Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Laura Cortese and Version 2 by Catherine Yang.
Leptin, an adipokine regulating body fat mass, represents a key molecule in obesity, able to modulate immune responses and foster chronic inflammatory response in peripheral tissues.
- leptin
- obesity
- immune regulation
- Physiology
1. Physiological Role of Leptin and Its Relationship with Obesity
Leptin is a hormone secreted by white adipocytes [1][2][16,17]. Through the blood-brain barrier, such a hormone reaches the hypothalamus to decrease food intake and to increase metabolism [1][16]. Leptin receptors, encoded by the LEPR gene [3][4][18,19], are expressed by hypothalamic satiety centres and are widely disseminated throughout the body—this occurrence reflects the pleiotropic nature of leptin that is involved in the control of many physiologic processes [5][20]. Ob-Rb, the ‘long’ isoform of the receptor, is predominantly expressed in the hypothalamus [6][7][8][21,22,23], while the short isoforms (Ob-Ra, Ob-Rc, Ob-Rd, and Ob-Rf) are expressed in the peripheral tissues [9][10][24,25]. Leptin receptor (LEPR) needs the activation of receptor associated kinases of Janus family (JAKs), which in turn induce downstream signalling involving different members of signal transducers and activators of transcription (STAT) family [11][26]. Leptin receptors activate a complex neural circuit involving anorexigenic (i.e., appetite-diminishing) and orexigenic (i.e., appetite-stimulating) neuropeptides to control food intake.
Moreover, leptin also stimulates the sympathetic nervous system inducing an increase in plasma norepinephrine and epinephrine concentrations via the ventromedial hypothalamus [12][27].
In addition to its pivotal role in the regulation of energy metabolism [13][28], leptin possesses other important physiological activities as the control of neuroendocrine and immune functions, and haematopoiesis [14][15][29,30]. The strict association between obesity and hematopoietic disruption evidenced the role of leptin on bone organization. The direct role for leptin in haematopoiesis has been demonstrated by the presence of Ob-R on bone marrow CD34+ cells as well as on lympho-haematopoietic and megakaryocytic cell lines [16][17][31,32]. Recently, Claycombe et al. [18][33] demonstrated that myelopoiesis recover after treatment with leptin in obese mice (ob/ob). Aberrant leptin levels in patients with haematological malignancies have been described, suggesting that leptin signalling is involved in the progression of haematological malignancies and could represent a useful prognostic value [19][34].
Relationship between leptin and obesity could be considered as a part of metabolic syndrome (MS), the pathological condition comprising of also dyslipidaemia, hyperglycaemia, and high blood pressure. It is noteworthy that obesity is related to the leptin receptor resistance mechanisms [20][35], including several aspects such as: (i) Interruption of leptin signalling in hypothalamic and other central nervous system (CNS) neurons; (ii) alteration of leptin transport across blood-brain barrier; (iii) hypothalamic inflammation, autophagy, and endoplasmic reticulum stress [21][22][36,37]. The development of leptin resistance and of hyperleptinemia have been widely demonstrated in humans and in domestic animals [23][38].
In the course of obesity and hyperleptinemia condition, an accumulation of epicardial adipose tissue has been demonstrated [24][39], suggesting its involvement in cardiovascular system damage. Chronic inflammation and the accumulation of epicardial fat is strongly concomitant with coronary artery disease, independent of visceral adiposity [24][39]. Furthermore, high circulating levels of leptin appeared to induce significant impairment of the haemostatic balance in cardiovascular diseases [25][40].
Moreover, leptin has been associated to hypertension and congestive heart failure (HF) in humans, dogs, and cats [23][26][38,41]. In addition, leptin accelerates atherosclerosis spreading [27][42].
