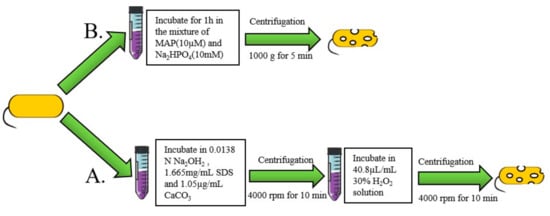

Figure 3. Chemical method for preparation of BGs. (A) Preparation of BGs using the model amphiphilic peptide (MAP). (B) The two-step method for preparation of BGs.
Rapid preparation of BGs using chemical agents eliminates the limitations associated with the use of lysis gene E, particularly genetic restriction-modification [2]. The chemical method is simple, rapid, and does not change the three-dimensional morphology of cells, except for producing holes. However, chemicals may denature surface immunogenic antigens. Excess holes may disrupt the controlled release property. Follow-up experiments should be performed to solve these challenges. Therefore, genetic engineering methods are still the most widely used techniques.
3. BGs-Based Vaccine
3.1. DNA Vaccines
Naked DNA vaccines are limited by their weak immunogenicity. To elicit stronger immune response, they are usually combined with various adjuvants, such as alum. However, the currently used adjuvants have various limitations and the adjuvant options available to any recombinant vaccine manufacturer are very limited. However, carrier transport can enhance its immunogenicity and efficiency [53]. Currently, nucleic acid vectors, including viruses (influenzavirus, adenovirus, poliovirus, etc), fungi (Saccharomyces), and bacteria (Bacillus Calmette Guerin), among others, are commonly used. As carriers, BGs can be internalized by various cells, such as mouse macrophage raw 264.7, HCDECs, DCs, and Caco-2 [54][55][56]. In vitro cell experiments have shown that 60% of macrophages (raw 264.7) can internalize BGs containing reporter plasmids and then express green fluorescent proteins [57]. DCs can also internalize BGs and secrete IL-12 to activate Th1 immune responses [58]. The internalization and activation of BGs by APC cells provides a new strategy for vaccination and in situ immunotherapy. BGs carrying plasmids adopt the diffusion strategy (plasmid DNA diffuses through the lytic pore into the BGs) [57], and the nucleic acid can nonspecifically bind with BGs (The negatively charged DNA binds to positively charged groups in the inner membrane, such as amines). Each BG can load 4000–5000 copies of plasmid DNA (from medium to large plasmids) [59]. These in vitro experiments have proven that BGs are feasible for nucleic acid delivery. In mice models, there have been many studies of BG-based nucleic acid vaccines. Zhou et al. used BGs as a delivery system to prepare an effective DNA vaccine for the prevention of C. psittaci infection [60]. This BG-based DNA vaccine induced a stronger humoral immunity (IgG upregulation) and cellular immunity (Th1 type immune related indicator upregulation) than the naked DNA vaccine and BGs. Jiao et al. used the Salmonella ghost to prepare DNA vaccines to prevent Neisseria gonorrhoea [61]. They found that BG-based DNA vaccines induced stronger humoral immunities (IgG upregulation) and lymphocyte proliferation, relative to naked DNA vaccines and BGs alone. In a model experiment with bone marrow-derived DCs (BMDCs), Jiao et al. cultured the vaccine with BMDCs [62]. They found that the BG-based DNA vaccine promoted greater DCs maturation and activation (up regulation of cell surface costimulatory molecules CD80, CD86, CD40, and MHC-II) than the naked DNA vaccine and BGs alone. These results show that DNA vaccines prepared with BG-loaded plasmids have a better stimulatory effect on both humoral and cellular immunities, relative to naked DNA vaccines. Cao et al. used DH5α ghosts loaded with plasmids containing five exogenous fragments (including invariant chain-like protein [Iclp] gene) to prepare double-targeted DNA vaccines for oral immunization of grass carp [63]. Plasmids with endogenous Iclp easily enter the MHC-II antigen presentation pathway. Immunization with the double-targeted DNA vaccine substantially increases the activities of three innate immune parameters (SOD, LZM and C3) in serum and intestinal mucus. Moreover, the relative survival rate of the experimental group reached 81.11%, demonstrating the efficiency of the vaccine against Vibrio strains. These studies show that antigenic genes in BGs can be internalized and expressed by APCs, and foreign genes can be derived from multiple plasmids or a plasmid containing the fusion gene fragment. The multigene BGs vaccine induced stronger immune responses than the naked DNA vaccine.
