Fungal secondary metabolites (SMs) comprise a vast collection of compounds expendable for these organisms under laboratory conditions. They exhibit enormous chemical diversity, and usually belong to four major families: terpenoids, polyketides, non-ribosomal peptides, or a combination of the last two. Their functions are very diverse and are normally associated with a greater fitness of the producing fungi in their environment, which often compete with other microorganisms or interact with host plants. Many SMs have beneficial applications, e.g., as antibiotics or medical drugs, but others, known as mycotoxins, are harmful to health.
- polyketides
- PKS
- terpenoids
- non-ribosomal peptides
- NRP
- PKS–NRPS hybrid genes
- gene clusters
- pigments
- antibiotics
- mycotoxins
1. Introduction
The production of metabolites by fungi began to receive attention in the first half of the last century [1], and acquired special relevance after the discovery of penicillin, a metabolite produced by the fungus Penicillium, which started the era of antibiotics [2]. Today, one of the most characteristic traits of fungi is their enormous metabolic versatility, which is reflected in the richness of secondary metabolism in many species [3]. Secondary metabolites (SMs) can be defined as chemical compounds resulting from specific biosynthetic pathways, whose production is not necessary for normal growth and development of the fungus in the laboratory. However, they are present in numerous species, and therefore their persistence in evolution implies a competitive benefit in nature. This entry reviews the major SM families, summarizes the genetic basis and regulatory mechanisms involved in their production, and provides with selected examples a general overview of their chemical diversity, possible roles in fungal life, and biological effects and applications in human life.
2. Chemical Families of SMs
2.1. Polyketides
PKs constitute the most abundant and diverse SM family. Their biochemical pathways begin with the addition of acetyl-CoA units to form different structures, followed by a broad diversity of chemical reactions [5][6]. All polyketide biosynthetic pathways are initiated by a characteristic enzyme, known as polyketide synthetase (PKS), initially discovered in bacteria. According to their structures and mechanisms, PKSs are classified into three classes, known as types I, II, and III [7]. Type I PKSs are giant multifunctional enzymes structurally related to fatty acid synthetases [8]. They usually have in common a set of conserved domains that always include three basic ones: acyltransferase (AT), which recognizes the monomer that will be used in the synthesis; ketosynthase (KS), which joins it to the elongating polyketide chain; and acyl carrier protein (ACP), which has a prosthetic group of phosphopantetheine that serves as a covalent binding site for the intermediate formed in the synthesis. These are accompanied by other optional domains. The keto groups formed in the elongating process can be reduced by ketoreductase (KR), dehydratase (DH), or enol reductase (ER) domains to produce different modifications depending on the specific PK in question. There are other possible domains, e.g., methyl transferase (MT), or condensation/heterocyclization (HC), that can introduce additional changes. PKSs can be iterative or non-iterative. In iterative PKSs the macro-enzyme functions as an extension module that elongates the product in successive reactive cycles. These PKSs can be reducing or non-reducing depending on the presence of the reducing KR, DH, and ER domains. Non-iterative PKSs are usually multimodular, with each module having its own domain combination responsible for a complete elongation cycle, and with a final module with a releasing thioesterase (TE) domain [9]. The products resulting from the PKS activity are modified by other enzymes, giving rise to the vast chemical diversity that characterizes this family. Some well-known examples of PKs synthesized by type I PKSs are described in Table 1 and Figure 1. In some cases, two different PKSs participate in the synthesis of the same compound, as occurs with zearalenone [10].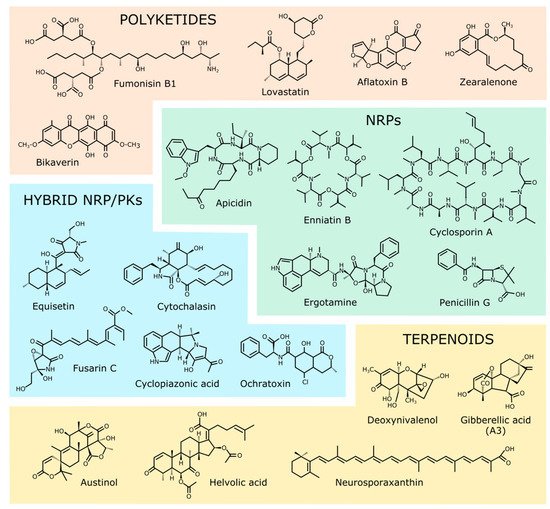
| Chemical Family | Metabolite | Function/Activity | Representative Producing Genera |
Reference |
|---|---|---|---|---|
| Polyketides (PKs) |
Fumonisin B1 | Mycotoxin | Fusarium | [11] |
| Lovastatin | HMG-CoA reductase inhibitor | Aspergillus | [12] | |
| Aflatoxin | Mycotoxin | Aspergillus | [13] | |
| Bikaverin | Antibiotic (protozoa) | Fusarium | [14] | |
| Zearalenone | Mycotoxin (estrogenic) | Fusarium | [10] | |
| Non ribosomal peptides (NRPs) |
Enniatin B | Mycotoxin (cytotoxic) | Fusarium | [15] |
| Cyclosporine A | Immunosuppressant | Tolypocladium | [16] | |
| Ergotamine | Ergot alkaloid | Claviceps | [17] | |
| Penicillin G | Antibiotic (bacteria) | Penicillium | [18] | |
| Apicidin | Histone deacetylase inhibitor | Fusarium | [19] | |
| Hybrid NRP/PKs | Equisetin | Antibiotic (bacteria) | Fusarium | [20][21] |
| Fusarin C | Mycotoxin | Fusarium | [22] | |
| Cytochalasin | Actin inhibitor | Penicillium, Chaetomium | [23] | |
| Cyclopiazonic acid | Mycotoxin | Aspergillus, Penicillium | [24] | |
| Ochratoxin A | Mycotoxin | Aspergillus, Penicillium | [25][26] | |
| Terpenoids | Gibberellic acid (GA3) | Plant hormone | Fusarium | [27][28] |
| Deoxynivalenol | Mycotoxin | Fusarium | [29] | |
| Neurosporaxanthin | Carotenoid pigment | Neurospora, Fusarium | [30][31] | |
| Austinol | Unknown | Aspergillus | [32] | |
| Helvolic acid | Antibiotic (bacteria) | Aspergillus | [33] |
2.2. Non-Ribosomal Peptides
Non-ribosomal peptides (NRPs) are low-molecular-weight peptides with extensive chemical variety [38]. As their name indicates, they are synthesized by a mechanism unrelated to protein synthesis in the ribosome. In addition to their smaller sizes compared to most proteins, they differ from these in their structures, which are frequently cyclical, and in the participation of atypical amino acids, such as hydroxylated or methylated variants, or their D forms. Unlike proteins, which undergo modifications only after their synthesis, NRPs undergo chemical changes in their amino acids during their formation or by other enzymes after they have been released. Like PKs, NRPs are produced by gigantic multi-enzyme complexes known as NRP synthetases (NRPSs). They are usually organized as modules, each consisting of several catalytic domains that function in a coordinated fashion [39]. A basic module includes an adenylation (A) and a thiolation domain (T). Selection and activation of the substrate is carried out by the A domain. The substrate is then transferred to the T domain, where it is