The role of leptin and adipokines on the cardiovascular system have been largely described to be dependent on two mechanisms involving the heart or the central nervous system [28][29][30][43,44,45]. Leptin acts by stimulating the migration and proliferation of vascular smooth muscle cells (VSMCs) [31][46]. Such hormones block the vasoconstrictor action of angiotensin II and inhibits the angiotensin II-induced increase in intracellular Ca2+ in VSMCs through Ob-Rb [32][47]. Leptin shows angiogenetic effects dependent on both proliferation and migration of vascular smooth muscle cells by promoting the upregulation of vascular endothelial growth factor (VEGF) expression [33][48] and the cytoskeleton reorganization [34][49].
Acute pancreatitis is associated with high levels of leptin in serum and pancreas [35][36][50,51], suggesting the role for such a hormone as a marker for adipose tissue necrosis [37][52]. Intriguingly, the pancreas could secrete leptin and its protective role in pancreatitis has been described [38][39][53,54]. In agreement with this hypothesis, beneficial effects of leptin on acute pancreatitis have been evidenced in ischemia/reperfusion [39][40][54,55].
2. Role of Leptin in the Relationship between Obesity and Immune-Modulation.
An interesting scenario on obesity is that immune response greedily needs “energy” to be implemented. In a pathophysiological perspective, this energy can be in excess or in deficit. In this regard, food opulence is frequently associated with autoimmune diseases [41][42][43][7,56,57], while hyponutrition induces susceptibility to infectious diseases [44][45][46][47][58,59,60,61]. Therefore, an excess of nutrients could drive the immune system towards self-reactivity, while a defect can determine insufficient anti-infectious immune responses. In this regard, the relationship between obesity and immune modulation appears of great relevance in both human and veterinary medicine [41][42][43][48][49][50][51][52][53][7,56,57,62,63,64,65,66,67].
In human and animal obesity, the secretion of leptin and other hormones from the adipose tissue appears to determine the dysregulation of the immune response [41][54][55][7,68,69] (Figure 1).
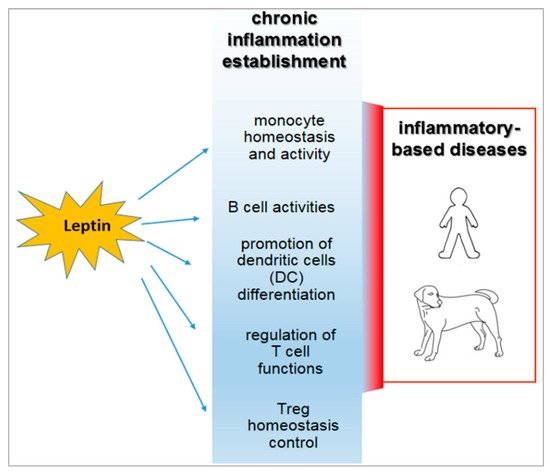
Figure 1.
Inflammatory roles of leptin in the course of obesity and their relevance in both human and veterinary medicine.
Moreover, leptin and its receptors are integral components of a complex physiological system evolved to regulate fuel stores and energy balance at an optimum level in mammals [56][70].
Leptin has structural similarities with the alpha-helix family of cytokines and its receptor (ObR) belongs to the superfamily of class I cytokine receptors [57][71]. Leptin receptors are expressed by immune system cells [58][59][60][72,73,74], and leptin possesses modulatory effects on both innate and adaptive immunity [61][62][75,76] (Figure 2). Such a hormone is currently considered a pro-inflammatory adipokine [41][63][64][7,8,12]. In this regard, leptin acts as an acute phase inflammatory cytokine like interleukin (IL)-1, IL-6, and tumour necrosis factor (TNF)-α [14][29] and is necessary for phagocytosis of bacteria by polymorph nuclear cells [65][77].
