Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Magdalena Klink and Version 3 by Catherine Yang.
Inducible nitric oxide synthase (iNOS), the enzyme responsible for nitric oxide (NO) production, is not present in most cells under normal conditions. The expression of its mRNA, as well as its protein synthesis and full enzymatic activity, undergoes multilevel regulation including transcriptional and posttranscriptional mechanisms, the availability of iNOS substrate and cofactors and oxygen tension.
- iNOS
- ovarian cancer
1. Introduction
Inducible nitric oxide synthase (iNOS, NOS2) is one of three isoforms belonging to the family of nitric oxide synthases, which are enzymes that catalyze the production of nitric oxide (NO) from L-arginine. iNOS is a unique enzyme since the iNOS transcript and protein are not present under normal conditions in most cells. Its expression is inducible and is frequently associated with inflammation and malignant diseases. iNOS expression, enzyme activation, and subsequent NO production comprise a multistage process that undergoes complex regulation on many levels from mRNA induction to the modulation of full enzymatic activity. In the tumor environment, a number of stimuli are constantly present, and large molecular changes occur in tumor and stromal cells, resulting in the permanent induction of iNOS expression. It is well established that iNOS, along with derived NO, is an important factor in both protumor and antitumor activity, which has been proved by a number of papers available on this topic. While the importance of iNOS expression in ovarian tumors is not obvious and far from being fully understood, the present review summarizes its possible involvement in the development and growth of ovarian cancer, its association with the chemoresistance of ovarian cancer cells to platinum compounds, and its potential as a prognostic factor in the course of this disease.
2. iNOS—Structure, Enzymatic Activity and Regulation in Normal Cells
Inducible nitric oxide synthase, which is similar to other NOS isoforms, is a homodimer with a molecular weight of approximately 130–135 kDa. It adopts a bidomain structure in which a carboxy-terminal “reductase” moiety is associated with flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD) and the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH), while an amino-terminal “oxygen” domain is a binding site for protoporphyrin IX (heme), tetrahydropterin (BH4) and L-arginine. Calmodulin is noncovalently bound to the iNOS complex [1][2][1,2] (Figure 1).
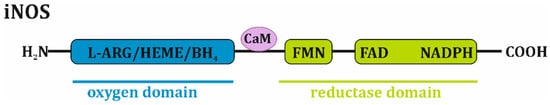
Figure 1. Domain-structured scheme of iNOS monomer. iNOS monomer is composed of oxygen domain that associates L-arginine (L-ARG), protoporphyrin IX (HEME) and tetrahydropterin (BH4), as well as reductase domain that consists of flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD) and reduced form of nicotinamide adenine dinucleotide phosphate (NADPH). Calmodulin (CaM) is noncovalently bound to the iNOS complex.
Monomeric iNOS is unable to bind the BH4 cofactor and the L-arginine substrate, thus, it cannot synthesize nitric oxide. The homodimerization process, which is required for enzyme activation, occurs in two oxygen domains, resulting in rapid conformational changes, and is dependent on the availability of L-arginine and BH4. Moreover, the presence of heme appears to be mandatory for dimer formation and to stabilize the whole enzymatic complex. Therefore, the physiological regulation of the enzymatic activity of iNOS is primarily based on the dietary intake of L-arginine, the regulation of its transport into cells, its use by other competing biological systems, such as arginase, and the regulation of BH4 synthesis and consumption. Moreover, iNOS catalytic activity is also downregulated by NO feedback since this molecule strongly binds to the heme group. The extracellular/pharmacological blocking of iNOS activity may occur in various manners and is mainly based on the use of L-arginine derivatives that compete with the substrate, the inhibition of NADPH, the inhibition of BH4 synthesis or binding, and the inhibition of heme binding [1][2][3][4][5][6][1,2,3,4,5,6].