covalently linked by a thiodiester bond into a phosphopantetheine unit. Each module contributes one aminoacyl or aryl residue to be used in the formation of the final NRP. Other enzymatic domains optionally present in the modules introduce modifications in the residues, which can lead to their epimerization (E) or methylation (M), or to other chemical changes, while the condensation domains (C) catalyze the union by peptide bonds of substrates linked to the adjacent phosphopantetheine. Reactions proceed until the full NRP is generated. The NRP’s release is often accompanied by a cycling reaction carried out by a thioesterase domain located at the carbon end of the multienzyme complex. In many cases, the NRP undergoes new chemical modifications by other enzymes, including oxidation, halogenation, or glycosylation reactions, among others, thereby increasing the chemical diversity of the resulting NRPs. A database of NRPs called Norine [40] is available to researchers, and at the time this article was written it included 1740 peptides formed by 544 different monomers. The number of monomers in each NRP varies from 2 to 26 [41]. Due to their numerous applications, different strategies have been used to improve the biotechnological production of many NRPs [38][42][43]. Some representative examples of fungal NRPs are described in Table 1 and Figure 1.2.3. Hybrid Non-Ribosomal Peptide/Polyketides
2.4. Terpenoids
3. Genetic Organization and Regulation of SM Genes
The genes responsible for SM synthesis are usually clustered in the fungal genomes sharing the same regulation [58]. Typical SM gene clusters contain a key pathway gene, coding for a PKS, a NRPS, or a terpene cyclase, which is often easily identified by its larger size. They also contain genes for other modifying enzymes frequently belonging to easily recognizable families, e.g., P450 monooxygenases [59], and in some cases genes for permeases involved in their excretion. SM biosynthetic pathways can be controlled by specific regulatory genes [60] frequently belonging to the Zn cluster family and normally included in the corresponding cluster. As a prototypical example, in F. fujikuroi the transcription factor Bik5 is necessary for the synthesis of bikaverin, supported in this case by a second regulatory protein Bik4 [61]. In other cases, regulatory genes are not found in the cluster, as occurs with the gibberellin cluster in the same species [27] or in penicillin and cephalosporin clusters in other fungi [62]. Due to the interest of SM production, much attention has been devoted to its regulatory mechanisms. SM clusters are regulated by a diversity of environmental cues and are controlled by different regulatory proteins, which are frequently involved in more general regulatory networks [63][64]. External signals controlling SM biosynthesis include the availability of nitrogen or carbon sources, pH, or light, mediated by global regulation systems that act simultaneously on different SM clusters as well as on other metabolic processes [65]. Regulation of SM production by nitrogen is very frequent [66], and different proteins participate in it, among which AreA-like proteins play a pivotal role. Carbon source availability affects many metabolic processes [67], including SM production [68], that usually involve a catabolite repressor of the CreA family. SM regulation by pH is usually controlled by proteins of the PacC family, with examples in different fungi [69]. Another general regulator of special interest is LaeA [70], which is associated with light regulation and development control with Velvet proteins forming a complex [71][72]. It has recently been observed that the main regulator by light in F. fujikuroi, WcoA, positively or negatively controls different SM clusters in this fungus [73]. This double positive/negative role on different pathways is frequent in these global regulatory systems, even when responding to the same signal. For example, AreA mutation results in derepression of gibberellin biosynthesis in F. fujikuroi [74], but repression of fumonisin production in F. verticillioides [75]. Examples of mutations on genes affecting fungal SM production—in some cases encoding enzymes, as found for glutamine synthetase in F. fujikuroi [76]—have been abundantly described in the literature. SM clusters may be in any chromosomal region, but in many cases they are found in subtelomeric regions [77]. Such locations may be associated with epigenetic silencing in the form of heterochromatin. Supporting this, some regulatory proteins modulate SM production at the level of chromatin structure by histone modifications [78], a conclusion reinforced by the effects of mutations of genes involved in such modifications, such as that for the methyltransferase Kmt6 in Fusarium sp. [79][80]. This level of regulation provides a selective advantage to the genomic organization as clusters since it allows the simultaneous inactivation of all the genes of a pathway through heterochromatinization. This is the way through which LaeA acts in the Velvet complex, but other regulatory proteins, such as AreA, also participate in this control mechanism [81]. Regulation occurs also at the level of cell compartmentalization: e.g., there are regulatory systems for controlling the enzymes of each metabolic pathway and their localization in the appropriate cellular compartments, such as peroxisomes, vacuoles, endoplasmic reticulum and Golgi, or cytosol [82].4. Biological Functions
As already stated, a distinctive feature of SMs is that they are dispensable for the fungus under controlled growth conditions, so that mutants unable to produce them are not affected in their viability in the laboratory. However, the persistence of these biosynthetic processes in fungi imply adaptive advantages in their natural environment. In some cases the functions are obvious and in other cases they are less clear. Some illustrative examples of known SM functions are mentioned below. Melanin protects against UV radiation, facilitating the survival of the fungus under strong sun exposure. However, it also exerts protective effects against other sources of stress, such as oxidative or thermal stress, as well as mechanical damage [83]. SMs can fulfill more specific physiological functions. Siderophores are NRPs that act as high-affinity iron chelators, which are used by some fungi to scavenge environmental iron or to sequestrate internal reactive iron [84]. Fusarubin and 5-deoxybostrycoidin-based melanin provide dark pigmentation to perithecia in F. fujikuroi [85] and F. graminearum [86], respectively. Other SMs are used for more than one purpose. β-carotene is useful as a protective agent against oxidative stress [87], but it is also