A Raffinose family oligosaccharides (RFOs) is one of the major translocated sugars in the vascular bundle of cucumber, but little RFOs can be detected in fruits. Alpha-galactosidases (α-Gals) catalyze the first catabolism step of RFOs. Six α-Gal genes exist in a cucumber genome, but their spatial functions in fruits remain unclear.
1. Introduction
Cucumber (
Cucumis sativus L.) is an important vegetable worldwide with great economic and nutritional value
[1,2][1][2]. Unlike most plants, in which sucrose (Suc) is the predominant sugar transported in the vascular bundle, raffinose (Raf) family oligosaccharides (RFOs) are the major sugars transported between “source” organs and “sink” organs in cucumber
[3,4,5,6,7][3][4][5][6][7]. Fruit is one of the most important sink organs in cucumber plants. However, in this organ, few RFOs are detected, and hexoses are the predominant free carbohydrates, indicating quick hydrolysis of imported RFOs in cucumber fruits
[8,9][8][9]. RFOs consisting of stachyose (Sta) and Raf are translocated into sieve elements and hydrolysed into sucrose (Suc) in sink tissues after long-distance translocation
[10,11][10][11]. There are two opinions about the location of RFOs hydrolyzation in fruits. Some researchers believe that the catabolism of Sta and Raf occurs in the pedicel. Evidence supporting this view include little RFOs, which were detected in fruits
[3,12,13][3][12][13]. While the activity of enzymes catalysing RFOs catabolism, such as α-galactosidase, Suc synthase (SS, synthetic activity), SPS and UDP- galactose pyrophosphorylase were detected in the pedicel
[6,14][6][14]. However, other researchers have suggested that RFOs are transported into the fruit and metabolized rapidly there. The evidence supporting this view include that RFOs exist in the cucurbits fruit
[8,10,15,16][8][10][15][16]; exudates from the fruit’s main bundles contains Sta as the major sugar
[12,17][12][17]; and active RFO metabolic enzymes, including alkaline a-galactosidase have been detected in the cucurbit’s fruits
[10,11,12,15,18,19][10][11][12][15][18][19].
α-Galactosidase (EC3.2.1.22), also known as α-d-galactoside galactohydrolase and melibiase, is an exoglycosidase that hydrolyses the terminal nonreducing α-galactosyl moieties from glycolipids and glycoproteins
[20,21][20][21]. α-galactosidases, distributed in various plant organs including seeds, seedlings, leaves and fruits, are involved in many physiological, biochemical processes, such as RFO unloading in fruits, seed development and germination, leaf development and senescence
[21,22,23,24][21][22][23][24]. When RFOs were unloaded in cucumber fruits, previous studies showed that α-galactosidase catalysed the initial step of RFOs decomposition
[8,18][8][18]. Taken together, α-galactosidase is a key determinant of fruit sink strength and an important regulator of source-sink balance in cucumber plants.
In higher plants, α-galactosidases are classified into two groups, acid α-galactosidases (GAL) and alkaline α-galactosidases (AGA), according to their activity in response to pH. There is a negative correlation between Sta levels and the activity of CsAGAs in cucumber peduncles, suggesting that CsAGAs rather than CsGALs may be responsible for the metabolism of imported Sta
[12]. Irving et al. (1997) also reported that the activity of AGA was higher than that of GAL in
Cucurbita maxima Duch. fruits at anthesis. Gao and Schaffer (1999) identified two AGAs in melon (
Cucumis melo L.). CmAGA1 (AAM75139.1) showed significant activity with both Raf and Sta, whereas CmAGA2 (AAM75140.1) was relatively specific for Sta
[19]. In addition, CmAGA1 enzyme activity is increased during the early stages of melon ovary development and fruit set, while CmAGA2 enzyme activity is declined during this period, indicating CmAGA1 may play a key role in melon photoassimilate unloading
[18,19][18][19]. However, ClAGA2, an alkaline α-galactosidase, was recently identified as the key factor controlling the hydrolysis of Sta and Raf in watermelon (
Citrullus lanatus);
claga2 mutants showed reduced content of Glu, Fru and Suc, but increased Raf content in the fruit fresh of watermelon
[5,6][5][6]. In addition, two single-nucleotide polymorphisms (SNPs) existing in the
ClAGA2 promoter affected the binding of the transcription factor ClNF-YC2 to regulate
ClAGA2 expression
[5]. In cucumber, there are six putative α-galactosidase genes (
α-Gals), with three acid forms (
CsGAL1,
CsGAL2,
CsGAL3) and three alkaline forms (
CsAGA1,
CsAGA2,
CsAGA3)
[25]. Those six α-Gals are universally expressed in different organs and have different substrate specificities, pH and temperature responding curves in vitro
[25]. However, little is known about which forms is responsible for RFOs hydrolysis when they are unloaded into cucumber fruits.