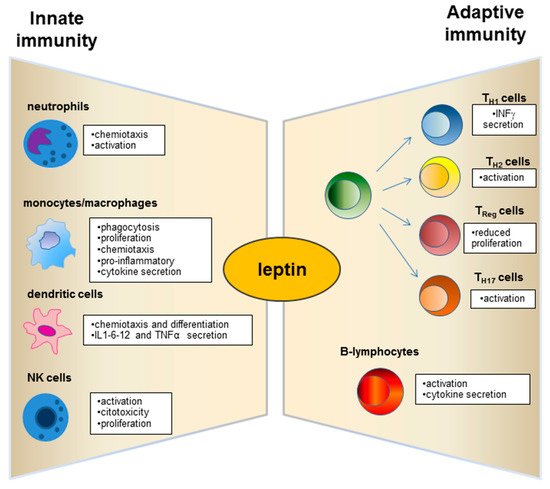
Figure 2.
Physiological role of leptin on innate and adaptive immunity.
Neutrophils express the short form of the leptin receptor [66][86] that can stimulate the expression of CD11b and prevent apoptosis.
Dendritic cells (DC), a specialized cell population for antigen uptake in body tissues, express leptin receptors (Ob-R) on their surface [67][87]. Leptin acts on these cells, favouring their differentiation, maturation, recruitment, and survival [67][68][87,88] and modulating the signalling pathways involved in these biological processes as observed in db/db mice lacking leptin receptors (Ob-R) [68][88]. Furthermore, an important role of leptin is exercised by the activation and recruitment of the DC (Figure 2).
Deficits of leptin receptors in Natural Killer (NK) cells correlate with decreased NK number and functions [69][70][89,90].
The role of leptin in adaptive immunity has been largely demonstrated from early studies on db/db mice that showed high level of thymocyte apoptosis [72][92].
A great research interest has moved to explore the leptin role on the T and B cell population (Figure 2). Leptin acts with several mechanisms on T lymphocytes and induces the expression of the long isoform of LEPR in CD4+ T cells [73][93]. Such adipokine promotes activation and proliferation of T lymphocytes and enhances their cytokine production [74][75][94,95]. In addition, the leptin supplementation to a mixed lymphocyte reaction has been observed to induce a proliferation of CD4+ T cells [75][95].
Leptin regulates the adaptive immunity, also influencing activities of T Helper (Th) 1 and 2 lymphocytes [41][63][44][76][7,8,58,96]. In particular, the hormone stimulates the Th1 production of cytokines such as IL-2, interferon (IFN)-γ, TNF-α, and IL-18, and drives the differentiation of the Th17 cells mainly involved in chronic inflammation establishment [77][78][97,98].
In addition, leptin influences B-cell activities, regulating and promoting cell cycle by Bcl-2 and cyclin D activation [79][99].
It is of note that leptin acts on the homeostasis of a specific CD4+CD25highFoxp3+ T immune regulatory cell population, usually referred to as Treg [41][80][81][82][83][84][7,100,101,102,103,104]. Such cells avoid the auto reactivity of the immune system against the “self” molecular components that belong to the individual itself [41][80][81][82][7,100,101,102]. Human Treg cells display heterogeneous gene expression, phenotype, and suppressive functions [85][105]. This occurrence strongly correlates with the different splicing variants of the transcriptional factor FoxP3 [86][106]—the full-length isoform (FoxP3fl), which contains the sequences involved in the interaction with retinoic acid-related orphan receptors α and γt (RORα and RORγt), is associated with Treg function in humans [87][107]. In contrast, the expression of the isoform lacking exon 2 (FoxP3Δ2) correlates with dysfunction of Treg cells, since it appears to be unable to interact with RORα and RORγt [88][108]. FoxP3Δ2 expression has been correlated with multiple sclerosis in humans [89][109]. Expression of the different FOXP3 isoforms is conditioned by metabolic aspects [90][110] and by the exposure of Treg to the pro-inflammatory micro-environment [91][111]. No data over this potential functional dichotomy are available from canine or feline models.
