You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Mohammed Jawad Ahmed Alathari and Version 2 by Bruce Ren.
Life was once normal before the first announcement of COVID-19’s first case in Wuhan, China, and what was slowly spreading became an overnight worldwide pandemic. Ever since the virus spread at the end of 2019, it has been morphing and rapidly adapting to human nature changes which cause difficult conundrums in the efforts of fighting it.
- SARS-CoV-2 detection
- COVID-19 detection
- coronavirus detection
- transmission human exchange
- next generation sequencing (NGS)
- RT-PCR
- LAMP
- biosensor application
1. Introduction and Overview of Coronaviruses:
The Coronaviridae family to which the Coronavirus belongs is also a part of the Nidovirales order and is the subfamily of Orthocoronavirinae. The subfamily of Orthocoronavirinae consists of four types which are delta (δ) beta (β), alpha (α), and gamma (γ) coronavirus as shown in Figure 1, these viruses share qualities where they are all enveloped, contain single-stranded RNA, have positive-sense and are not segmented viruses that cause minor or critical illnesses in some breathing creatures which includes human beings. The name of Coronavirus is driven from its club-shaped spikes, protruding from the virion surface resulting in a solar corona shape [1][2][3][1,2,3].
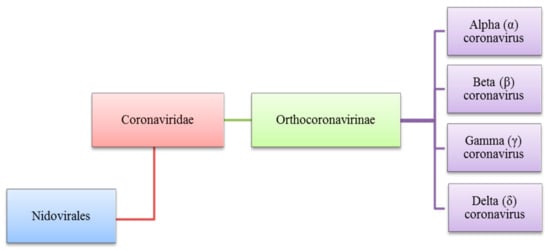
Figure 1. Diverse types of coronaviruses within Nidovirales, Coronaviridae family, Orthocoronavirinae subfamily and the respective genera, (α), (β), (γ) and (δ).
Preliminary studies on Coronaviruses were reported in the 1930s. In contrast, the human type of the virus named (HCoVs) was first discovered in the 1960s. Until recently, seven types of the virus have been discovered including the HCoVs-NL63 and HCoVs-229E 229E (α-Corona viruses) and HCoVs-OC43, HCoVs-HKU1 (β-Corona viruses), severe acute respiratory syndrome-CoV (SARS-CoV), and Middle East respiratory syndrome-CoV (MERS-CoV), and SARS-CoV-2 which was discovered in 2019 [4][5][6][4,5,6].
1.1. Coronaviruses That Infect the Human Body Respiratory System
Coronaviruses were mostly known to infect animals of mammal species and birds; it is only recently that they have infected humans. The latest research reported seven types of the Coronavirus that can infect humans, four of which are (229E, NL63 HKU1, and OC43). These viruses are reported to cause a respiratory system infection, in which symptoms include coughing, sore throat and coryza. Other reported symptoms that are less likely to occur are pneumonia, bronchiolitis, and bronchitis, whereas three of them MERS-CoV, SARS-CoV and SARS-CoV-2 result in illnesses with fluctuating severity to the human respiratory system. The severity ranges from the common cold to incurable pneumonia [7][8][9][10][7,8,9,10].
In 2002, severe acute respiratory syndrome (SARS) was reported first in China and soon after affected other countries worldwide. The virus caused infection in human respiratory system organs that were incurable and therefore fatal in most cases [11][12][13][11,12,13]. The virus’s fatality range is 50% higher in seniors than younger adults, with young adults having a ~3–6% fatality percentage [14][15][14,15]. A coronavirus known as SARS-CoV was found as the etiological agent of SARS [16].
A decade later a new variation of the virus known as (MERS-CoV) Middle East respiratory syndrome started spreading in the Middle East in 2012; the first fatality reported was at the hospital in Jeddah, Kingdom of Saudi Arabia [17][18][19][17,18,19]. This case was the starting point for a series of infections in surrounding countries and later worldwide [20]. The virus causes lower respiratory system infection and has a 35% fatality percentage. Since the virus outbreak, 2279 cases were reported, 806 of which were fatal. After reports of an outbreak in 27 countries, the WHO listed MERS-CoV as a priority disease requiring urgent research. Notably, this virus’s nature is continuously evolving. Hence, it can efficiently fight human antiviral responses. In retrospect, there are only supportive treatments for the virus [21][22][21,22].