It is well established that NO synthesis by iNOS includes two catalytic steps. First, L-arginine is hydroxylated by molecular oxygen (O2) and NADPH to Nω-hydroxy-L-arginine, which is next oxidized to L-citrulline, H2O and NO. Moreover, in the absence of L-arginine and BH4, the monomeric form of iNOS reduces molecular oxygen to the superoxide anion (·O2−). Thus, BH4 prevents superoxide anion synthesis via NOS. However, the reduction of oxygen to superoxide is an obligatory step during NO synthesis. The presence of both molecules (NO and ·O2−) in the reaction milieu leads to their rapid reaction to form the highly reactive radical peroxynitrite anion (ONOO−), which is characterized by strong nitrosative and oxidative properties. Another factor controlling iNOS enzymatic activity is oxygen tension since L-arginine is converted in an O2-dependent manner [1][4][7][8][1,4,7,8].
The induction of iNOS expression is a complex mechanism that undergoes multilevel control and is primarily regulated by both transcriptional and posttranscriptional mechanisms that are highly cell- and species-dependent. The human iNOS (NOS2) gene is 43,764 bp in size and located on chromosome 17. It encodes two transcripts: NOS2_001 and NOS2_201. The first transcript is 4176 nt in length, encodes a protein of 1153 amino acids and is thought to be a major iNOS mRNA. The NOS2_201 transcript is 4089 nt in length and encodes a protein of 1114 amino acids that is a splice variant of NOS2_001, but its involvement in protein synthesis is poorly recognized [5]. Extracellular signals control transcription factor activity and, consequently, iNOS gene expression and subsequent mRNA translocation. These extracellular signals are primarily pro-inflammatory cytokines (interleukin 1β, IL-1β; interferon γ, IFN-γ; tumor necrosis factor α, TNF-α), bacterial products (lipopolysaccharide, LPS; lipoarabinomannan, LAM), hypoxia and oxidative stress. The promoter sequence for human iNOS contains a TATA box approximately 30 bp from the transcription start site. Next to this are binding sites for transcription factors (NF-kB, NF-IL6), octamer factors, transcription factors induced by TNF-α, and transcription factors induced by IFN-γ (IRF-1, STAT-1α). It is worth noting that the maximal induction of the human iNOS gene is believed to occur through the binding of NF-κB and IRF-1 together to promoter sequences. Interestingly, a 1000 bp fragment of the human iNOS promoter showed low basal activity but no induction by cytokines. Only an iNOS promoter fragment larger than 3.8 kb showed any significant response to cytokines. Therefore, the promoter region relevant to cytokine-induced NF-κB/STAT-1α transcriptional factors for the human iNOS gene is located 3.8 kb upstream of the 5′ flanking region. A classical cytokine-induced enhancer is located between position 5.2 and 6.5. However, the notably high induction of iNOS expression (8-10-fold) was also found with the promoter fragment ranging from 7.2 kb to 16 kb [5][9][10][11][5,9,10,11]. Pathways that greatly contribute to transcriptional factor activation are the NF-κB signaling pathway, the Janus tyrosine kinase-Signal transducer and activator of transcription (JAK-STAT) pathway, and the mitogen-activated protein kinase (MAPK) pathway. Among them, NF-κB is a central factor for the activation or inhibition of iNOS gene expression. IL-1β, TNF-α, and oxidative stress induce NF-κB activity, its translocation to the nucleus and its subsequent binding to the promoter sequence. In contrast, transforming growth factor-β1 (TGF-β1) and antioxidants inhibit NF-κB activity through the induction of its ubiquitin-dependent proteasomal degradation, blocking its translocation to the nucleus or enhancing I-κB expression [5][11][12][13][5,11,12,13]. Interestingly, different polymorphisms in the sequence of the human iNOS promoter and their correlation with various human diseases, such as asthma or Alzheimer’s disease have been described, and the relationship between specific polymorphisms and iNOS expression, activity, and NO production [12] have been reported.