The vascular systems, consisting of xylem and phloem, play key roles in the translocation of solutes
[9,26,27][9][26][27]. Xylem and phloem play partly different roles: xylem mainly transports water and solute minerals, and phloem mainly transports photosynthetic products from source to sink organs
[26,27][26][27]. Different from many other plants, phloem tissues in cucumber include two systems: fascicular phloem and extrafascicular phloem
[28,29,30][28][29][30]. For unloading RFOs in fruits, previous work indicated that Sta may be quickly broken down to Suc by a-Gals and subsequently hydrolysed to hexose after reaching the vascular bundles of fruits
[3,9][3][9]. In addition, there are higher Raf content and lower Sta content in stalk than pedicel
[9]. The distinct distribution of Sta and Raf indicated the hydrolysis of Sta may differ from Raf hydrolysis.
2. The Distribution Pattern of Soluble Sugars
To investigate the location of RFOs hydrolysis, we examined the distribution of soluble sugar (Sta, Raf, Suc, Glu and Fru) in cucumber fruits and selected the vascular and non-vascular tissues of pedicel (PE), stalk (ST), basal part (BF), middle part (MF) and top part (TF) of fruits for further analysis (
Figure 1A,B).
Figure 1. High accumulation of soluble sugar in vascular tissue of fruits. (A,B) The schematic diagram shows the sampling location in fruit. Vascular tissue and non-vascular tissue of pedicel (PE), stalk (ST), basal part (BF), middle part (MF) and top part (TF) of fruit were used for the analysis of soluble sugar content. The red circles show the vascular bundle; the white circles show the mesocarp. (C–G) The stachyose (Sta) (C), raffinose (Raf) (D), sucrose (Suc) (E), glucose (Glu) (F) and fructose (Fru) (G) content in different tissue of fruit were analysed by HPLC. The bars represent standard deviation (SD) of five biological replicates. Bars marked with different letters denote significant differences (p < 0.05) from Student’s t-test.
The content of Sta in non-vascular tissues was highest in pedicel and gradually decreased from BF to TF; while in vascular tissues, Sta content was higher in PE, ST, and BF (
Figure 1C). In non-vascular tissues, the Raf content was gradually decreased from PE to TF; while in vascular tissues, Raf content was high in ST and TF, but low in PE, BF and MF (
Figure 1D). The content of Suc, Glu and Fru was gradually increased from PE to TF in both vascular and non-vascular tissues (
Figure 1E–G). Taken together, RFOs decreased from PE to TF while the content of Suc, Glu and Fru was increased from PE to TF (
Figure 1C–G). In addition, RFO content was significantly higher in vascular tissues than non-vascular tissues (
Figure 1C–G). To verify whether the distribution of soluble sugars is conserved in
Cucumis plants, we selected a fresh-eating cucumber cultivar “83-16” (North European greenhouse ecotype), a Vietnamese melon (named melon below), and a non-sweet melon cultivar, for further analysis. The RFOs content was gradually decreased from PE to TF, while Glu, Fru contents were gradually increased from PE to TF in vascular tissues of “83-16” and melon. In addition, RFOs content in vascular tissues of melon fruits was significantly higher than in mesocarp, which is consistent with the trend in cucumber (
Figure 1D). Those results indicated that RFOs hydrolysis could occur mainly in vascular tissues and is conserved in
Cucumis plants including cucumber.
