Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Binghua Wu and Version 2 by Nora Tang.
Volatile benzenoid compounds are found in diverse aromatic bouquets emitted by most moth-pollinated flowers. The night-blooming Jasminum sambac is widely cultivated worldwide in the tropics and subtropics for ornamental and industrial purposes owing to its fragrant flowers.
- Jasminum sambac
- BAHD acyltransferases family
- benzyl alcohol O-acetyltransferase (BEAT)
1. Introduction
Plants utilize a wide array of specialized metabolites to interact with changing environments and to safeguard their survival and reproduction [1][2][3][1,2,3]. These structurally different molecules produced by plants are usually classified based on their biosynthetic origin, such as terpenoids, benzenoids/phenylpropanoids, fatty acid derivatives and amino acid derivatives. Large portions of the plant-specialized metabolites are lipophilic small molecules with high vapor pressure at ambient temperatures, and thus called volatile organic compounds (VOCs). More than 1700 VOCs have been identified from 90 different plant families, and mostly are synthesized and released, with high abundance and diversity, from the flowers in many flowering plants [4]. Floral VOCs play important roles in the attraction of pollinators and protection against pathogens, parasites, and herbivores [5][6][7][5,6,7].
Flowers of the night-blooming plant Jasminum sambac (L.) Aiton (Oleacese) emit a strong fragrance which has long been used as raw material in the perfume industry and in scented tea. While jasmine absolute is the common name of the aroma product made by distillation from subsequent hexane and ethanol extractions of the flowers, the VOCs are enriched in benzyl acetate, linalool and (E,E)-α-Farnesene [8]. Floral VOCs in several Jasminum species have also been reported [9][10][11][12][13][14][9,10,11,12,13,14]. Although variation in the composition of the floral scent exists depending on the species/varieties, cultivation practices, and season, benzyl acetate is the predominant benzenoid ester, accounting for almost 90% of the volatile benzenoid/phenylpropanoids. However, the biosynthetic enzymes and the encoding genes are rarely explored in this genus.
Phytochemically, most floral benzenoid esters are produced by acyl-CoA-dependent acylation performed by a group of acyltransferases, the Benzylalcohol O-acetyl-, Anthocyanin O-hydroxycinnamoyl-, Hydroxycinnamoyl/benzoyl-CoA: Anthranilate N-hydroxycinnamoyl/benzoyl-, and Deacetylvindoline 4-O- acetyltransferases (BAHD) superfamily [15][16][17][18][15,16,17,18]. These enzymes catalyze the transfer of an acyl group from activated acyl donor molecules, Coenzyme A or CoA thioesters, to acyl acceptor molecules, oxygen- and nitrogen-containing substrates, producing esters and amides, respectively [19]. A benzyl–alcohol acetyl-CoA acetyltransferase (BEAT) from Clarkia breweri involved in floral VOC synthesis was first identified and cloned in 1998 [17]. Since then, more than 69 biochemically characterized BAHD acyltransferases have been reported, and they share two conserved motifs, HXXXD and DFGWG, but with low overall sequence identity at the amino acid level of 25–34% [19][20][19,20]. Phylogenetically, the plant BAHD family could be divided into five clades [15] or further refined to eight clades [21]. The versatile reactions catalyzed by these divergent enzymes make it difficult to predict their function solely based on the encoding sequences.
2. Two BAHD Acetyltransferases in Jasminum sambac (L.) Aiton
After blooming at night, J. sambac flowers produce and emit various volatile benzenoid compounds among others, with benzylacetate occupying ~one-third to one-half of headspace volatiles and ~10 to 30% in the extracts (Table 1) [8][9][14][8,9,14]. Two highly expressed JsBEAT1 and JsBEAT2 were full-length cDNA cloned, and the encoded proteins could convert benzyl–alcohol to benzylacetate by using acetyl-CoA as the acyl donor in vitro. In addition, JsBEAT4 and 5 were also moderately expressed (having a transcript abundance of ~one-seventh of JsBEAT1 and 2). Since we did not characterize these two genes, it could not be excluded that these two might also contribute to the formation of benzylacetate in the J. sambac flowers. Although not tested, both JsBEAT1 and JsBEAT2 may also be capable of making other minor acetylated scent compounds found in the J. sambac flowers, in addition to benzylacetate. The question remains as to what extent may individual JsBEAT contribute to the floral benzenoids production, which requires further experiments using gene knockout or other approaches.