3.2. Protein Antigen Vaccines
BGs serve as carriers for protein vaccines in two ways. (I) Multi epitope peptide BGs: through genetic engineering, the antigen and protein target is displayed on the surface to improve its immunogenicity and targeting. (II) Non-recombinant BGs mixture: when the antigen is co-incubated with BGs, the protein non-specifically binds the intima. However, to obtain strong immune responses, optimal doses of proteins and BGs should be investigated further. Tuntufye HN et al. found that BG-based recombinant vaccines of ferri-siderophore receptors protected chickens against avian pathogenic E. coli APEC infection [64]. They found that both the recombinant BGs vaccine and the non-recombinant mixture vaccine could significantly increase IgG immune responses and reduce mortality after infection. Moreover, the mortality rate for recombinant BGs chickens was lower, relative to that of the non-recombinant mixture vaccine. Sai Gong et al. developed a vaccine against hand-foot-and-mouth disease by expressing antigenic proteins of Entero virus 71 and Coxsackie virus in the outer membrane protein A(OMPA) of E. coli O157: H7 [15]. The vaccine increased IgG and IgA secretion, thereby inducing mucosal immunity. Moreover, the vaccine candidate protected mice against E. coli infection. In addition to expressing protein antigens on the outer membrane of BGs, Riedmann et al. enhanced cellular immune responses via intestinal or lung inoculation of BGs developed by fusing the Haemophilus influenzae (NTHi) antigen (OMP26) into the S layer or periplasmic space of E. coli [41]. In conclusion, endomembrane proteins, periplasmic space proteins and outer membrane proteins on BGs are antigenic, and they can induce immunogenicity. Compared to the protein subunit vaccine, BGs adjuvants significantly enhance the immunogenicity of antigenic proteins [19].
Currently, there is increasing attention on how to improve the protein loading capacities of BGs (Figure 5). To achieve this, attempts have been made to express streptavidin in the inner membrane of BGs, whereas the target protein is phthalated with biotin. The specific interaction between biotin and streptavidin immobilizes the target protein in the inner membrane. In other studies, the protein was attached to the bacterial membrane through genetic engineering. For example, Sührer et al. attached galactosidase anchors on BGs containing cytochrome b5 to immobilize the enzyme [65]. In other studies, BGs have been sealed by fusions with membrane vesicles [66].
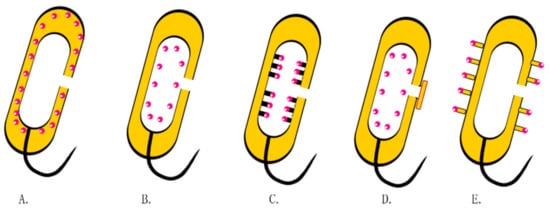

Figure 4. Drug loading strategies in BGs. (A) Expressions of proteins or antigens in the periplasmic space through genetic engineering. (B) Non-specific binding of drugs to the inner membrane. (C) Attachment of exogenous proteins or antigens onto the inner membrane using streptavidin. (D) BGs encapsulated with membranous vesicles. (E) Multiepitope peptide BGs.
BGs can either be mixed with the antigen of interest, or the antigen can be expressed in a Gram-negative bacterium to be turned into BGs, thereby creating a bacterial shell with integrated antigens. Given the success of nucleic acid vaccines for COVID-19, BG-based vaccine research should fully utilize the natural intrinsic adjuvant effect of BGs in its vaccine candidates.
References
- Peng, R.; Ji, H.; Jin, L.; Lin, S.; Huang, Y.; Xu, K.; Yang, Q.; Sun, D.; Wu, W. Macrophage-Based Therapies for Atherosclerosis Management. J. Immunol. Res. 2020, 2020, 1–11.