3. The Distribution of Soluble Sugars in Phloem Sap
To further verify whether vascular tissues are the main location of RFOs hydrolysis, we selected the fascicular phloem sap of PE, ST, BF, MF and TF for soluble sugar measurement (
Figure 2A).
Figure 2. The distribution of soluble sugar in phloem sap of fruits. (A) The schematic diagram shows the collected sites of phloem sap. (B–F) The Sta (B), Raf (C), Suc (D), Glu (E) and Fru (F) content of phloem sap in pedicel (PE), stalk (ST), basal part(BF), middle part(MF) and top part(TF) of fruit. The bars represent standard deviation (SD) of five biological replicates. Bars marked with different letters denote significant differences (p < 0.05) from Student’s t-test.
Sta content of phloem sap was highest and up to 5 mg/mL in PE and ST; Sta content was gradually reduced from BF to TF (
Figure 2B). Similar with Sta, Raf content of phloem sap was highest in PE, ST and gradually reduced from BF to TF (
Figure 2C). It was odd that Suc content was lowest in ST and only about 0.2 mg/mL (
Figure 2D). In contrast with Sta and Raf, the content of Suc, Glu and Fru in phloem sap was low in PE, ST and gradually increased from BF to TF (
Figure 2D–F).
We also analysed soluble sugar content in “83-16” and melon. In “83-16”, Sta and Raf contents were highest in S, PE and reduced from ST to TF; the content of Glu and Fru was low in S, PE and gradually increased from ST to TF. In melon, Sta content was high in PE, ST and was sharply reduced in MF and TF; Raf content was gradually decreased from PE to TF. Suc content was low in PE, ST and was high in MF, TF; Glu content was similar in PE, ST, MF and TF; Fru content was lowest in PE. Those results showed that RFO hydrolysis in phloem sap mainly occurred in the pedicel and stalk and displayed a similar trend in cucumber and melon.
4. The Expression Pattern of α-Gals in Fruits
In watermelon, a previous study showed that
α-Gals play key roles in RFO hydrolysis and are highly expressed in vascular bundles
[5]. So, the high expression of
α-Gals in vascular tissues probably contributes to the hydrolysis of RFOs in cucumber. Our previous study showed that there are six α-Gals, three acid forms (
CsGAL1,
CsGAL2 and
CsGAL3) and three alkaline forms (
CsAGA1,
CsAGA2,
CsAGA3) in cucumber
[25]. To verify which α-Gals is involved in RFO hydrolysis in cucumber, we analysed their expression in fruits. We selected the vascular tissues and non-vascular tissues of ST, BF, MF and TF for analysis. By qRT-PCR, we found that
CsAGAs were highly expressed in vascular tissues:
CsAGA1 and
CsAGA3 were highly expressed in TF; the expression of
CsAGA2 was gradually decreased from ST to TF (
Figure 3A–C). Different from
CsAGAs,
CsGALs did not show the preferred expression in vascular tissues (
Figure 3D–F). Notably
CsAGA2 expression displayed a similar trend with Sta distribution in fruits. To visualize the spatial expression pattern of alkaline forms α-Gals (
CsGAL1,
CsGAL2,
CsGAL3) in vascular tissues, we conducted in situ hybridizations. As shown in
Figure 3G–I, there were clear hybridization signals in EP (external phloem) and IP (internal phloem), while no obvious signal in mesocarp cells using the anti-sense probe. Among them, in situ hybridization signal of
CsAGA2 was stronger than
CsAGA1 and
CsAGA3 (
Figure 3G–I). In contrast to the anti-sense probe, there were no obvious signals, either in phloem or in mesocarp (
Figure 3G–I). We further compared the α-galactosidase activity of acid forms and alkaline forms in ST and BF. In ST and BF, α-Gals activity of alkaline forms was higher than acid forms when Sta or Raf acted as the substrate (
Figure 3J,K). Those combined results implied three alkaline forms α-Gals, but not acid forms α-Gals, might be involved in RFOs hydrolysis in vascular bundles.