Table 1. Major floral volatile benzenoids/phenylpropanoids in J. sambac petal extracts post anthesis.
| Time | 3-Hexenal | Concentration (mg g−1 FW) * | Benzoic Acid-2-Propenyl Ester | ||||
|---|---|---|---|---|---|---|---|
| Benzyl Alcohol |
Benzyl Acetate |
Methyl Anthranilate | cis-3-Hexenyl Benzoate | Benzyl Benzoate | |||
| 20:00 | 16.5 ± 1.5 a | 41.3 ± 2.3 c | 34.2 ± 1.7 e | 10.7 ± 0.6 f | 140.8 ± 1.3 d | 14.3 ± 0.2 e | 50.4 ± 8.4 c |
| 23:00 | 15.3 ± 0.9 a | 48.3 ± 3.5 b | 199.4 ± 11.3 b | 50.1 ± 3.1 d | 208.9 ± 13.3 a | 23.9 ± 1.0 c | 69.0 ± 7.04 b |
| 02:00 | 14.2 ± 1.1 a | 48.3 ± 3.2 b | 227.2 ± 10.0 a | 87.2 ± 1. 7 b | 228.2 ± 7.8 a | 25.2 ± 0.62 b | 77.6 ± 2.5 a |
| 05:00 | 10.9 ± 0.6 c | 55.4 ± 3.4 a | 148.8 ± 5.9 c | 95. 4 ± 3.4 a | 170.2 ± 8.2 b | 29.7 ± 1.1 a | 80.9 ± 2.0 a |
| 08:00 | 11.1 ± 0.7 bc | 52.6 ± 1.2 a | 75.9 ± 1.3 d | 62.9 ± 2.9 c | 134.8 ± 2.5 c | 19.8 ± 0.6 d | 42. 5± 0.6 d |
| 11:00 | 12.5 ± 1.1 b | 42.8 ± 0.3 c | 33.4 ± 0. 8 ef | 47.9 ± 1.1 d | 68.7 ± 2.1 d | 10.1 ± 0.2 fg | 6.5 ± 0.2 e |
| 14:00 | 7.7 ± 1.3 d | 15.2 ± 1.2 d | 27.8 ± 0.5 fg | 26.6 ± 1.2 e | 44.5 ± 3.6 e | 8.9 ± 0.8 g | 8.2 ± 1.0 e |
| 17:00 | 2.3 ± 0.4 e | 9.0 ± 0.2 e | 24.1 ± 0.8 g | 23.1 ± 1.7 e | 13.8 ± 0.7 f | 11.2 ± 1.1 f | 7.5 ± 0.8 e |
* Relative to an internal standard isobutylbenzene. Means ± SD followed by same letter indicates no significant difference within each column (p < 0.05, n = 3).