used as a source of derivatives with more specific functions. In mucormycotina fungi, such as B. trispora or P. blakesleeanus, β-carotene is cleaved to produce sexual hormones called trisporic acids, which are needed to carry out the sexual cycle [88][89]. However, in F. fujikuroi [30] and Ustilago maydis [90] the same carotene is cleaved to produce retinal, the prosthetic group of rhodopsins. Many secondary metabolites play roles in the interactions of fungi with other organisms, both in terms of competition and pathogenesis [91]. There are many examples where fungi produce antibiotics to avoid competition. For example, Beauveria bassiana produces the polyketide oosporein to limit bacterial growth in the parasitized insect [92]. It is well known that the interaction between fungi and plants, either mutualistic or pathogenic, involves the simultaneous production of molecular signals from the interacting species [93][94]. Moreover, some SM gene clusters are expressed in the host plant but not in others. The F. fujikuroi FUB1 gene, coding for a PKS for the synthesis of the toxin fusaric acid, is expressed when infecting its host but not when infecting other plants [95]. The participation of SMs in pathogenesis is not easily predictable. In Pyricularia oryzae, the causative agent of rice blast disease, melanins are required for pathogenesis, but no role is apparently played by tenuazonic acid, a hybrid NRP/PKS mycotoxin, by nectriapyrones, polyketide compounds with antibacterial activity, or by pyriculols, phytotoxic polyketide compounds [96]. The dependence on specific needs in their ecological niches explains why many fungal SM clusters are not expressed under laboratory conditions. However, their functions may be investigated by activating them in a targeted way [97][98].5. Biological Properties and Applications
Many SMs possess useful biological properties or have biotechnological applications [101], while others are detrimental or have disadvantageous effects [102][103]. SMs useful for humans include a large diversity of antibiotics. In addition to the historical example of penicillin, there are numerous SMs with a very varied spectrum of antibiosis. Among them are other antibacterials, such as cephalosporin obtained from Acremonium chrysogenum [104], antifungals, such as griseofulvin produced by Penicillium or other fungi [105], or antiprotozoals, such as bikaverin synthesized by Fusarium species [14]. Other compounds have medical or pharmaceutical applications, such as immunosuppressant cyclosporin A, produced by Tolypocladium inflatum [16]; cholesterol-lowering statins, with lovastatin from Aspergillus terreus as the best known example [12]; vitamin-A precursor β-carotene, industrially obtained from Blakeslea trispora [106]; and anticancer drugs, such as the indole alkaloid camptothecin and taxol, produced by the endophytic fungus Entrophospora infrequens [107] and Taxomyces andreanae [108], respectively. An outstanding case in biotechnological applications is the aforementioned gibberellins, growth-regulating plant hormones with agricultural applications, which are mostly represented by gibberellic acid obtained from F. fujikuroi [27].
Frequently, secondary metabolites absorb visible light and have striking colors, ranging across all ranges of the spectrum: e.g., bikaverin and fusarubin have a reddish pigmentation [28]. In some cases, although it is not related to their biological function, different SMs are used commercially as pigments. Among them some carotenoids stand out, such as astaxanthin. This pigment, produced by the yeast Xhantophyllomyces dendrorhous [109] and some algae, is used in aquaculture as feed additive to provide an orange color to certain fish and crustaceans. Other well-known fungal pigments are polyketides produced by Monascus purpurea [110], a fungus used in rice fermentation since ancient times in Chinese and Japanese cuisine. These polyketides include monascorubramine as well as rubropunctatin and its derivatives, which are of various yellowish, orange, or reddish colors, and to which numerous healthy properties are attributed, such as anticancer, antidiabetic, or antiobesity properties.
Regardless of their real roles in nature, many SMs are toxic to humans, and their presence in plant foods, due to contamination by producing fungi before or after the harvest, constitutes an important public health problem [102][103][111]. These harmful SMs are known generically as mycotoxins. One specially studied for its high toxicity is aflatoxin B1, produced by various species of Aspergillus [112]. Its consumption is associated with a syndrome known as acute aflatoxicosis, as well as with liver cancer or other damaging effects [103]. Many well-known mycotoxins are produced by the Fusarium species [113], among them fumonisins, zearalenones, trichothecenes, and fusarins. Fumonisins inhibit sphingolipid metabolism and also have carcinogenic properties. A correlation between their consumption and esophageal cancer is well documented [102]. Trichothecenes inhibit protein synthesis and produce toxic syndromes in humans and animals. A well-known trichothecene is deoxynivalenol, which produces alimentary toxic aleukia, acute gastroenteritis, and growth impairment, among other effects [103]. Fusarins, especially fusarin C, are mutagenic in the Ames test, presumably due to their transformation into more toxic derivatives in the body [114]. Another mutagenic mycotoxin is ochratoxin A, which provokes renal cancer [102]. Zearalenones have lower toxicity, but produce an estrogenic syndrome in pigs, presumably due to their resemblance to this family of hormones [103].
An interesting consequence of the large metabolic diversity of fungi is that different species produce specific patterns of SMs, which allow for their use in taxonomic studies. The identification of fungal species based on the metabolites produced is known as chemotaxonomy [115]. This tool is especially relevant in the case of lichens, which are symbiotic associations between a fungus and a photoautotrophic partner, usually an alga. Lichens show an enormous capacity to produce SMs, which is mainly due to the fungal partner [116]. In many cases, these metabolites provide protection against the harmful effects of UV in their natural habitats [117]. The availability of powerful analytical techniques for metabolite identification allows for the creation of databases, which facilitate the assignment of lichens based on the metabolites detected [118].
6. Conclusions and Prospects
References
- Raistrick, H. A Region of Biosynthesis. Proc. R. Soc. Lond. B Biol. Sci. 1950, 136, 481–508. Raistrick, H. A Region of Biosynthesis. Proc. R. Soc. Lond. B Biol. Sci. 1950, 136, 481–508.