- Sun, D.; Chen, J.; Wang, Y.; Ji, H.; Peng, R.; Jin, L.; Wu, W. Advances in Refunctionalization of Erythrocyte-Based Nanomedicine for Enhancing Cancer-Targeted Drug Delivery. Theranostics 2019, 9, 6885–6900.
- Moghimipour, E.; Abedishirehjin, S.; Baghbadorani, M.A.; Handali, S. Bacteria and Archaea: A New Era of Cancer Therapy. J. Control. Release 2021, 338, 1–7.
- Ding, C.; Cicuttini, F.; Li, J.; Jones, G. Targeting IL-6 in the Treatment of Inflammatory and Autoimmune Diseases. Expert Opin. Investig. Drugs 2009, 18, 1457–1466.
- Xie, S.; Zhang, P.; Zhang, Z.; Liu, Y.; Chen, M.; Li, S.; Li, X. Bacterial Navigation for Tumor Targeting and Photothermally-Triggered Bacterial Ghost Transformation for Spatiotemporal Drug Release. Acta Biomater. 2021, 131, 172–184.
- Holay, M.; Guo, Z.; Pihl, J.; Heo, J.; Park, J.H.; Fang, R.H.; Zhang, L. Bacteria-Inspired Nanomedicine. ACS Appl. Bio Mater. 2021, 4, 3830–3848.
- Hosseinidoust, Z.; Mostaghaci, B.; Yasa, O.; Park, B.-W.; Singh, A.V.; Sitti, M. Bioengineered and Biohybrid Bacteria-Based Systems for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 27–44.
- Senevirathne, A.; Hewawaduge, C.; Lee, J.H. Immunization of Chicken with Flagellin Adjuvanted Salmonella enteritidis Bacterial Ghosts Confers Complete Protection against Chicken Salmonellosis. Poult. Sci. 2021, 100, 101205.
- Carvalho, S.B.; Peixoto, C.; Carrondo, M.J.T.; Silva, R.J.S. Downstream Processing for Influenza Vaccines and Candidates: An Update. Biotechnol. Bioeng. 2021, 118, 2845–2869.
- Sabbaghi, A.; Miri, S.M.; Keshavarz, M.; Zargar, M.; Ghaemi, A. Inactivation Methods for Whole Influenza Vaccine Production. Rev. Med. Virol. 2019, 29, e2074.
- Sheweita, S.A.; Batah, A.M.; Ghazy, A.A.; Hussein, A.; Amara, A.A. A New Strain of Acinetobacter Baumannii and Characterization of Its Ghost as a Candidate Vaccine. J. Infect. Public Health 2019, 12, 831–842.
- Michalek, J.; Hezova, R.; Turanek-Knötigova, P.; Gabkova, J.; Strioga, M.; Lubitz, W.; Kudela, P. Oncolysate-Loaded Escherichia Coli Bacterial Ghosts Enhance the Stimulatory Capacity of Human Dendritic Cells. Cancer Immunol. Immunother. 2017, 66, 149–159.
- Wang, G.; Zhao, G.; Chao, X.; Xie, L.; Wang, H. The Characteristic of Virulence, Biofilm and Antibiotic Resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 2020, 17, 6278.
- Muhammad, A.; Champeimont, J.; Mayr, U.B.; Lubitz, W.; Kudela, P. Bacterial Ghosts as Carriers of Protein Subunit and DNA-Encoded Antigens for Vaccine Applications. Expert Rev. Vaccines 2012, 11, 97–116.
- Gong, S.; Nan, N.; Sun, Y.; He, Z.; Li, J.; Chen, F.; Li, T.; Ning, N.; Wang, J.; Li, Z.; et al. Protective Immunity Elicited by VP1 Chimeric Antigens of Bacterial Ghosts against Hand-Foot-and-Mouth Disease Virus. Vaccines 2020, 8, 61.
- Batah, A.M.; Ahmad, T.A. The Development of Ghost Vaccines Trials. Expert Rev Vaccines 2020, 19, 549–562.