At the amino acid level, JsBEAT1 shares a 48.86, 28.24, and 25.89% identity with JsBEAT4, JsBEAT2, and JsBEAT5, respectively, while JsBEAT2 is 27.40 to 32.22% identical to JsBEAT4 and JsBEAT5. Phylogenetically, the four petal-expressing acyltransferases are grouped into the clade V of the BAHD family. This clade includes some previously described members such as the benzyl alcohol/phenylethanol benzoyl-CoA benzoyltransferases (BPBT) from P. hybrida and C. breweri flowers and leaves, which are responsible for volatile benzenoids production via the β-oxidative benylbenzoate synthetic pathway [22][23][23,24]. Another example comes from the Concord grape; the VlAMAT catalyzes the formation of methyl anthranilate from anthraniloyl-CoA and methanol in the berry [24][31]. Additionally, other reported BAHD-clade V acyltransferases catalyze the formation of O- or N-acylation on diverse substrates with different acyl-CoA donors, and may be involved in defense against herbivores, UV-protection, and pathogen resistance, as well as in cell-wall biosynthesis [15][19][15,19]. The two JsBEAT could equally enhance the floral benzenoid volatile production, especially the accumulation of benzylbenzoate and benzylacetate (Figure 16) in the transgenic P. hybrida plants, suggesting that they may be functionally redundant in the J. sambac flowers. Or else they may possess a subtle, not-yet-determined catalytic preference depending on the substrate availability and subcellular compartmentalization. Indeed, the subcellular localizations of both JsBEAT1 and 2 in transgenic P. hybrida were found unexpectedly in granule cytosolic structures, other than in the bulk cytoplasm [19] as seen in Jasminum petal protoplasts. The nature of the observed cellular granule structures is not known; however, peroxisomes or mitochondria or membrane vesicles are considered as possible candidates, as such the two enzymes may be recruited by specific unknown proteins from the heterologous P. hybrida flower tissue. These possibilities require further investigation.
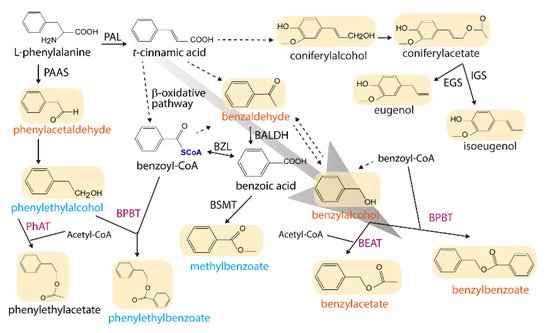
Figure 1. A simplified metabolic network for major floral volatile benzenoid/phenylpropanoid compounds in P. hybrida W115, depicting the effects of ectopic expression of the two JsBEATs. The volatile compounds are shades and the names in tangerine orange, cyan blue, and black indicate the compound is increased, decreased and no change or not detected, respectively. Solid arrow indicates a described conversion while a broken arrow denotes multiple steps and/or uncharacterized reactions. Enzyme abbreviations: BALDH, benzaldehyde dehydrogenase; BEAT, benzylalcohol acetyl-CoA acetyltransferase; BPBT, benzylalcohol/phenylethanol benzoyl-CoA benzoyltransferase; BSMT, benzoic acid/salicylic acid carboxyl methyltransferase; BZL, benzoate:CoA ligase; EGS, eugenol synthase; IGS, isoeugenol synthase; PAAS, phenylacetaldehyde synthase; PAL, phenylalanine ammonia lyase; PhAT, phenylalcohol acetyl-CoA acetyltransferase. Enzymes in violet are members of the BAHD acyltransferase family. The background gray arrow highlights the volatile flux driven by the overexpression.
The apparent Km for both acyl donor (acetyl-CoA, ranging from 317.3 to 546.0 μM) and acyl acceptor (benzyl alcohol, ranging from 278.7 to 447.3 μM) are rather high in our enzymatic assay using GST-tagged or 10 × His-tagged proteins, as compared to the canonical CbBEAT, CbBEBT and CbCHAT [22][25][23,32], indicating that both acetyl-CoA and benzyl alcohol may not be the preferred substrates for the two JsBEATs. The apparent Km for a preferred substrate in many plant BAHDs is reported ranging from several to two hundred μM ([26][27][33,34]). However, some BAHDs may exhibit higher Km (>300 μM for the preferred substrates) [28][35]. Thus, it will be necessary to further test the enzymatic activity of the two JsBEAT using different donor or acceptor substrates to determine the preferent substrates.
In summary, two highly expressed BAHD acyltransferases, capable of acylating benzylalcohol and other alcohol compounds, are likely responsible for the volatile benzenoid production in the nigh blooming fragrant flowers of J. sambac, with somewhat functional redundance.