- Bennett, J.W.; Chung, K.T. Alexander Fleming and the Discovery of Penicillin. Adv. Appl. Microbiol. 2001, 49, 163–184.Bennett, J.W.; Chung, K.T. Alexander Fleming and the Discovery of Penicillin. Adv. Appl. Microbiol. 2001, 49, 163–184.
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal Secondary Metabolism - from Biochemistry to Genomics. Nat. Rev. Microbiol. 2005, 3, 937–947.Keller, N.P.; Turner, G.; Bennett, J.W. Fungal Secondary Metabolism - from Biochemistry to Genomics. Nat. Rev. Microbiol. 2005, 3, 937–947.
- Robey, M.T.; Caesar, L.K.; Drott, M.T.; Keller, N.P.; Kelleher, N.L. An Interpreted Atlas of Biosynthetic Gene Clusters from 1,000 Fungal Genomes. Proc. Natl. Acad. Sci. USA 2021, 118, e2020230118.
- Staunton, J.; Weissman, K.J. Polyketide Biosynthesis: A Millennium Review. Nat. Prod. Rep. 2001, 18, 380–416.
- Rawlings, B.J. Biosynthesis of Polyketides. Nat. Prod. Rep. 1997, 14, 523–556.
- Shen, B. Polyketide Biosynthesis beyond the Type I, II and III Polyketide Synthase Paradigms. Curr. Opin. Chem. Biol. 2003, 7, 285–295.
- Herbst, D.A.; Townsend, C.A.; Maier, T. The Architectures of Iterative Type I PKS and FAS. Nat. Prod. Rep. 2018, 35, 1046–1069.
- Weissman, K.J. Uncovering the Structures of Modular Polyketide Synthases. Nat. Prod. Rep. 2015, 32, 436–453.
- Kim, Y.-T.; Lee, Y.-R.; Jin, J.; Han, K.-H.; Kim, H.; Kim, J.-C.; Lee, T.; Yun, S.-H.; Lee, Y.-W. Two Different Polyketide Synthase Genes Are Required for Synthesis of Zearalenone in Gibberella zeae. Mol. Microbiol. 2005, 58, 1102–1113.
- Nelson, P.E.; Desjardins, A.E.; Plattner, R.D. Fumonisins, Mycotoxins Produced by Fusarium Species: Biology, Chemistry, and Significance. Annu. Rev. Phytopathol. 1993, 31, 233–252.
- Mulder, K.C.L.; Mulinari, F.; Franco, O.L.; Soares, M.S.F.; Magalhães, B.S.; Parachin, N.S. Lovastatin Production: From Molecular Basis to Industrial Process Optimization. Biotechnol. Adv. 2015, 33, 648–665.
- Klich, M.A. Aspergillus flavus: The Major Producer of Aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722.
- Limón, M.C.; Rodríguez-Ortiz, R.; Avalos, J. Bikaverin Production and Applications. Appl. Microbiol. Biotechnol. 2010, 87, 21–29.
- Prosperini, A.; Berrada, H.; Ruiz, M.J.; Caloni, F.; Coccini, T.; Spicer, L.J.; Perego, M.C.; Lafranconi, A. A Review of the Mycotoxin Enniatin B. Front. Public Health 2017, 5, 304.
- Survase, S.A.; Kagliwal, L.D.; Annapure, U.S.; Singhal, R.S. Cyclosporin A-a Review on Fermentative Production, Downstream Processing and Pharmacological Applications. Biotechnol. Adv. 2011, 29, 418–435.
- Florea, S.; Panaccione, D.G.; Schardl, C.L. Ergot Alkaloids of the Family Clavicipitaceae. Phytopathology 2017, 107, 504–518.
- Peñalva, M.A.; Rowlands, R.T.; Turner, G. The Optimization of Penicillin Biosynthesis in Fungi. Trends Biotechnol. 1998, 16, 483–489.
- Jin, J.-M.; Lee, S.; Lee, J.; Baek, S.-R.; Kim, J.-C.; Yun, S.-H.; Park, S.-Y.; Kang, S.; Lee, Y.-W. Functional Characterization and Manipulation of the Apicidin Biosynthetic Pathway in Fusarium semitectum. Mol. Microbiol. 2010, 76, 456–466.
- Ratnaweera, P.B.; de Silva, E.D.; Williams, D.E.; Andersen, R.J. Antimicrobial Activities of Endophytic Fungi Obtained from the Arid Zone Invasive Plant Opuntia dillenii and the Isolation of Equisetin, from Endophytic Fusarium sp. BMC Complement. Altern. Med. 2015, 15, 220.
- Kakule, T.B.; Jadulco, R.C.; Koch, M.; Janso, J.E.; Barrows, L.R.; Schmidt, E.W. Native Promoter Strategy for High-Yielding Synthesis and Engineering of Fungal Secondary Metabolites. ACS Synth. Biol. 2015, 4, 625–633.
- Niehaus, E.-M.; Díaz-Sánchez, V.; von Bargen, K.W.; Kleigrewe, K.; Humpf, H.-U.; Limón, M.C.; Tudzynski, B. Fusarins and Fusaric Acid in Fusaria. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Martín, J.-F., García-Estrada, C., Zeilinger, S., Eds.; Springer: New York, NY, USA, 2014; pp. 239–262. ISBN 978-1-4939-1190-5.
- Skellam, E. The Biosynthesis of Cytochalasans. Nat. Prod. Rep. 2017, 34, 1252–1263.
- Tokuoka, M.; Seshime, Y.; Fujii, I.; Kitamoto, K.; Takahashi, T.; Koyama, Y. Identification of a Novel Polyketide Synthase-Nonribosomal Peptide Synthetase (PKS-NRPS) Gene Required for the Biosynthesis of Cyclopiazonic Acid in Aspergillus oryzae. Fungal Genet. Biol. 2008, 45, 1608–1615.
- Gil-Serna, J.; García-Díaz, M.; González-Jaén, M.T.; Vázquez, C.; Patiño, B. Description of an Orthologous Cluster of Ochratoxin A Biosynthetic Genes in Aspergillus and Penicillium Species. A Comparative Analysis. Int. J. Food Microbiol. 2018, 268, 35–43.
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191.