The posttranscriptional regulation of human iNOS mRNA primarily involves mRNA stability and/or degradation. The 3′-untranslated region (3′-UTR) of human iNOS contains four AUUUA motifs and one AUUUUA motif, which are known to destabilize the mRNAs of proto-oncogenes, nuclear transcription factors, and cytokines. AU-rich elements (ARE) mediate mRNA decay primarily by the protein requirement of exosomes containing mRNA. Moreover, the TGF-β-mediated activation of nucleases acting on AU-rich sequences leads to mRNA destabilization and the inhibition of iNOS induction. Therefore, the posttranscriptional regulation of mRNA stability is dependent on the 3′-UTR region. Moreover, several RNA-binding proteins (HuR, AUF1, KSPR, TIAR, TTP, PTB) are involved in either the stability or the degradation of human iNOS mRNA. Using DLD-1 cells, the only human model available for this type of research, it was shown that in unstimulated cells, KSPR binds to the 3′-UTR of iNOS mRNA and recruits exosomes to the mRNA, resulting in its rapid degradation. In cells stimulated with cytokines, TTP interacts with KSPR, preventing the binding of KSPR to mRNA, which in turn enhances HuR binding to the 3′-UTR iNOS sequence. The interaction of HuR with the 3′-UTR region increases iNOS mRNA stability and thus enhances iNOS expression. AUF1 consists of four isoforms, all of which can bind to ARE and promote iNOS mRNA degradation by competing with HuR for the same AU-rich elements. In contrast, TIAR enhances mRNA stability and increases iNOS expression [5][11][14][15][16][5,11,14,15,16].
Apart from effects on mRNA stability, another potential mechanism of iNOS posttranscriptional regulation involves short, noncoding RNAs known as microRNAs (miRNAs). It has been suggested that miRNAs block translation by the posttranscriptional repression of human iNOS. This was described in a study by Guo et al. [17], in which miR-939 was found to bind to the 3′-UTR in in vitro and in vivo models. miR-939 binding decreased cytokine-induced iNOS protein expression but did not affect its mRNA level and stability. Similarly, miR-26a and miR-146a affected the expression of iNOS protein either directly by interacting with the 3′-UTR (miR-26a) or indirectly by modulating the level of inflammatory cytokines (miR-146a) [18][19][18,19].
Finally, the posttranscriptional regulation of iNOS expression may involve mRNA translation and protein stability. Human cardiomyocytes express factors that can inhibit iNOS mRNA translation by interacting with the 5′- and/or 3′-UTR sequences of iNOS mRNA [20]. Furthermore, Felley-Bosco et al. [21] reported that the overexpression of caveolin-1 (Cav-1) in human DLD-1 cells destabilized iNOS. These data suggest that the direct interaction of Cav-1 with iNOS increased the proteasomal degradation of this enzyme.
Human iNOS expression and activity are observed in a large number of normal cells and various tissues, e.g., macrophages, neutrophils, Kupffer cells, chondrocytes, hepatocytes, and the vasculature [22]. Despite the complex, multilevel regulation of iNOS induction and activity, it is constantly upregulated in cancer cells, most likely due to vast alterations in their cellular biology.
3. iNOS Expression and Regulation in Ovarian Tumors
The constitutive overexpression of iNOS has been demonstrated in a number of tumors including breast, brain, prostate, lung, pancreas, ovarian, bladder, gastric, and colorectal tumors as well as melanoma and Kaposi’s sarcoma. In most tumors, it is possible to observe the increased expression and enzymatic activity of iNOS in comparison to those in adjacent normal tissue. However, it must be noted that the cellular level and activity of iNOS strongly depend on the histological type and grade of the tumor as well as the clinical stage of the disease. Moreover, the expression of iNOS in the tumor microenvironment (tumor cells along with stromal cells) varies primarily depending on the complexity of the particular tumor environment as well as on the presence of primary or metastatic lesions. Although its precise role has still not been fully established, iNOS-derived NO has a biphasic effect on tumor-related processes, such as tumorigenesis/malignant transformation, tumor progression, angiogenesis, metastasis, and chemoresistance. This topic has been widely discussed, with vast and comprehensive knowledge available in the form of reports and reviews, therefore it was not the purpose of this paper. The most important factors that strongly influence the dichotomous effects of NO are its concentration, duration, cell cycle status, and cell redox condition and the presence of oxygen and oxygen radicals that allow the formation of reactive nitrogen species (RNS) [23][24][25][23,24,25].
