Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Juan José Ramos-Rodríguez and Version 2 by Yvaine Wei.
Alzheimer’s disease (AD) is the most common cause of dementia. It is characterized by cognitive decline and progressive memory loss. Currently, the amyloid cascade hypothesis remains the leading theory in the pathophysiology of AD. This hypothesis states that amyloid-β (Aβ) deposition triggers a chemical cascade of events leading to the development of AD dementia.
- Alzheimer’s disease
- senile plaques
- tau protein
- diagnosis
- biomarker
- treatment
- acetylcholinesterase inhibitors
- immunotherapy
1. Introduction
Alzheimer’s disease (AD) is the leading cause of dementia in addition to being the most common neurodegenerative disease in the developed countries [1]. Anatomically, it is characterized by cerebral atrophy that especially affects the hippocampus and the entorhinal cortex, causing progressive memory loss and inability to carry out daily activities [2].
AD has been known since 1906 when psychiatrist and neurologist Alois Alzheimer described the findings after an autopsy performed on a 51-year-old patient after following the case of Auguste Deter who suffered from memory loss, inability to speak properly, disorientation and hallucinations between 1901 and 1906. Alois Alzheimer observed in the brain autopsy of this patient the presence of extraneuronal senile plaques (SP) and intraneuronal neurofibrillary tangles (NFTs), all accompanied by severe brain atrophy. These findings led him to believe that this was not a previously known dementia [3].
The prevalence of AD depends directly on the age range studied, showing a higher prevalence, for all ages, in women (5.1%) than in men (3.8%) [4]. Epidemiological studies indicate that prevalence increases sharply from the age of 65 [5][6][7][8,9,10], as shown in Figure 1.
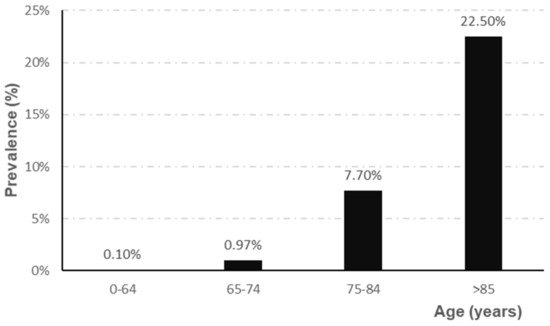

Figure 1. Evolution of the prevalence of AD according to age range (adapted from Garre-Olmo et al. [7]).
2. Etiology of Alzheimer’s Disease
2.1. Etiology of Alzheimer’s Disease
AD is a disease of unknown cause. However, it is believed that its etiology may be multifactorial, where risk factors play an important role. There is a small percentage of cases of AD of genetic origin, which constitutes less than 3% of all AD subtypes and has been called familial AD. Familial AD is characterized by earlier development (about 10–12 years earlier) compared to forms of idiopathic AD, known as sporadic AD [8][13]. Familial AD shows a dominant inheritance pattern [9][14]. The main mutations responsible for familial AD are in the APP gene, as well as in the proteolytic enzymes that generate the peptides Aβ, presenilin 1 and 2. There are other mutations that can increase the risk of developing this dementia. These include mutations in the APOE gene, where the APOE4 variant predisposes to this dementia [10][15].
In addition to genetic factors, there are other factors that may contribute to the development of AD, such as high blood pressure [11][12][16,17], overweight and hypercholesterolemia [13][14][18,19], sedentary lifestyle, tobacco use, low level of education [15][20], diabetes mellitus or hyperinsulinemia [16][17][18][21,22,23]. All these factors together are involved in the development of 33.3% of AD cases [15][20].
3. Clinical Stages of Alzheimer’s Disease
2.2. Clinical Stages of Alzheimer’s Disease
The global and functional clinical stages of AD are summarized as follows. There is an initial phase, commonly known as the prodromal phase, whose main characteristic is a mild cognitive impairment, i.e., with subtle memory loss, which often goes unnoticed [19][24]. As the disease progresses, different cortical functions are altered, causing difficulties in the development of basic activities of daily living [20][25]. When the disease reaches the final stages, patients with AD become totally dependent, compromising their lives and causing significant changes for the relatives, who become caregivers [21][26].
However, there is a long transition period between the appearance of alterations at the brain level and the presentation of the first clinical symptoms [19][24]. The severity of AD according to its clinical symptomatology can be classified as mild, moderate or severe. As the disease worsens, so do the symptoms, which can range from mild cognitive impairment, increased memory loss, personality variations, problems in carrying out everyday tasks to confusion, psychomotor difficulty, loss of speech and ultimately death of the patient [2].