- Ganeshpurkar, A.; Ganeshpurkar, A.; Pandey, V.; Agnihotri, A.; Bansal, D.; Dubey, N. Harnessing the Potential of Bacterial Ghost for the Effective Delivery of Drugs and Biotherapeutics. Int. J. Pharm. Investig. 2014, 4, 1.
- Mendel, S.; Holbourn, J.M.; Schouten, J.A.; Bugg, T.D.H. Interaction of the Transmembrane Domain of Lysis Protein E from Bacteriophage ΦX174 with Bacterial Translocase MraY and Peptidyl-Prolyl Isomerase SlyD. Microbiology 2006, 152, 2959–2967.
- Langemann, T.; Koller, V.J.; Muhammad, A.; Kudela, P.; Mayr, U.B.; Lubitz, W. The Bacterial Ghost Platform System: Production and Applications. Bioeng. Bugs 2010, 1, 326–336.
- Lubitz, P.; Mayr, U.B.; Lubitz, W. Applications of Bacterial Ghosts in Biomedicine. In Pharmaceutical Biotechnology; Guzmán, C.A., Feuerstein, G.Z., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2009; Volume 655, pp. 159–170. ISBN 978-1-4419-1131-5.
- Chung, T.-C.; Jones, C.H.; Gollakota, A.; Kamal Ahmadi, M.; Rane, S.; Zhang, G.; Pfeifer, B.A. Improved Escherichia Coli Bactofection and Cytotoxicity by Heterologous Expression of Bacteriophage ΦX174 Lysis Gene E. Mol. Pharm. 2015, 12, 1691–1700.
- Szostak, M.P.; Hensel, A.; Eko, F.O.; Klein, R.; Auer, T.; Mader, H.; Haslberger, A.; Bunka, S.; Wanner, G.; Lubitz, W. Bacterial Ghosts: Non-Living Candidate Vaccines. J. Biotechnol. 1996, 44, 161–170.
- Witte, A.; Wanner, G.; Sulzner, M.; Lubitz, W. Dynamics of PhiX174 Protein E-Mediated Lysis of Escherichia Coli. Arch. Microbiol. 1992, 157, 381–388.
- Haeusser, D.P.; Margolin, W. Splitsville: Structural and Functional Insights into the Dynamic Bacterial Z Ring. Nat. Rev. Microbiol. 2016, 14, 305–319.
- Witte, A.; Brand, E.; Mayrhofer, P.; Narendja, F.; Lubitz, W. Mutations in Cell Division Proteins FtsZ and FtsA Inhibit ΦX174 Protein-E-Mediated Lysis of Escherichia Coli. Arch. Microbiol. 1998, 170, 259–268.
- Marchart, J.; Dropmann, G.; Lechleitner, S.; Schlapp, T.; Wanner, G.; Szostak, M.P.; Lubitz, W. Pasteurella Multocida- and Pasteurella Haemolytica-Ghosts: New Vaccine Candidates. Vaccine 2003, 21, 3988–3997.
- Jalava, K. Bacterial Ghosts as Vaccine Candidates for Veterinary Applications. J. Control. Release 2002, 85, 17–25.
- Hajam, I.A.; Dar, P.A.; Won, G.; Lee, J.H. Bacterial Ghosts as Adjuvants: Mechanisms and Potential. Vet. Res. 2017, 48, 37.
- Paukner, S.; Stiedl, T.; Kudela, P.; Bizik, J.; Al Laham, F.; Lubitz, W. Bacterial Ghosts as a Novel Advanced Targeting System for Drug and DNA Delivery. Expert Opin. Drug Deliv. 2006, 3, 11–22.
- Barisani-Asenbauer, T.; Montanaro, J.; Inic-Kanada, A.; Ladurner, A.; Stein, E.; Belij, S.; Bintner, N.; Schlacher, S.; Schuerer, N.; Mayr, U.B.; et al. Escherichia Coli Nissle 1917 Bacterial Ghosts Retain Crucial Surface Properties and Express Chlamydial Antigen: An Imaging Study of a Delivery System for the Ocular Surface. DDDT 2015, 9, 3741.