Figure 3. The expression of α-galactosidase genes in fruit. (A–C) Relative expression showed that α-galactosidase genes of alkaline forms (CsAGA1 (A), CsAGA2 (B) and CsAGA1 (C)) were preferred expressed in phloem tissue. (D,E) Relative expression showed that α-galactosidase genes of acid forms (CsGAL1 (D), CsGAL2 (E) and CsGAL3 (F)) did not show the prefer expression in phloem tissue by qRT-PCR. The bars represent standard deviation (SD) of three biological replicates. 18S rRNA acted as reference gene and relative amounts were normalized with respect to the expression in vascular of stalk. (G–I) In situ hybridizations showed that α-galactosidase genes of alkaline forms (CsAGA1 (G), CsAGA2 (H) and CsAGA3 (I)) were prefer expressed in phloem. The anti-sense probe was used for detecting the expression of α-galactosidase genes of alkaline forms (right). The sense probe was used for in situ hybridization in fruit as a negative control (left). Vascular bundles were labelled, with the IP (internal phloem) marked with white arrows and EP (external phloem) marked with red arrows. Bars = 100 μm. (J,K) Alpha-galactosidase activity of alkaline forms was higher than acid forms when Sta (J) or Raf (K) acted as the substrate. Alpha-galactosidase activities in stalk (ST) and basal part (BF) of fruit were measured.
With the similar soluble sugar distribution in the fruit of cucumber, “83-16” and melon, the expression pattern of
α-Gals may also be similar. To verify it, we characterized the expression of
α-Gals in “83-16” and melon by qRT-PCR. In “83-16”,
CsAGA2 was highly expressed in vascular tissues, but other five
α-Gals did not show the vascular preferred expression. Similar with cucumber, three alkaline forms
α-Gals were highly expressed in vascular tissues, while acid forms
α-Gals did not display the vascular preferred expression in melon. The high expression level of alkaline forms
α-Gals, especially
CsAGA2 in vascular tissues implies their functional involvement in RFOs hydrolysis, so we chose CsAGA2 for further functional verification.
5. CsAGA2 Mediates Stachyose Hydrolysis
Our previous works showed that
CsAGAs are universally expressed in different cucumber organs, and
α-Gal genes may regulate other physiological processes including RFOs hydrolyzation
[25]. To avoid potential artifacts caused by constitutive silencing, we silenced
CsAGAs expression using an β-estradiol inducible expression system. As the content of RFOs is high in pedicel, we treated the pedicel with 150 μM β-estradiol once a day.
After β-estradiol treatment, we characterized
CsAGA2 expression by qRT-PCR. The result showed that
CsAGA2 expression was significantly down-regulated in the pedicel of RNAi-
CsAGA2 plants after β-estradiol treatment (
Figure 4B). To exclude the potential nonspecific inhibition of
CsAGA1 and
CsAGA3 expression, we also examined the expression of both
CsAGA1 and
CsAGA3, and found no significant difference in RNAi-
CsAGA2 plants with β-estradiol treatment compared with wild type or RNAi-
CsAGA2 plants without β-estradiol treatment (
Figure 4A,C). Those results showed that β-estradiol treatment could specifically silence the expression of
CsAGA2 in RNAi-
CsAGA2 plants.