In December 2019, seven years after the appearance of MERS-CoV, a new case of virus infection was reported in Wuhan, China. The virus was named by the World Health Organization (WHO) the 2019 novel Coronavirus (2019-nCoV). The name was later changed to SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), based on its similarities to SARS-CoV. The WHO named the disease caused by the virus infection as COVID-19 [23]. Based on recent data, over 216 M people have reported positive for COVID-19 worldwide by August 2021 [24]. This highly dangerous pandemic has affected all aspects of human day-to-day activities and impeded the most routine activities to a stage that was not possible to predict [25]. In retrospect, international transportations were limited and quarantine and social distancing rules were enforced in most nations [26].
However, the enforced strategies are not a permanent solution as they will have unfavorable impacts on the economy, education, food system including mental wellbeing [27]. In response to the enforced strategies, many have lost their income sources, and others are threatened to lose their job at any minute. It is notable that enforcing strict rules is not controllable and governments are yet to handle this long term [28]. The transmission speed of the virus is soaring and it is a challenge to manage the issues it prevails [29]. This issue is even more crucial when associated with healthcare providers [30]. Having rapid diagnostic technologies is vital to navigating this fatal outbreak [31][32][31,32]. Table 1 compares coronaviruses that infect the human body respiratory system; the table compares coronavirus names, year of finding, emergence, type, host, cellular receptor, incubation period, respiratory system infection, symptoms, and mortality rate. The most recently discovered virus in India was the Delta Variant which is a mutation of the SARS-CoV-2 virus. The Delta Variant virus is hosted in humans. The cellular receptor of the virus is ACE2 and the incubation period is 5–6 days. The symptoms accompanied are headaches, sore throat, runny nose, cough, loss of taste and loss of smell.
Table 1. Comparative Coronaviruses that infect the human body respiratory system.
| Reference | Coronavirus | Analyte | Target Genes | Detection Methods | Limit of Detection | Concentration Range |
Detection Time | Tested Sample | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [33] | [134] | SARS-CoV | RNA | Polymerase | RT-PCR | 10 copies/reaction | N.A. | (5) h | Nasal aspirate | ||||
| [34] | [135] | SARS-CoV | RNA | NA | RT-PCR | 2 nM | N.A. | (~2) h | Throat swab samples | ||||
| [35] | [136] | MERS-CoV | RNA | (N) gene | rRT-PCR | 10 copies/reaction or 0.0013 TCID50/ml | 10–10 | 8 | copies/-reaction | (~2) h | Serum, nasopharyngeal/- oropharyngeal swab, and sputum samples | ||
| [36] | [137] | COVID-19 | RNA | (E)-gene | rRT-PCR | 275.7 copies/reaction | N.A. | (~1) h | Swab samples | ||||
| [37] | [138] | COVID-19 | RNA | (N) gene | rRT-PCR | 10 copies/reaction | N.A. | (~30) min | Plasmids containing the complete N gene | ||||
| [38] | [139] | MERS-CoV | RNA | (N) gene | RT-LAMP | 10 copies/μL | 5 × 10 | 1 | –5 × 10 | 8 | copies/-reaction | (35) min | Throat swab specimens |
| [39] | [140] | SARS-CoV | RNA | (ORF1b) and (N) gene | LAMP | 10 | 4 | copies/reaction | N.A. | (20–25) min | Synthetic RNA solutions | ||
| [40] | [141] | COVID-19 | RNA | (ORF1b) and (N) gene | RT-LAMP | 20 copies/reaction | N.A. | (20–30) min | Nasopharyngeal swab and bronchoalveolar lavage fluid samples | ||||
| [41] | [126] | COVID-19 | RNA | (ORF1ab), (N) and (E) gene | RT-LAMP | 5 copies/reaction | N.A. | (30) min | Nasopharyngeal swab specimens | ||||
| [42] | [142] | COVID-19 | RNA | (S) gene | NGS | 125 GCE/mL | N.A. | N.A. | Nasopharyngeal swab | ||||
| [43] | [132] | COVID-19 | RNA | (N) and (E) gene | CRISPR/Cas13a | ~100 copies/µL | 3.2 × 10 | 5 | –1.65 × 10 | 3 | copies/µL | (30) min | Nasal swab |
1.2. SARS-CoV and SARS-CoV-2 Genomic Structure and Proteins
The genomic structure and proteins of SARS-CoV and SARS-CoV-2 viruses have similarities more than differences. Both viruses reported being spherical single-stranded RNA viruses that are known to have protein spikes that are protruding from the virion surface. This characteristic in the virus shape gave it its name, Coronavirus, driven from the Latin word corona due to its resemblance to the crown shape [44][45][38,39].