- Farjadian, F.; Moghoofei, M.; Mirkiani, S.; Ghasemi, A.; Rabiee, N.; Hadifar, S.; Beyzavi, A.; Karimi, M.; Hamblin, M.R. Bacterial Components as Naturally Inspired Nano-Carriers for Drug/Gene Delivery and Immunization: Set the Bugs to Work? Biotechnol. Adv. 2018, 36, 968–985.
- Koller, V.J.; Dirsch, V.M.; Beres, H.; Donath, O.; Reznicek, G.; Lubitz, W.; Kudela, P. Modulation of Bacterial Ghosts—Induced Nitric Oxide Production in Macrophages by Bacterial Ghost-Delivered Resveratrol. FEBS J 2013, 280, 1214–1225.
- Zhu, W.; Zhang, Y.; Liu, X. Efficient Production of Safety-Enhanced Escherichia Coli Ghosts by Tandem Expression of PhiX 174 Mutant Gene E and Staphylococcal Nuclease A Gene. Microbiol. Res. 2015, 176, 7–13.
- Tian, Q.; Zhou, W.; Si, W.; Yi, F.; Hua, X.; Yue, M.; Chen, L.; Liu, S.; Yu, S. Construction of Salmonella Pullorum Ghost by Co-Expression of Lysis Gene E and the Antimicrobial Peptide SMAP29 and Evaluation of Its Immune Efficacy in Specific-Pathogen-Free Chicks. J. Integr. Agric. 2018, 17, 197–209.
- Hjelm, A.; Söderström, B.; Vikström, D.; Jong, W.S.P.; Luirink, J.; de Gier, J.-W. Autotransporter-Based Antigen Display in Bacterial Ghosts. Appl. Environ. Microbiol. 2015, 81, 726–735.
- Senevirathne, A.; Hewawaduge, C.; Park, J.-Y.; Park, S.; Lee, J.H. Parenteral Immunization of Salmonella Typhimurium Ghosts with Surface-Displayed Escherichia Coli Flagellin EnhancesTLR-5 Mediated Activation of Immune Responses That Protect the Chicken against Salmonella Infection. Microb. Pathog. 2020, 147, 104252.
- Ekong, E.E.; Okenu, D.N.; Mania-Pramanik, J.; He, Q.; Igietseme, J.U.; Ananaba, G.A.; Lyn, D.; Black, C.; Eko, F.O. A Vibrio Cholerae Ghost-Based Subunit Vaccine Induces Cross-Protective Chlamydial Immunity That Is Enhanced by CTA2B, the Nontoxic Derivative of Cholera Toxin. FEMS Immunol. Med Microbiol. 2009, 55, 280–291.
- Hatfaludi, T.; Liska, M.; Zellinger, D.; Ousman, J.P.; Szostak, M.; Jalava, K.; Lubitz, W. Bacterial Ghost Technology for Pesticide Delivery. J. Agric. Food Chem. 2004, 52, 5627–5634.
- Chen, J.; Li, N.; She, F. Helicobacter Pylori Outer Inflammatory Protein DNA Vaccine-Loaded Bacterial Ghost Enhances Immune Protective Efficacy in C57BL/6 Mice. Vaccine 2014, 32, 6054–6060.
- Muhammad, A.; Kassmannhuber, J.; Rauscher, M.; Falcon, A.A.; Wheeler, D.W.; Zhang, A.A.; Lubitz, P.; Lubitz, W. Subcutaneous Immunization of Dogs With Bordetella bronchiseptica Bacterial Ghost Vaccine. Front. Immunol. 2019, 10, 1377.
- Riedmann, E.M.; Lubitz, W.; McGrath, J.; Kyd, J.M.; Cripps, A.W. Effectiveness of Engineering the Nontypeable Haemophilus Influenzae Antigen Omp26 as an S-Layer Fusion in Bacterial Ghosts as a Mucosal Vaccine Delivery. Hum. Vaccines 2011, 7, 99–107.