Figure 4. CsAGA2 mainly regulated stachyose hydrolysis but not Raf. (A–C) qRT-PCR analysis of the expression of CsAGA1 (A), CsAGA2 (B) and CsAGA3 (C) in RNAi-CsAGA2 plants with or without β-estradiol treatment. The expression of CsAGA2, but not CsAGA1 (A) and CsAGA3 (C), was down regulated in RNAi-CsAGA2 plants with 150 μM β-estradiol treatment but not regulated in RNAi-CsAGA2 plants without β-estradiol treatment (B). Three lines of RNAi-CsAGA2 were chosen for qRT-PCR analysis. The bars represent standard deviation (SD) of three biological replicates. 18S rRNA act as reference gene and relative amounts were normalized with respect to the expression in WT. (D,E,I) The fruit length (D,I) and fruit diameter (E,I) were decreased in RNAi-CsAGA2 plants treated with β-estradiol compared with WT with β-estradiol treatment and RNAi-CsAGA2 plants without β-estradiol treatment. The fruits at 1DPA (day post-anthesis), 2DPA, 3DPA, 4DPA, 5DPA, 6DPA, 7DPA and 8DPA stages were selected for measurement. The bars represent standard deviation (SD) of fifteen biological replicates. (F,G) The fruit fresh weight (F) and dry weight (G) were reduced in RNAi-CsAGA2 plants treated with β-estradiol compared with WT with β-estradiol treatment and RNAi-CsAGA2 plants without β-estradiol treatment. The fruit at 7DPA stage were selected for measurement. The bars represent standard deviation (SD) of 15 biological replicates. Bars marked with different letters denote significant differences (p < 0.05) from Student’s t-test. (H) The analysis of soluble sugar content in RNAi-CsAGA2 plants with or without β-estradiol treatment. Soluble sugar of phloem sap was collected for measurement. Sta, Raf, Suc, Glu and Fru were measured. The bars represent standard deviation (SD) of fifteen biological replicates. Bars annotated with asterisks are significantly different according to Fisher’s least significant difference test after ANOVA (**, p < 0.01). (I) The fruit phenotype comparison of RNAi-CsAGA2 plants with or without β-estradiol treatment. Bar = 1 cm.
After β-estradiol treatment, fruit development was obviously blocked in RNAi-
CsAGA2 plants (
Figure 4I). To evaluate the fruit morphology in RNAi-
CsAGA2, we quantified the fruit length and diameter from 1DPA (day post-anthesis) stage to 8DPA stage. The fruit length was significantly reduced, while fruit diameter had no significant change in RNAi-
CsAGA2 plants with β-estradiol treatment compared with wild type treated with β-estradiol, or RNAi-
CsAGA2 plants without β-estradiol treatment (
Figure 4D,E). Furthermore, we characterized the fresh weight and dry weight of fruits. At 8DPA stage, the fresh weight and dry weight of fruits were significantly decreased in RNAi-
CsAGA2 plants with β-estradiol treatment compared with wild type treated with β-estradiol or RNAi-
CsAGA2 plants without 0 μM β-estradiol treatment (
Figure 4F,G).
As
CsAGA2 is highly expressed in vascular tissues where RFO hydrolysis mainly occurs, we characterized the soluble sugar contents of phloem sap. As expected, Sta content was higher, while Suc, Glu and Fru contents were lower in RNAi-
CsAGA2 plants treated with β-estradiol than without β-estradiol treatment (
Figure 4H). Interestingly, Raf content was decreased in RNAi-
CsAGA2 after β-estradiol treatment (
Figure 4H). Besides, the non-sweet motif (CTTAGGTTGGTGTTAGTG) also existed in the
CsAGA2 promoter
[5]. Those results indicated that
CsAGA2 was the main gene contributing to Sta hydrolysis, and the other two alkaline forms α-Gals may play key roles in Raf hydrolysis.
5. CsAGA1 Mediates Both Stachyose and Raffinose Hydrolysis
To verify the role of CsAGA1 and CsAGA3 in Raf hydrolysis, we generated the RNAi-CsAGA1 and RNAi-CsAGA3 plants using the similar strategy as RNAi-CsAGA2. In RNAi-CsAGA1 plants, the expression of CsAGA1, but not CsAGA2 and CsAGA3, was specifically suppressed after β -estradiol treatment (Figure 5A–C). Similarly, the fruit development was obviously blocked in RNAi-CsAGA1 plants after β -estradiol treatment (Figure 5I). Further more, we quantified the fruit length and diameter. The fruit length was decreased and fruit diameter had no significant change in RNAi-CsAGA1 plants after β -estradiol treatment (Figure 5D,E). The fruit fresh weight and dry weight were reduced in RNAi-CsAGA1 plants after β -estradiol treatment (Figure 5F,G). To characterize the effect of CsAGA1 in RFOs hydrolysis, we compared the soluble sugar content in RNAi-CsAGA1 plants treated with or without β -estradiol. Sta and Raf contents were higher in RNAi-CsAGA1 plants treated with β -estradiol than in those without β -estradiol (Figure 5H). Suc, Glu and Fru contents were reduced in RNAi-CsAGA1 plants after β -estradiol treatment (Figure 5H). Those results indicated that CsAGA1 played an essential role in RFOs (especially Raf) hydrolysis. Similarly, we characterized the phenotype of RNAi-CsAGA3 plants. In RNAi-CsAGA3 plants, the expression of CsAGA3, but not CsAGA1 and CsAGA2, was suppressed after β -estradiol treatment (Figure 6A–C). The fruit length and diameter had no significant difference in RNAi-CsAGA3 plants after β -estradiol treatment (Figure 5D,E). The fresh weight, dry weight and soluble sugar contents had no significant difference in RNAi- CsAGA3 plants after β-estradiol treatment (Figure 5F–H).