SARS-CoV-2 genome consists of 29,903 nucleotides that are significantly similar to SARS such as SARS-CoV that infects bats by 81% nucleotides resemblance. Both viruses have a unique replication approach considering their large RNA genome with none segmented positive-sense RNA genome featuring a shape of 5′cap and 3′ poly (A) tail. The unique structure of the virus allows the replicase polyproteins to understand the genome for translating 2/3 of the genome encodes nonstructural proteins (NSPs). In contrast, the remaining 1/3 encodes for structural and auxiliary proteins. There are four main structural proteins known as (S) spike, (M) membrane, (E) envelope and nucleocapsid (N) proteins. Furthermore, PP1a and PP1b are viral replicas created by the virus are later customized into 16 mature NSPs [46][47][40,41]. The pre-mentioned proteins of coronavirus and genomic structure of SARS-CoV and SARS-Cov-2 are illustrated in Figure 2.
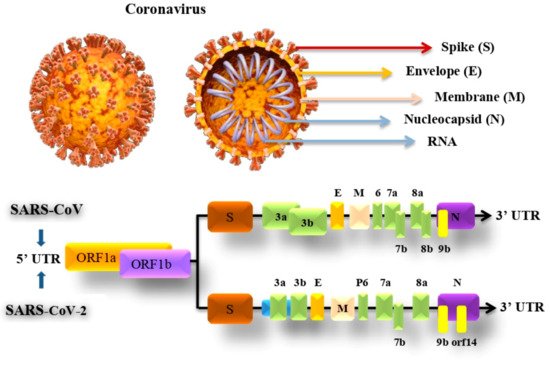
Figure 2. SARS-CoV and SARS-CoV-2 genomic structure and proteins.
The S protein in the virus that weighs about 180 kDa molecular units and is 20 nm long is possibly a target for inhibition of viral entrance and the growth of antibody-based therapeutics to prevent the disease. The protein comprises two subunits which are S1 and S2. The S1 receptor is for binding, which contains the receptor-binding domain (RBD), which identifies and binds to its receptor angiotensin-converting enzyme 2 (ACE2), which is found in the epithelial cells of the lungs. The S2 subunit for membrane fusion, by undertaking specific conformational modifications, induces membrane fusion, which enables viral entry into the host cell. This protein is the chief focus of neutralizing antibodies, which in response prevents infection and further dissemination by stopping binding to ACE2 [48][49][42,43].
The E protein is a small membrane protein that manages virion construction and has added effects on the affected host cells. During the host cell infection, the E protein controls the stress response in the cell which, as a result, modifies the virulence of the virus leading to caspase-mediated cell death. The M protein is the amplest in the viral envelope, this protein is essential to the virion particles creation. N protein helps in creating the virial nucleocapsid by forming complexes with the genome RNA within the viral membrane. Both M and N proteins play an important role in RNA replication [50][44].
2. Detection Techniques of COVID-19 Virus
The recent pandemic of COVID-19 has drawn attention to the high importance of rapid and accurate diagnostic assays. In parallel to the outbreak, researchers from different areas worldwide have worked together for such assays, concurrently to other many assays that are approved along with the ones that are yet clinically validated. Various techniques of Coronavirus detection have been studied to detect potential patients, in this paper we elaborate on two main methods which are Ribonucleic Acid (RNA)-based techniques and Biosensor techniques.
2.1. Based on Ribonucleic Acid (RNA) Method
Molecular diagnostic assays are the leading group of tests used to diagnose COVID-19, mainly using RNA to detect the SARS-CoV-2 virus in patients. The latest detection techniques of COVID-19 RNA, including those still in the development stage, are CRISPR and next-generation sequencing (NGS). Another detection method which is the current golden standard technique is reverse transcription-polymerase chain reaction (RT-PCR) as well as the second most used method which is known as loop-mediated isothermal amplification (LAMP). This section thoroughly explains the previously mentioned techniques and their applications in the efforts of COVID-19 detection.