- Ran, X.; Meng, X.-Z.; Geng, H.-L.; Chang, C.; Chen, X.; Wen, X.; Ni, H. Generation of Porcine Pasteurella Multocida Ghost Vaccine and Examination of Its Immunogenicity against Virulent Challenge in Mice. Microb. Pathog. 2019, 132, 208–214.
- Wang, S.; Li, Z.; Zhang, J.; Xi, L.; Cui, Y.; Zhang, W.; Zhang, J.; Zhang, H. A Safe Non-Toxic Brucella Abortus Ghosts Induce Immune Responses and Confer Protection in BALB/c Mice. Mol. Immunol. 2020, 124, 117–124.
- Jiang, N.; Luo, L.; Xing, W.; Li, T.; Yuan, D.; Xu, G.; Li, W.; Ma, Z.; Jin, L.; Ji, M. Generation and Immunity Effect Evaluation of Biotechnology-Derived Aeromonas Veronii Ghost by PhiX174 Gene E-Mediated Inactivation in Koi (Cyprinus Carprio Koi). Fish Shellfish Immunol. 2019, 86, 327–334.
- Panoff, J.M.; Chuiton, C. Horizontal Gene Transfer: A Universal Phenomenon. Hum. Ecol. Risk Assess. 2004, 10, 939–943.
- Soleymani, S.; Tavassoli, A.; Hashemi Tabar, G.; Kalidari, G.A.; Dehghani, H. Design, Development, and Evaluation of the Efficacy of a Nucleic Acid-Free Version of a Bacterial Ghost Candidate Vaccine against Avian Pathogenic E. Coli (APEC) O78:K80 Serotype. Vet. Res. 2020, 51, 144.
- Amara, A.A.; Salem-Bekhit, M.M.; Alanazi, F.K. Sponge-Like: A New Protocol for Preparing Bacterial Ghosts. Sci. World J. 2013, 2013, 1–7.
- Rabea, S.; Salem-Bekhit, M.M.; Alanazi, F.K.; Yassin, A.S.; Moneib, N.A.; Hashem, A.E.M. A Novel Protocol for Bacterial Ghosts’ Preparation Using Tween 80. Saudi Pharm. J. 2018, 26, 232–237.
- Hu, J.; Dong, H.; Fu, L.; Zuo, J.; Hu, S. Comparison of Three Methods for Preparation of Bacterial Ghosts from Avian Pathogenic Escherichia Coli. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2017, 33, 2009–2016.
- Palm-Apergi, C.; Hällbrink, M. A New Rapid Cell-Penetrating Peptide Based Strategy to Produce Bacterial Ghosts for Plasmid Delivery. J. Control. Release 2008, 132, 49–54.
- Yu, S.; Peng, W.; Si, W.; Yin, L.; Liu, S.; Liu, H.; Zhao, H.; Wang, C.; Chang, Y.; Lin, Y. Enhancement of Bacteriolysis of Shuffled Phage PhiX174 Gene E. Virol. J. 2011, 8, 206.
- Vinod, N.; Oh, S.; Park, H.J.; Koo, J.M.; Choi, C.W.; Kim, S.C. Generation of a Novel Staphylococcus Aureus Ghost Vaccine and Examination of Its Immunogenicity against Virulent Challenge in Rats. Infect. Immun. 2015, 83, 2957–2965.
- Xu, Y.; Pak-Wai, Y.; Jenny, L. Intranasal DNA Vaccine for Protection against Respiratory Infectious Diseases: The Delivery Perspectives. Pharmaceutics 2014, 6, 378.
- Kudela, P.; Koller, V.J.; Mayr, U.B.; Nepp, J.; Lubitz, W.; Barisani-Asenbauer, T. Bacterial Ghosts as Antigen and Drug Delivery System for Ocular Surface Diseases: Effective Internalization of Bacterial Ghosts by Human Conjunctival Epithelial Cells. J. Biotechnol. 2011, 153, 167–175.
- Paukner, S.; Kohl, G.; Lubitz, W. Bacterial Ghosts as Novel Advanced Drug Delivery Systems: Antiproliferative Activity of Loaded Doxorubicin in Human Caco-2 Cells. J. Control. Release 2004, 94, 63–74.