- Tudzynski, B. Gibberellin Biosynthesis in Fungi: Genes, Enzymes, Evolution, and Impact on Biotechnology. Appl. Microbiol. Biotechnol. 2005, 66, 597–611.
- Studt, L.; Tudzynski, B. Gibberellins and the Red Pigments Bikaverin and Fusarubin. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Martín, J.-F., García-Estrada, C., Zeilinger, S., Eds.; Springer: New York, NY, USA, 2014; pp. 209–238. ISBN 978-1-4939-1190-5.
- Chen, Y.; Kistler, H.C.; Ma, Z. Fusarium graminearum Trichothecene Mycotoxins: Biosynthesis, Regulation, and Management. Annu. Rev. Phytopathol. 2019, 57, 15–39.
- Avalos, J.; Pardo-Medina, J.; Parra-Rivero, O.; Ruger-Herreros, M.; Rodríguez-Ortiz, R.; Hornero-Méndez, D.; Limón, M.C. Carotenoid Biosynthesis in Fusarium. J. Fungi 2017, 3, 39.
- Avalos, J.; Corrochano, L.M. Carotenoid Biosynthesis in Neurospora. In Neurospora: Genomics and Molecular Biology; Kasbekar, D.P., McCluskey, K., Eds.; Caister Academic Press: Poole, UK, 2013; pp. 227–241.
- Lo, H.-C.; Entwistle, R.; Guo, C.-J.; Ahuja, M.; Szewczyk, E.; Hung, J.-H.; Chiang, Y.-M.; Oakley, B.R.; Wang, C.C.C. Two Separate Gene Clusters Encode the Biosynthetic Pathway for the Meroterpenoids Austinol and Dehydroaustinol in Aspergillus nidulans. J. Am. Chem. Soc. 2012, 134, 4709–4720.
- Mitsuguchi, H.; Seshime, Y.; Fujii, I.; Shibuya, M.; Ebizuka, Y.; Kushiro, T. Biosynthesis of Steroidal Antibiotic Fusidanes: Functional Analysis of Oxidosqualene Cyclase and Subsequent Tailoring Enzymes from Aspergillus fumigatus. J. Am. Chem. Soc. 2009, 131, 6402–6411.
- Hertweck, C.; Luzhetskyy, A.; Rebets, Y.; Bechthold, A. Type II Polyketide Synthases: Gaining a Deeper Insight into Enzymatic Teamwork. Nat. Prod. Rep. 2007, 24, 162–190.
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone Synthase and Its Functions in Plant Resistance. Phytochem. Rev. 2011, 10, 397–412.
- Nosanchuk, J.D.; Stark, R.E.; Casadevall, A. Fungal Melanin: What Do We Know about Structure. Front. Microbiol. 2015, 6, 1463.
- Eisenman, H.C.; Casadevall, A. Synthesis and Assembly of Fungal Melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940.
- Süssmuth, R.D.; Mainz, A. Nonribosomal Peptide Synthesis-Principles and Prospects. Angew. Chem. Int. Ed. Engl. 2017, 56, 3770–3821.
- Finking, R.; Marahiel, M.A. Biosynthesis of Nonribosomal Peptides. Annu. Rev. Microbiol. 2004, 58, 453–488.
- Caboche, S.; Pupin, M.; Leclère, V.; Fontaine, A.; Jacques, P.; Kucherov, G. NORINE: A Database of Nonribosomal Peptides. Nucleic Acids Res. 2008, 36, D326–D331.
- Flissi, A.; Ricart, E.; Campart, C.; Chevalier, M.; Dufresne, Y.; Michalik, J.; Jacques, P.; Flahaut, C.; Lisacek, F.; Leclère, V.; et al. Norine: Update of the Nonribosomal Peptide Resource. Nucleic Acids Res. 2020, 48, D465–D469.
- Soukup, A.A.; Keller, N.P.; Wiemann, P. Enhancing Nonribosomal Peptide Biosynthesis in Filamentous Fungi. Methods Mol. Biol. 2016, 1401, 149–160.
- Walsh, C.T. Insights into the Chemical Logic and Enzymatic Machinery of NRPS Assembly Lines. Nat. Prod. Rep. 2016, 33, 127–135.
- Boettger, D.; Hertweck, C. Molecular Diversity Sculpted by Fungal PKS-NRPS Hybrids. Chembiochem 2013, 14, 28–42.
- Sørensen, J.L.; Sondergaard, T.E.; Covarelli, L.; Fuertes, P.R.; Hansen, F.T.; Frandsen, R.J.N.; Saei, W.; Lukassen, M.B.; Wimmer, R.; Nielsen, K.F.; et al. Identification of the Biosynthetic Gene Clusters for the Lipopeptides Fusaristatin A and W493 B in Fusarium graminearum and F. pseudograminearum. J. Nat. Prod. 2014, 77, 2619–2625.
- Du, L.; Sánchez, C.; Shen, B. Hybrid Peptide-Polyketide Natural Products: Biosynthesis and Prospects toward Engineering Novel Molecules. Metab. Eng. 2001, 3, 78–95.
- Theobald, S.; Vesth, T.C.; Andersen, M.R. Genus Level Analysis of PKS-NRPS and NRPS-PKS Hybrids Reveals Their Origin in Aspergilli. BMC Genom. 2019, 20, 847.
- Fisch, K.M. Biosynthesis of Natural Products by Microbial Iterative Hybrid PKS–NRPS. RSC Adv. 2013, 3, 18228.
- Sacchettini, J.C.; Poulter, C.D. Creating Isoprenoid Diversity. Science 1997, 277, 1788–1789.
- Lange, B.M.; Rujan, T.; Martin, W.; Croteau, R. Isoprenoid Biosynthesis: The Evolution of Two Ancient and Distinct Pathways across Genomes. Proc. Natl. Acad. Sci. USA 2000, 97, 13172–13177.
- Heddergott, C.; Calvo, A.M.; Latgé, J.P. The Volatome of Aspergillus fumigatus. Eukaryot. Cell 2014, 13, 1014–1025.
- Kellogg, B.A.; Poulter, C.D. Chain Elongation in the Isoprenoid Biosynthetic Pathway. Curr. Opin. Chem. Biol. 1997, 1, 570–578.
- Christianson, D.W. Unearthing the Roots of the Terpenome. Curr. Opin. Chem. Biol. 2008, 12, 141–150.