iNOS expression is significantly increased in ovarian cancer compared to its expression in normal ovarian tissue or benign tumors [26]. As determined in three groups of patients suffering from ovarian cystic tumors, 88% of malignant tumors were characterized by the strongly increased expression of iNOS in comparison to that in nonneoplastic (5%) or benign tumors (6%) [27]. The positive expression of iNOS was observed in both epithelial cells and tumor-associated macrophages (TAM) in malignant, borderline and benign tumors [28]. The importance of iNOS-positive stromal cells (omental adipose stromal cells) in the promotion of ovarian cancer cell proliferation and their resistance to paclitaxel was shown by Salimian Rizi et al. [29]. However, it should be emphasized that similar to other tumors, both iNOS and NO play rather multifaceted roles in ovarian cancer, and despite obtainable reports in this subject, it is difficult to draw any final conclusions [30].
The expression of iNOS in tumor cells is upregulated (Figure 2) by extracellular signals, such as pro-inflammatory cytokines and hypoxia. The ovarian cancer tumor microenvironment is characterized by high levels of IL-6, IL-1, and TNF-α, which strongly activate the JAK/STAT and MAPK signaling pathways known to be iNOS inducers [31][32][31,32]. It should be mentioned that cancer cells are characterized by the constant overexpression and overactivation of STAT3 [33], serine-threonine protein kinase (AKT) [34] and MAPK [35][36][35,36] signaling proteins, allowing them to survive, grow, proliferate and resist various chemotherapeutics. Many in vitro studies have shown that hypoxia alone or in parallel with pro-inflammatory cytokines increases the level of iNOS mRNA. The main mechanism for hypoxic iNOS stimulation includes two pathways. In the first pathway, hypoxia-inducible factor (HIF), an essential factor in the cellular response to hypoxic conditions, directly binds to the hypoxia-responsive element (HRE) present in the promoter region of iNOS. In the second pathway, hypoxia activates NF-κB through the activation of the inhibitory κB kinase, leading to classical NF-κB signal transduction and the induction of iNOS. It is worth noting that both of these pathways are interdependent and can be altered by multiple factors (including NO) [37].
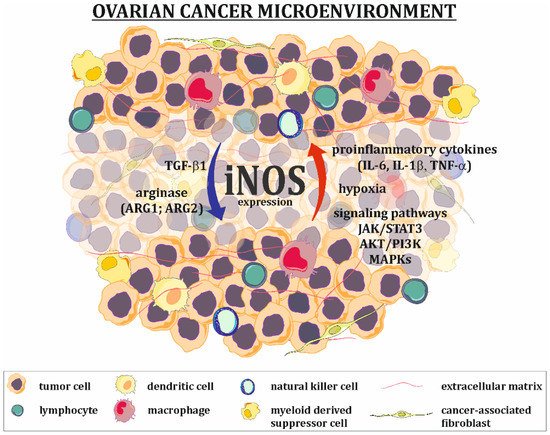
Figure 2. Regulation of iNOS expression in ovarian cancer microenvironment. Factors present in the ovarian cancer microenvironment, such as proinflammatory cytokines (IL-1β, IL-6, TNF-α), as well as hypoxia and overactivation of some signaling pathways (e.g., JAK/STAT3, AKT/PI3K, MAPKs), are responsible for upregulation of iNOS expression in tumor cells, while agents like TGF-β1 and arginase downregulate its expression.
The agents that downregulate iNOS expression/activation are primarily arginase and TGF-β1 (Figure 2). Arginase exists in two forms: ARG1 and ARG2. It catalyzes the conversion of arginine to ornithine and urea. One of the functions of arginase is the regulation of iNOS activity by lowering L-arginine bioavailability and the downregulation of iNOS expression, which is manifested by low levels of this amino acid [38][39][38,39]. The positive expression of TGF-β1 in ovarian tumors, as well as in ovarian cancer cells present in ascites fluid, has been well documented [40][41][42][40,41,42]. This cytokine suppresses the expression of iNOS at the mRNA level [43].