2.1.1. Next-Generation Sequencing (NGS)
Technologies along with bioinformatics played a significant role in changing the conventional research on viral pathogens and attracting attention to the field of virus diagnosis [51][117]. Recent research and developments in next-generation sequencing (NGS), alternatively named high-throughput sequencing (HTS) offered to the science field countless applications, and the current outbreak is urging these applications to be wildly used. An advantage of applying NGS for the detection of infectious diseases in the clinical diagnosis is that it is neither culturing nor clinical hypothesis dependent. NGS-based testing discloses all kinds of existing microorganisms in the sample such as fungi, bacteria, parasites and of course viruses, unlike conventional testing methods that require clinicians to do the extra work of addressing patients’ symptoms with possible explanations and requests for testing those particular pathogens [52][118].
NGS is based on massively parallel sequencing, meaning that billions of short DNA fragments are sequenced simultaneously producing short sequence “reads” rendering dramatically reduced time and cost of sequencing [53][119]. This technology is reported to be one of the best applications in the current outbreak. Since the emergence of the virus, false-negative results were reported for patients admitted with acute respiratory distress syndrome. It was also reported that this technology was the only one that discovered the etiological pathogen by applying metagenomic RNA sequencing and analyzing the phylogenetic of the complete generated genome allowing us to conclude that the founded new strain of RNA belonged to the Coronaviridae family and was later specified as COVID-19 after nucleotide similarity and genome matching with the existing pathogen’s genome [51][117]. As a result, NGS technology shows promising results in the efforts of SARS-CoV-2 virus detection.
2.1.2. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
RT-PCR is the most reliable and gold standard method for the identification of COVID-19 infection [54][120]. because of its advantages as a precise, and simple quantitative assay [55][121]. Moreover, real-time RT-PCR showed higher sensitivity compared to RT-PCR assay, which helped greatly in early infection diagnosis [56][122]. Based on laboratory testing, using this method requires collecting samples for RNA extraction followed by reverse transcription from suspected patients’ upper respiratory tract fluids such as nasal aspirate, nasopharyngeal swab, or pharyngeal swab or the lower respiratory tract (sputum, tracheal aspirate) [57][123]. After completing the reverse transcription, the cDNA regions are amplified to sufficient levels for pathogen presence detection. This process depends on DNA primer–probe sets complementary to specific regions of the SARS-CoV-2 cDNA, as well as scientists worldwide, who are competing to create these sets since the first SARS-CoV-2 genome was publicly shared. Tests were constructed by finding a number of SARS-CoV-2 particular regions, such as (N/S/E) genes, ORF1ab, and RNA-dependent RNA polymerase (RdRp) [52][118].
There are two types of RT-PCR testing; the first type uses a single tube containing the required primers to execute the whole process of RT-PCR reaction, the other type is known as two-step, whereby it requires more than one tube to complete the test by doing separate reverse transcription and amplification reactions while offering higher flexibility and accuracy than the other test type. It demands fewer starting materials as well as allowing multiple targets quantification using cDNA stocks. Nevertheless, the first type is more appealing for its quick setup and limited sample handling [58][124].
Accuracy and specificity are the reason why RT-PCR is widely used despite its expensive price, time-consuming test duration as well as the need for experienced staff [59][125].
2.1.3. Loop-Mediated Isothermal Amplification (LAMP)
The second in popularity for COVID-19 detection after PCR testing is LAMP testing, PCR testing requires two primers (one in the front and one in the back section), which presents a challenge for LAMP testing that occurs in designing primers and their conflict prevention. Whereas, the LAMP technique needs four or six primers that divide the target sequence into three regions in the forward section and the other three in the back section making a total of six regions. LAMP process is favored because it is executed in an isothermal condition which is an affordable and rapid method. Furthermore, numerous researches reported the application of the LAMP method for coronaviruses detection [41][60][61][126,127,128].
LAMP works on molecular amplification techniques, by rapidly amplifying the genomic material using high efficiency. This technique primarily uses targeted DNA that is synthesized at a stable temperature of 60–65 °C achieved by using explicitly modeled primer sand enzyme (DNA polymerase) that uses strand displacement activity rather than heat denaturation which is used in PCR techniques and within 60 min or less the targeted sequence is amplified to more than 109 copies resulting in the shape of cauliflower including a stem and a loop form of DNA with many inverted repetitions [62][129].
Researchers combined the techniques of reverse transcription and LAMP (RT-LAMP) and produced a detection method that is characterized by being one-step with the high-throughput method of detection for finding the RNA of SARS-CoV-2 with 100 copies/reaction of an RNA virus. By applying this method the results will be given in 30 min which makes it suitable for POC test and screening application for its simplicity and rapidness compared to RT-qPCR and its needlessness for complicated equipment [63][130].