- Quin, M.B.; Flynn, C.M.; Schmidt-Dannert, C. Traversing the Fungal Terpenome. Nat. Prod. Rep. 2014, 31, 1449–1473.
- Schmidt-Dannert, C. Biosynthesis of Terpenoid Natural Products in Fungi. Adv. Biochem. Eng. Biotechnol. 2015, 148, 19–61.
- Christianson, D.W. Structural Biology and Chemistry of the Terpenoid Cyclases. Chem. Rev. 2006, 106, 3412–3442.
- Matsuda, Y.; Awakawa, T.; Mori, T.; Abe, I. Unusual Chemistries in Fungal Meroterpenoid Biosynthesis. Curr. Opin. Chem. Biol. 2016, 31, 1–7.
- Rokas, A.; Mead, M.E.; Steenwyk, J.L.; Raja, H.A.; Oberlies, N.H. Biosynthetic Gene Clusters and the Evolution of Fungal Chemodiversity. Nat. Prod. Rep. 2020, 37, 868–878.
- Zhang, X.; Guo, J.; Cheng, F.; Li, S. Cytochrome P450 Enzymes in Fungal Natural Product Biosynthesis. Nat. Prod. Rep. 2021, 38, 1072–1099.
- Yin, W.; Keller, N.P. Transcriptional Regulatory Elements in Fungal Secondary Metabolism. J. Microbiol. 2011, 49, 329–339.
- Wiemann, P.; Willmann, A.; Straeten, M.; Kleigrewe, K.; Beyer, M.; Humpf, H.U.; Tudzynski, B. Biosynthesis of the Red Pigment Bikaverin in Fusarium fujikuroi: Genes, Their Function and Regulation. Mol. Microbiol. 2009, 72, 931–946.
- Brakhage, A.A. Molecular Regulation of Beta-Lactam Biosynthesis in Filamentous Fungi. Microbiol. Mol. Biol. Rev. 1998, 62, 547–585.
- Keller, N.P. Fungal Secondary Metabolism: Regulation, Function and Drug Discovery. Nat. Rev. Microbiol. 2019, 17, 167–180.
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and Role of Fungal Secondary Metabolites. Annu. Rev. Genet. 2016, 50, 371–392.
- Brakhage, A.A. Regulation of Fungal Secondary Metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32.
- Tudzynski, B. Nitrogen Regulation of Fungal Secondary Metabolism in Fungi. Front. Microbiol 2014, 5, 656.
- Szilágyi, M.; Miskei, M.; Karányi, Z.; Lenkey, B.; Pócsi, I.; Emri, T. Transcriptome Changes Initiated by Carbon Starvation in Aspergillus nidulans. Microbiology 2013, 159, 176–190.
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of Microbial Secondary Metabolites: Regulation by the Carbon Source. Crit. Rev. Microbiol. 2010, 36, 146–167.
- Li, B.; Chen, Y.; Tian, S. Function of PH-Dependent Transcription Factor PacC in Regulating Development, Pathogenicity, and Mycotoxin Biosynthesis of Phytopathogenic Fungi. FEBS J. 2021.
- Bok, J.W.; Keller, N.P. LaeA, a Regulator of Secondary Metabolism in Aspergillus spp. Eukaryot. Cell 2004, 3, 527–535.
- Bayram, O.; Krappmann, S.; Ni, M.; Bok, J.W.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.-J.; Keller, N.P.; Yu, J.-H.; et al. VelB/VeA/LaeA Complex Coordinates Light Signal with Fungal Development and Secondary Metabolism. Science 2008, 320, 1504–1506.
- Bayram, O.; Braus, G.H. Coordination of Secondary Metabolism and Development in Fungi: The Velvet Family of Regulatory Proteins. FEMS Microbiol. Rev. 2012, 36, 1–24.
- Pardo-Medina, J.; Gutiérrez, G.; Limón, M.C.; Avalos, J. Impact of the White Collar Photoreceptor WcoA on the Fusarium fujikuroi Transcriptome. Front. Microbiol. 2021, 11, 619474.
- Tudzynski, B.; Homann, V.; Feng, B.; Marzluf, G.A. Isolation, Characterization and Disruption of the AreA Nitrogen Regulatory Gene of Gibberella fujikuroi. Mol. Gen. Genet. 1999, 261, 106–114.
- Kim, H.; Woloshuk, C.P. Role of AREA, a Regulator of Nitrogen Metabolism, during Colonization of Maize Kernels and Fumonisin Biosynthesis in Fusarium verticillioides. Fungal Genet. Biol. 2008, 45, 947–953.
- Wagner, D.; Wiemann, P.; Huß, K.; Brandt, U.; Fleißner, A.; Tudzynski, B. A Sensing Role of the Glutamine Synthetase in the Nitrogen Regulation Network in Fusarium fujikuroi. PLoS ONE 2013, 8, e80740.
- Palmer, J.M.; Keller, N.P. Secondary Metabolism in Fungi: Does Chromosomal Location Matter? Curr. Opin. Microbiol. 2010, 13, 431–436.
- Strauss, J.; Reyes-Dominguez, Y. Regulation of Secondary Metabolism by Chromatin Structure and Epigenetic Codes. Fungal Genet. Biol. 2011, 48, 62–69.
- Studt, L.; Rösler, S.M.; Burkhardt, I.; Arndt, B.; Freitag, M.; Humpf, H.-U.; Dickschat, J.S.; Tudzynski, B. Knock-down of the Methyltransferase Kmt6 Relieves H3K27me3 and Results in Induction of Cryptic and Otherwise Silent Secondary Metabolite Gene Clusters in Fusarium fujikuroi. Environ. Microbiol. 2016, 18, 4037–4054.
- Adpressa, D.A.; Connolly, L.R.; Konkel, Z.M.; Neuhaus, G.F.; Chang, X.L.; Pierce, B.R.; Smith, K.M.; Freitag, M.; Loesgen, S. A Metabolomics-Guided Approach to Discover Fusarium graminearum Metabolites after Removal of a Repressive Histone Modification. Fungal Genet. Biol. 2019, 132, 103256.