- Stein, E.; Inic-Kanada, A.; Belij, S.; Montanaro, J.; Bintner, N.; Schlacher, S.; Mayr, U.B.; Lubitz, W.; Stojanovic, M.; Najdenski, H.; et al. In Vitro and In Vivo Uptake Study of Escherichia Coli Nissle 1917 Bacterial Ghosts: Cell-Based Delivery System to Target Ocular Surface Diseases. Invest. Ophthalmol. Vis. Sci. 2013, 54, 6326.
- Kudela, P.; Paukner, S.; Mayr, U.B.; Cholujova, D.; Schwarczova, Z.; Sedlak, J.; Bizik, J.; Lubitz, W. Bacterial Ghosts as Novel Efficient Targeting Vehicles for DNA Delivery to the Human Monocyte-Derived Dendritic Cells. J. Immunother. 2005, 28, 136–143.
- Haslberger, A.G.; Kohl, G.; Felnerova, D.; Mayr, U.B.; Fürst-Ladani, S.; Lubitz, W. Activation, Stimulation and Uptake of Bacterial Ghosts in Antigen Presenting Cells. J. Biotechnol. 2000, 83, 57–66.
- Walcher, P.; Mayr, U.B.; Azimpour-Tabrizi, C.; Eko, F.O.; Jechlinger, W.; Mayrhofer, P.; Alefantis, T.; Mujer, C.V.; DelVecchio, V.G.; Lubitz, W. Antigen Discovery and Delivery of Subunit Vaccines by Nonliving Bacterial Ghost Vectors. Expert Rev. Vaccines 2004, 3, 681–691.
- Zhou, P.; Wu, H.; Chen, S.; Bai, Q.; Chen, X.; Chen, L.; Zeng, X.; Liu, L.; Chen, L. MOMP and MIP DNA-Loaded Bacterial Ghosts Reduce the Severity of Lung Lesions in Mice after Chlamydia Psittaci Respiratory Tract Infection. Immunobiology 2019, 224, 739–746.
- Jiao, H.; Yang, H.; Zheng, W.; Zhang, Q.; Zhao, D.; Li, G. Enhancement of Immune Responses by Co-administration of Bacterial Ghosts-mediated Neisseria Gonorrhoeae DNA Vaccines. J. Appl. Microbiol. 2020, 130, 1770–1777.
- Jiao, H.; Yang, H.; Zhao, D.; Chen, J.; Zhang, Q.; Liang, J.; Yin, Y.; Kong, G.; Li, G. Design and Immune Characterization of a Novel Neisseria Gonorrhoeae DNA Vaccine Using Bacterial Ghosts as Vector and Adjuvant. Vaccine 2018, 36, 4532–4539.
- Cao, J.; Zhu, X.-C.; Liu, X.-Y.; Yuan, K.; Zhang, J.-J.; Gao, H.-H.; Li, J.-N. An Oral Double-Targeted DNA Vaccine Induces Systemic and Intestinal Mucosal Immune Responses and Confers High Protection against Vibrio Mimicus in Grass Carps. Aquaculture 2019, 504, 248–259.
- Tuntufye, H.N.; Ons, E.; Pham, A.D.N.; Luyten, T.; Van Gerven, N.; Bleyen, N.; Goddeeris, B.M. Escherichia Coli Ghosts or Live E. coli Expressing the Ferri-Siderophore Receptors FepA, FhuE, IroN and IutA Do Not Protect Broiler Chickens against Avian Pathogenic E. Coli (APEC). Vet. Microbiol. 2012, 159, 470–478.
- Sührer, I.; Langemann, T.; Lubitz, W.; Weuster-Botz, D.; Castiglione, K. A Novel One-Step Expression and Immobilization Method for the Production of Biocatalytic Preparations. Microb Cell Fact 2015, 14, 180.
- Paukner, S.; Kohl, G.; Jalava, K.; Lubitz, W. Sealed Bacterial Ghosts—Novel Targeting Vehicles for Advanced Drug Delivery of Water-Soluble Substances. J. Drug Target. 2003, 11, 151–161.
More