4. Neuropathological Features
2.3. Neuropathological Features
Currently, AD has no accurate diagnosis. Its definite diagnosis is still limited to post-mortem examination of brain tissue. In order to diagnose this pathology, its three defining characteristics must be present [22][27]: amyloid-β pathology, tau pathology and neuroinflammation, neuronal death and brain atrophy. Currently, the amyloid cascade hypothesis continues to be the explanatory model for describing the onset and histopathological evolution of AD. This hypothesis states that amyloid-β (Aβ) deposition in the brain parenchyma triggers a chemical cascade of events leading to the development of AD dementia.
4.1. Amyloid-β Pathology (Aβ Pathology)
2.3.1. Amyloid-β Pathology (Aβ Pathology)
SP are characteristic alterations of AD very frequent in the brain of patients with dementia and possibly the origin of denervation of the disease.
SP are the result of the progressive accumulation of parenchymal Aβ [3]. Aβ is a peptide of between 39–43 amino acids derived from progressive processing of the Aβ precursor protein (APP) by the β- and γ-secretase complexes, where presenilins would be the catalytic component [23][28]. APP is one of the proteins found in greater proportion in the central nervous system [24][29]. Aβ is derived from the proteolytic breakdown of the APP protein which, when processed by the β- and γ-secretases, results in three products, among which is the Aβ which promotes the formation of SP (Figure 2).
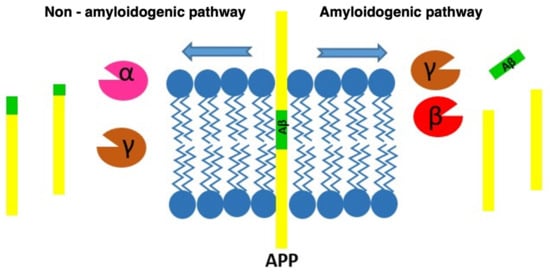

Figure 2. Diagram of the processing routes by which the APP can be degraded, showing how the β-amyloid peptide is produced.
4.2. Tau Pathology
2.3.2. Tau Pathology
NFTs are intraneuronal deposits mainly composed of hyperphosphorylated tau protein which form filamentous aggregates in neuronal somas and proximal dendrites [25][26][58,59].
The tau protein is mostly found in the central nervous system and in the peripheral nervous system. It is a small protein that is attached to microtubules, offering stability when interacting with them (Figure 3). Tau is a protein found abundantly in the axons where microtubules predominate. These microtubules play an important role in the structure of neurons, axon transport and synaptic plasticity of neurons [24][29].
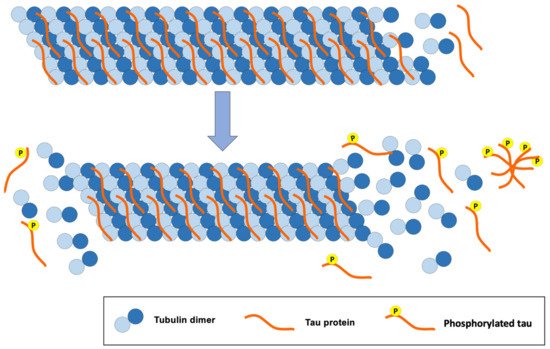

Figure 3. Consequences of tau protein hyperphosphorylation for the structure of tubulin microtubules, a classic pathology of AD.
The structure of microtubules is modified when inadequate phosphorylation of the tau protein occurs. Likewise, hyperphosphorylation of this protein disrupts the synapses between neurons, causing cellular alterations that lead to the loss of synapses, neuronal ramifications and neuronal death [24][29].
5. Diagnosis of AD
2.4. Diagnosis of AD
New diagnostic methods are currently gaining prominence, including fluid and image-based biomarkers which have been incorporated in diagnostic criteria and recommendations for AD [27][28][75,76]. The main fluid-based biomarkers are those present in cerebrospinal fluid (CSF) and plasma [29][77]. In CSF, the molecular markers that have been shown to be most clinically useful are Aβ42 and phosphorylated tau. Thus, it has been observed that as humans age, Aβ42 levels decrease and phosphorylated tau levels increase, reflecting possible deposition in the brain leading to SP and the formation of NFTs, respectively [30][78]. Although this is a widely accepted diagnostic method, sampling requires lumbar puncture which is a relatively invasive procedure, justifying the search for other less aggressive methods such as plasma biomarkers [31][79].6. Alzheimer’s Disease Treatment and Management
2.5. Alzheimer’s Disease Treatment and Management
6.1. Currently Available Pharmacological Treatment
2.5.1. Currently Available Pharmacological Treatment
A curative treatment of AD remains to be discovered. Currently, the therapeutic armory available focuses on symptom control in milder cases of the disease [32][33][34][94,95,96] (Figure 4).