- Pfannmüller, A.; Leufken, J.; Studt, L.; Michielse, C.B.; Sieber, C.M.K.; Güldener, U.; Hawat, S.; Hippler, M.; Fufezan, C.; Tudzynski, B. Comparative Transcriptome and Proteome Analysis Reveals a Global Impact of the Nitrogen Regulators AreA and AreB on Secondary Metabolism in Fusarium fujikuroi. PLoS ONE 2017, 12, e0176194.
- Kistler, H.C.; Broz, K. Cellular Compartmentalization of Secondary Metabolism. Front. Microbiol. 2015, 6, 68.
- Freitas, D.F.; da Rocha, I.M.; Vieira-da-Motta, O.; de Paula Santos, C. The Role of Melanin in the Biology and Ecology of Nematophagous Fungi. J. Chem. Ecol. 2021, 47, 597–613.
- Bushley, K.E.; Ripoll, D.R.; Turgeon, B.G. Module Evolution and Substrate Specificity of Fungal Nonribosomal Peptide Synthetases Involved in Siderophore Biosynthesis. BMC Evol. Biol. 2008, 8, 328.
- Studt, L.; Wiemann, P.; Kleigrewe, K.; Humpf, H.-U.; Tudzynski, B. Biosynthesis of Fusarubins Accounts for Pigmentation of Fusarium fujikuroi Perithecia. Appl. Environ. Microbiol. 2012, 78, 4468–4480.
- Frandsen, R.J.N.; Rasmussen, S.A.; Knudsen, P.B.; Uhlig, S.; Petersen, D.; Lysøe, E.; Gotfredsen, C.H.; Giese, H.; Larsen, T.O. Black Perithecial Pigmentation in Fusarium Species Is Due to the Accumulation of 5-Deoxybostrycoidin-Based Melanin. Sci. Rep. 2016, 6, 26206.
- Avalos, J.; Limón, M.C. Biological Roles of Fungal Carotenoids. Curr. Genet. 2015, 61, 309–324.
- Medina, H.R.; Cerdá-Olmedo, E.; Al-Babili, S. Cleavage Oxygenases for the Biosynthesis of Trisporoids and Other Apocarotenoids in Phycomyces. Mol. Microbiol. 2011, 82, 199–208.
- Tagua, V.G.; Medina, H.R.; Martín-Domínguez, R.; Eslava, A.P.; Corrochano, L.M.; Cerdá-Olmedo, E.; Idnurm, A. A Gene for Carotene Cleavage Required for Pheromone Biosynthesis and Carotene Regulation in the Fungus Phycomyces blakesleeanus. Fungal Genet. Biol. 2012, 49, 398–404.
- Estrada, A.F.; Brefort, T.; Mengel, C.; Díaz-Sánchez, V.; Alder, A.; Al-Babili, S.; Avalos, J. Ustilago maydis Accumulates β-Carotene at Levels Determined by a Retinal-Forming Carotenoid Oxygenase. Fungal Genet. Biol. 2010, 46, 803–813.
- Demain, A.L.; Fang, A. The Natural Functions of Secondary Metabolites. Adv. Biochem. Eng. Biotechnol. 2000, 69, 1–39.
- Fan, Y.; Liu, X.; Keyhani, N.O.; Tang, G.; Pei, Y.; Zhang, W.; Tong, S. Regulatory Cascade and Biological Activity of Beauveria bassiana Oosporein That Limits Bacterial Growth after Host Death. Proc. Natl. Acad. Sci. USA 2017, 114, E1578–E1586.
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary Metabolites in Fungus-Plant Interactions. Front. Plant Sci. 2015, 6, 573.
- Kai, K. Bioorganic Chemistry of Signaling Molecules in Microbial Communication. J. Pestic. Sci. 2019, 44, 200–207.
- Niehaus, E.-M.; von Bargen, K.W.; Espino, J.J.; Pfannmüller, A.; Humpf, H.-U.; Tudzynski, B. Characterization of the Fusaric Acid Gene Cluster in Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2014, 98, 1749–1762.
- Motoyama, T.; Yun, C.-S.; Osada, H. Biosynthesis and Biological Function of Secondary Metabolites of the Rice Blast Fungus pyricularia Oryzae. J. Ind. Microbiol. Biotechnol. 2021, kuab058.
- Brakhage, A.A.; Schroeckh, V. Fungal Secondary Metabolites—Strategies to Activate Silent Gene Clusters. Fungal Genet. Biol. 2011, 48, 15–22.
- Scherlach, K.; Hertweck, C. Triggering Cryptic Natural Product Biosynthesis in Microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760.
- López-Díaz, C.; Rahjoo, V.; Sulyok, M.; Ghionna, V.; Martín-Vicente, A.; Capilla, J.; Di Pietro, A.; López-Berges, M.S. Fusaric Acid Contributes to Virulence of Fusarium oxysporum on Plant and Mammalian Hosts. Mol. Plant. Pathol. 2018, 19, 440–453.
- Wiemann, P.; Sieber, C.M.; von Bargen, K.W.; Studt, L.; Niehaus, E.M.; Espino, J.J.; Huss, K.; Michielse, C.B.; Albermann, S.; Wagner, D.; et al. Deciphering the Cryptic Genome: Genome-Wide Analyses of the Rice Pathogen Fusarium fujikuroi Reveal Complex Regulation of Secondary Metabolism and Novel Metabolites. PLoS Pathog. 2013, 9, e1003475.
- Demain, A.L. Valuable Secondary Metabolites from Fungi. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Martín, J.-F., García-Estrada, C., Zeilinger, S., Eds.; Fungal Biology; Springer: New York, NY, USA, 2014; pp. 1–15. ISBN 978-1-4939-1190-5.
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public Health Impacts of Foodborne Mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372.
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, Toxicology, and Exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237.
- Schmitt, E.K.; Hoff, B.; Kück, U. Regulation of Cephalosporin Biosynthesis. Adv. Biochem. Eng. Biotechnol. 2004, 88, 1–43.
- Petersen, A.B.; Rønnest, M.H.; Larsen, T.O.; Clausen, M.H. The Chemistry of Griseofulvin. Chem. Rev. 2014, 114, 12088–12107.
- Avalos, J.; Nordzieke, S.; Parra, O.; Pardo-Medina, J.; Limón, M.C. Carotenoid Production by Filamentous Fungi and Yeasts. In Biotechnology of Yeasts and Filamentous Fungi; Sibirny, A.A., Ed.; Springer: Cham, Switzerland, 2017; pp. 225–279. ISBN 978-3-319-58828-5.