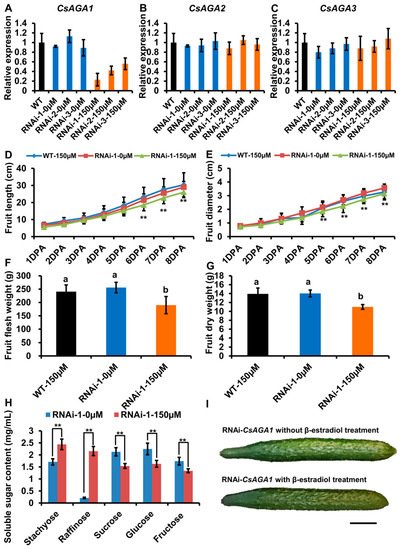
Figure 5. CsAGA1 mainly regulates Raf hydrolysis but not Sta. ( A – C ) qRT-PCR analysis of the expression of CsAGA1 ( A ), CsAGA2 ( B ) and CsAGA3 ( C ) gene in RNAi (RNA interfere)-CsAGA1 plants with or without β -estradiol treatment. The expression of CsAGA1 was down regulated in RNAi-CsAGA1 plants with β -estradiol treatment, but not in RNAi-CsAGA1 plants without β -estradiol treatment ( A ). The expression of CsAGA2 ( B ) and CsAGA3 ( C ) were not regulated in RNAi-CsAGA1 plants after β -estradiol treatment. Three lines of RNAi-CsAGA2 were chosen for qRT-PCR analysis. The bars represent standard deviation (SD) of three biological replicates. 18S rRNA act as reference gene and relative amounts were normalized with respect to the expression in WT. (D,E,I) The fruit length (D,I) and fruit diameter (E,I) were decreased in RNAi-CsAGA1 plants treated with β -estradiol compared with WT treated 150 µ M β -estradiol, RNAi-CsAGA1 plants without β -estradiol treatment. The fruit at 1DPA (day post anthesis), 2DPA, 3DPA, 4DPA, 5DPA, 6DPA, 7DPA and 8DPA stages were selected for measurement. The bars represent standard deviation (SD) of fifteen biological replicates. ( F , G ) The fruit fresh weight ( F ) and dry weight ( G ) were reduced in RNAi-CsAGA1 plants treated with β -estradiol compared with WT treated with β -estradiol, RNAi-CsAGA1 plants without β -estradiol treatment. The fruits at 7DPA stage were selected for measurement. The bars represent standard deviation (SD) of fifteen biological replicates. Bars marked with different letters denote significant differences (p < 0.05) from Student’s t-test. ( H ) The analysis of soluble sugar contents in RNAi-CsAGA1 plants with or without β -estradiol treatment. Soluble sugar contents of phloem sap were collected for measurement. Sta, Raf, Suc, Glu and Fru contents were measured. The bars represent standard deviation (SD) of fifteen biological replicates.Bars annotated with asterisks are significantly different according to Fisher’s least significant difference test after ANOVA (**, p < 0.01). ( G ) The fruit phenotype comparison of RNAi-CsAGA1 plants with or without β-estradiol treatment. Bar = 1 cm.