Nutrient availability is essential for the maintenance of tissue homeostasis. In this context, the intracellular “sensor” of nutrients [92][112] is represented by the mammalian target of rapamycin, the mTOR molecule [93][113]. This serine–threonine kinase “senses” the extracellular bioavailability of amino acids, glucose, growth factors, and hormones [81][92][93][94][101,112,113,114], promotes cell metabolism and growth when the conditions are favourable; or catabolic processes when conditions are not favourable. In this context, mTOR is strongly correlated with Treg homeostasis and functions [95][115]. High levels of leptin correlate with a reduced number and with decreased functions of Treg cells in human autoimmune diseases [41][42][7,56]. The relationship between metabolism and cell plasticity is of great relevance, particularly for the homeostasis of immune system cells that are highly “sensitive” to bioavailable nutrients [96][97][98][99][116,117,118,119].
Adiposity has been associated with increased concentrations of leptin and other proinflammatory adipokines, cytokines, and acute-phase proteins [100][128]. The role of adiponectin in dogs still appears controversial and few data are available in the veterinary literature on the possible impact of obesity on the immune response. The effects of weight loss on canine adipokines and cytokines have been reported [101][102][103][104][105][2,3,129,130,131]. Several studies showed that plasma leptin concentrations correlate with body fat content in experimentally induced obese beagles [106][107][132,133]. In this regard, Sagawa et al. highlighted that the positive relationship between plasma leptin concentration and body fat content in dogs is similar to correlations reported for humans and rodents [106][132]. Ishioka et al. [108][134] showed that plasma leptin represents an index of adiposity in dogs regardless of their age, gender, and breed variations. It is well known that plasma leptin concentrations increase with weight gain and decrease with weight loss in dogs. In this regard, Jeusette et al. [109][135] described a decrease in ghrelin and an increase in leptin and insulin concentrations in obese beagle dogs. The same authors [109][135] suggested that ghrelin and leptin could play a role in dogs in the adaptation to a positive or negative energy balance, as observed in humans. Proinflammatory state directly influences glucose metabolism, resulting in decreased insulin sensitivity [100][128]. In fact, high-plasma leptin concentrations have been correlated to insulin resistance in humans [110][136] and in insulin-resistant dogs [109][135]. Serum leptin concentrations correlated with percentage of body fat and decreased with weight loss, whereas the involvement of other inflammatory markers in canine obesity and weight loss is still less understood. Induction of canine obesity has been shown to increase concentrations of TNF-α [111][137] which decreases after a weight loss program in obese dogs [101][2]. However, acute phase proteins appeared to be unaltered after the weight loss program [103][129], while the production of C-reactive protein decreased in obese dogs [101][103][104][105][112][2,129,130,131,138].
Van de Velde et al. [113][139] investigated the effect of a short-term increase in body weight on immunological variables in adult healthy beagle dogs in which weight gain and increased body condition score (BCS) were accompanied by a significantly higher leptin concentration. Subsequently, the same authors [114][140] described that T-cell proliferation is affected after weight gain in Beagle dogs.
Recently, concentrations of IL-6 and monocyte chemoattractant protein 1, but not IL-8, were found to be increased in overweight dogs [115][141], whereas other authors described decreasing concentrations of IL-8 and other interleukins with weight loss in dogs [105][131]. Piantedosi et al. [116][142] revealed no significant differences in serum TNF-α and IL-6 concentrations between obese and normal weight dogs.
Increased inflammatory response has been correlated with clinical exacerbation, and the immunotherapeutic role of Tregs appears to be relevant in leishmaniosis [117][149].
Tregs function, macrophage activation, and the proinflammatory state appear to be involved in the pathogenesis of canine leishmaniasis. Naturally L. infantum infected dogs expressed alteration in leptin gene transcription and low levels of circulating Treg [118][150]. In the same model, ineffective immune response to parasites appeared to be associated with high Treg levels [119][151]. Di Loria et al. [120][152] showed an increase in leptin mRNA expression in dogs naturally infected by L. infantum.