- Amna, T.; Amina, M.; Sharma, P.R.; Puri, S.C.; Al-Youssef, H.M.; Al-Taweel, A.M.; Qazi, G.N. Effect of Precursors Feeding and Media Manipulation on Production of Novel Anticancer Pro-Drug Camptothecin from Endophytic Fungus. Braz. J. Microbiol. 2012, 43, 1476–1490.
- Boruta, T. Uncovering the Repertoire of Fungal Secondary Metabolites: From Fleming’s Laboratory to the International Space Station. Bioengineered 2018, 9, 12–16.
- Rodríguez-Sáiz, M.; de la Fuente, J.L.; Barredo, J.L. Xanthophyllomyces dendrorhous for the Industrial Production of Astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658.
- Feng, Y.; Shao, Y.; Chen, F. Monascus Pigments. Appl. Microbiol. Biotechnol. 2012, 96, 1421–1440.
- Pitt, J.I.; Miller, J.D. A Concise History of Mycotoxin Research. J. Agric. Food Chem. 2017, 65, 7021–7033.
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Aflatoxin Biosynthesis, Genetic Regulation, Toxicity, and Control Strategies: A Review. J. Fungi 2021, 7, 606.
- Desjardins, A.E.; Proctor, R.H. Molecular Biology of Fusarium Mycotoxins. Int. J. Food. Microbiol. 2007, 119, 47–50.
- Zhu, B.; Jeffrey, A.M. Fusarin C: Isolation and Identification of Two Microsomal Metabolites. Chem. Res. Toxicol. 1993, 6, 97–101.
- Frisvad, J.C.; Andersen, B.; Thrane, U. The Use of Secondary Metabolite Profiling in Chemotaxonomy of Filamentous Fungi. Mycol. Res. 2008, 112, 231–240.
- Molnár, K.; Farkas, E. Current Results on Biological Activities of Lichen Secondary Metabolites: A Review. Z. Naturforsch C. J. Biosci. 2010, 65, 157–173.
- Nguyen, K.-H.; Chollet-Krugler, M.; Gouault, N.; Tomasi, S. UV-Protectant Metabolites from Lichens and Their Symbiotic Partners. Nat. Prod. Rep. 2013, 30, 1490–1508.
- Olivier-Jimenez, D.; Chollet-Krugler, M.; Rondeau, D.; Beniddir, M.A.; Ferron, S.; Delhaye, T.; Allard, P.-M.; Wolfender, J.-L.; Sipman, H.J.M.; Lücking, R.; et al. A Database of High-Resolution MS/MS Spectra for Lichen Metabolites. Sci. Data 2019, 6, 294.
- Schueffler, A.; Anke, T. Fungal Natural Products in Research and Development. Nat. Prod. Rep. 2014, 31, 1425–1448.
- Li, M.; Yu, R.; Bai, X.; Wang, H.; Zhang, H. Fusarium: A Treasure Trove of Bioactive Secondary Metabolites. Nat. Prod. Rep. 2020, 37, 1568–1588.
- Medema, M.H.; de Rond, T.; Moore, B.S. Mining Genomes to Illuminate the Specialized Chemistry of Life. Nat. Rev. Genet. 2021, 22, 553–571.
- Khaldi, N.; Seifuddin, F.T.; Turner, G.; Haft, D.; Nierman, W.C.; Wolfe, K.H.; Fedorova, N.D. SMURF: Genomic Mapping of Fungal Secondary Metabolite Clusters. Fungal Genet. Biol. 2010, 47, 736–741.
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. AntiSMASH 5.0: Updates to the Secondary Metabolite Genome Mining Pipeline. Nucleic Acids Res. 2019, 47, W81–W87.
- Umemura, M.; Koike, H.; Machida, M. Motif-Independent de Novo Detection of Secondary Metabolite Gene Clusters-toward Identification from Filamentous Fungi. Front. Microbiol. 2015, 6, 371.
- Winter, J.M.; Behnken, S.; Hertweck, C. Genomics-Inspired Discovery of Natural Products. Curr. Opin. Chem. Biol. 2011, 15, 22–31.
- Wiemann, P.; Keller, N.P. Strategies for Mining Fungal Natural Products. J. Ind. Microbiol. Biotechnol. 2014, 41, 301–313.
- Kjærbølling, I.; Mortensen, U.H.; Vesth, T.; Andersen, M.R. Strategies to Establish the Link between Biosynthetic Gene Clusters and Secondary Metabolites. Fungal. Genet. Biol. 2019, 130, 107–121.
- Sanchez, J.F.; Somoza, A.D.; Keller, N.P.; Wang, C.C.C. Advances in Aspergillus Secondary Metabolite Research in the Post-Genomic Era. Nat. Prod. Rep. 2012, 29, 351–371.
- Nielsen, J.C.; Grijseels, S.; Prigent, S.; Ji, B.; Dainat, J.; Nielsen, K.F.; Frisvad, J.C.; Workman, M.; Nielsen, J. Global Analysis of Biosynthetic Gene Clusters Reveals Vast Potential of Secondary Metabolite Production in Penicillium Species. Nat. Microbiol. 2017, 2, 17044.
- Hoogendoorn, K.; Barra, L.; Waalwijk, C.; Dickschat, J.S.; van der Lee, T.A.J.; Medema, M.H. Evolution and Diversity of Biosynthetic Gene Clusters in Fusarium. Front. Microbiol. 2018, 9, 1158.
- Mattern, D.J.; Valiante, V.; Unkles, S.E.; Brakhage, A.A. Synthetic Biology of Fungal Natural Products. Front. Microbiol. 2015, 6, 775.
- Kornfuehrer, T.; Eustáquio, A.S. Diversification of Polyketide Structures via Synthase Engineering. Medchemcomm 2019, 10, 1256–1272.
- Hwang, S.; Lee, N.; Cho, S.; Palsson, B.; Cho, B.-K. Repurposing Modular Polyketide Synthases and Non-Ribosomal Peptide Synthetases for Novel Chemical Biosynthesis. Front. Mol. Biosci. 2020, 7, 87.