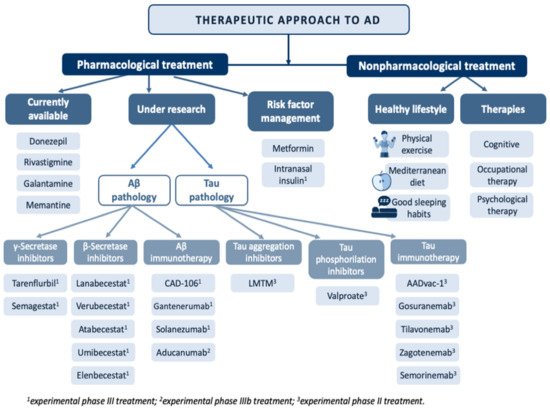

Figure 4.
Overview of the main treatments in the therapeutic approach to Alzheimer’s disease.
Among the pharmacological alternatives available, acetylcholinesterase inhibitors (AChEIs) stand out [35][97]. The mechanism of action of AChEIs is based on the inhibition of acetylcholinesterase (AChE) activity, which degrades acetylcholine (ACh), increasing its availability, which is reduced in AD.
6.2. Pharmacological Treatment under Investigation
2.5.2. Pharmacological Treatment under Investigation
Treatments affecting Aβ pathology focus on three therapeutic targets. The first is aimed at reducing the overproduction of Aβ42 through γ-secretase inhibitors, β-secretase inhibitors or α-secretase enhancers. The second therapeutic target focuses on reducing the Aβ load in SPs by using aggregation inhibitors or drugs that interact with the metals that are deposited on them. The third therapeutic target is aimed at boosting Aβ clearance by active or passive immunotherapy [36][104].6.3. Risk Factor Management
2.5.3. Risk Factor Management
AD is a multifactorial pathology where risk factors play an important role in the pathophysiology of the disease. One of the most important is type 2 diabetes mellitus as insulin resistance in the central nervous system has been observed in AD patients. Therefore, increasing the availability or sensitivity to insulin at the brain level could be a possible therapeutic alternative for the management of AD patients [37][116]. In fact, administration of oral antidiabetic drugs, and more specifically of metformin, has been shown to have a neuroprotective, anti-inflammatory and antioxidant action [38][117]. In addition, one of the therapeutic approaches under study is the administration of intranasal insulin [39][118]. In addition to diabetes, some cardiovascular events such as stroke, hypertension, hypercholesterolemia, heart failure or atrial fibrillation and other risk factors such as high homocysteine or smoking have been associated with the development of AD [40][41][42][43][44][45][119,120,121,122,123,124]. Thus, the design of preventive strategies that allow early diagnosis and treatment of cardiovascular risk factors could contribute significantly to the prevention of AD in the elderly.
6.4. Nonpharmacological Treatment
2.5.4. Nonpharmacological Treatment
In addition to all the treatments mentioned so far, in the therapeutic approach to AD, it is worth highlighting the role of different health professionals such as occupational therapists and psychologists. Occupational therapy represents an important part of the treatment as it increases the autonomy of these patients, allowing the development of activities of daily living through cognitive and behavioral exercises [46][125]. Psychological therapy is fundamental in AD patients as it provides them with the necessary tools to deal with processes such as depression or anxiety, two very common clinical manifestations in AD patients that have important repercussions at both the cognitive and functional levels [47][126]. Finally, it is important to emphasize that the best treatment for any disease lies in a good prevention strategy. In this regard, adopting a healthy lifestyle that includes physical exercise [48][127], a Mediterranean diet and a good sleep habit has proven to be one of the effective strategies as it has been associated with a reduction in cognitive decline and a lower risk of developing AD [49][128]. Cognitive therapy has also been positioned as the non-pharmacological treatment with the best results in the prevention of AD. This therapy is characterized by the development of cognitive stimulation and training exercises.