43. Targeting of iNOS in Ovarian Cancer
Since iNOS and the NO it produces have a dual nature and express both pro- and antitumor activity, it is probable that iNOS inhibitors or enhancers can be both beneficial and detrimental to ovarian cancer cell activity and survival. However, to date, few studies have explored the role of iNOS level manipulation in ovarian cancer. Thus, more research regarding the importance of this enzyme as a therapeutic target for ovarian cancer treatment is strongly needed. In a published report, the culture of SKOV-3 and MDAH2774 ovarian cancer cells with L-NAME (an L-arginine analog) significantly reduced the ability of cells to produce vascular endothelial growth factor (VEGF) and completely blocked their capacity for angiogenesis, in the in vitro angiogenesis assay. Therefore, the authors noted the positive effect of iNOS inhibition on blocking the metastatic potential of ovarian cancer cells. It should be noticed that the action of L-NAME was related to the decrease of the NO level, which is known to up-regulate the strongly pro-angiogenic factors IL-8 and VEGF-A [44][79]. Another study showed that a lack of iNOS activity is worse for cancer cells. The addition of 1400W, an iNOS inhibitor, to OVCAR-3 and Caov-3 ovarian cancer cell cultures or their treatment with NOS2 siRNA significantly reduced cell growth. Moreover, targeting iNOS also had a positive effect on the immune cell content in the tumor environment, since NOS2 siRNA decreased the number of M2-type TAM in OVCAR-3 and Caov-3 xenografts [45][80]. Similarly, silencing iNOS gene expression in epithelial the ovarian cancer cell lines MDAH2774 and SKOV-3 resulted in increased caspase-3 activity and a significant increase in cell apoptosis assessed by TUNEL. What is also interesting iNOS expression was in strict correlation with myeloperoxidase (MPO) expression, and silencing MPO gene also resulted in significant induction of ovarian cancer cells apoptosis [46][81]. On the other hand, it should also be mentioned that the beneficial effect of iNOS expression in tumor relapse was also described. Studies using a murine ovarian carcinoma model showed that IFN-γ gene therapy (liposomal IFN-γ gene) together with cisplatin-induced high levels of NO in ascites and kept the mice alive. In contrast, iNOS KO mice treated in the same way showed no NO in ascites and died in the course of treatment [47][82]. A different study also showed that iNOS-expressing micro-encapsulated cells significantly inhibited tumor mass (induced by SKOV-3) in mice [48][83]. The key mechanism by which NO promote angiogenesis is the induction of VEGF-A production [49][85] (Table 13).Table 13.
Summary of the beneficial and detrimental effects of iNOS targeting in ovarian cancer.
| Ovarian Cancer Model | Treatment | Effect | Ref. |
|---|
| iNOS Inhibition | |||
| Ovarian cancer cell lines | L-NAME -NOS inhibitor | Reduction of VEGF production | [44][79] |
| Ovarian cancer cell lines | 1400W- iNOS inhibitor iNOS siRNA |
Inhibition of cell growth | [45][80] |
| Ovarian cancer cell lines | iNOS siRNA | Increased activity of caspase 3 Induction of cell apoptosis |
[46][81] |
| Xenograft model | iNOS siRNA | Decrease in number of M2-type of TAM in tumor | [45][80] |
| iNOS Induction | |||
| Murine ovarian carcinoma | IFN-γ + cisplatin | High level of NO in ascites Enhanced survival of mice |
[47][82] |
| Murine ovarian carcinoma | iNOS-expressed micro-encapsulated cells | Inhibition of tumor growth | [48][83] |
| Mouse ovarian teratoma | IFN-β | Inhibition of tumor growth Enhanced survival of mice |
[50][84] |
| Ovarian cancer cell lines | DLX4 | Stimulation of STAT1 activity Stimulation of VEGF-A production |
[49][85] |
| Xenograft model | DLX4 | Stimulation of tumor angiogenesis | [49][85] |