2.1.4. Clusters of Regularly Interspaced Short Palindromic Repeats (CRISPR)
Clusters of regularly interspaced short palindromic repeats (CRISPR) possess a frequent series of nucleotides and tiny-scaled spacer sequences. CRISPR-associated proteins known as CAS acts as the nuclease enzymes. Both are used as a bacterial defense system to protect from unknown invaders and are extensively used in RNA modification, therapy using gene alteration, and viral genome detection. Over the last few years, CRISPR has been widely utilized in the field of Vitro diagnostics due to its allele accuracy which is significant in its successful implementation and delivering high accuracy detection and treatment [64][131].
Several types of research were conducted using CRISPER-based detection systems for SARS-CoV-2 virus detection. For example, a study reported the successful development of a CRISPR-Cas13a-based mobile phone assay that is non-amplified to detect SARS-CoV-2 by testing extracts of RNA taken from nasal swaps. The assay attained ~100 copies/µL sensitivity in less than an hour [43][132]. Additionally, researches showed detection of SARS-CoV-2 using CRISPR approaches in optimal conditions provides rapid and efficient results. Hence, CRISPR-based approaches are a promising, robust and precise method for SARS-CoV-2 diagnosis [65][133].
2.1.5. RNA Corona Virus Detection Methods Analysis Based on RT-PCR, LAMP, and CRISPR
This paper studied the detection of Coronavirus using RNA via RT-PCR, LAMP, NGS, and CRISPR Table 1 compares the four techniques by analyzing eleven reported studies. LAMP technique reported fastest detection time of 20–25 min using test sample of Synthetic RNA solution followed by rRT-PCR which takes 30 min using test sample of Plasmids containing the complete N gene. RT-PCR, LAMP, RT-LAMP, and rRT-PCR collectively use Polymerase, (N) Gene, (E) Gene, and ORF1b as target genes, whereas, whereas CRISPR uses (N) and (E) Gene. On the other hand, NGS uses (S) Gene. NGS Detection method has not had much information due to its recent discovery and not many tests were conducted using it.
Table 1. RNA Coronavirus detection methods analysis based on RT-PCR, LAMP, NGS and CRISPR.
2.2. Based on Biosensor Techniques
Nowadays, researchers are focusing on developing detection tools using biosensors techniques due to their sensitivity, mobility and miniaturization. This section explains four widely used biosensors techniques which are electrochemical biosensors, electronic biosensors, piezoelectric biosensors and optical biosensors.
2.2.1. Electrochemical Biosensors
Electrochemical biosensors are biological concentrations of information that are converted into an analytically relevant signal by using a current or voltage [66][143]. These biosensing devices can read biochemical information to detect biological materials such as protein and nucleic acid. It also holds qualities such as simple instrumentation, high sensitivity, economic, and the capacity for miniaturization [67][68][144,145]. Applications using this type of biosensing device are utilized widely in many areas, whereby it represents a standardized platform for constructing biosensors that include semiconductors and screen-printed electrodes [69][146]. In brief, these biosensors observe the dielectric changes in properties depicted in dimension, shape, and charge distribution. At the same time, the antibody–antigen complex is formed on the electrode surface, which is categorized into four main groups: potentiometric, amperometric, cyclic voltammetry, and impedimetric transducers [70][147].
In a recent study focused on SARS-CoV-2 detection, researchers proposed the detection of COVID-19 N-gene using an antifouling electrochemical biosensor. The biosensor is assembled based on electropolymerized polyaniline (PANI) nanowires combined with lately designed peptides. The biosensor detects the N-gene by using biotin-labeled probes that are immobilized onto peptide-coated PANI nanowires, creating an electrochemical interface that is antifouling and susceptible. The recorded detection limit of this biosensor was shallow at (3.5 fm) [71][148].
2.2.2. Electronic Biosensors
A biosensing device based on field-effect transistors (FETs) consists of a three-electrode structure containing the source, drain, and gate and was developed for the detection of small molecules and the diagnosis of viral diseases. The FET is an electric biosensor that detects changes in surface potential after the target molecule binds to the biorecognition element immobilized on the highly conductive chip surfac [72][149]. For instance, graphene, zinc oxide, gallium nitride, disulfide, and molybdenum are used in FET-based biosensors where heterogeneous analyte concentrations can be detected rapidly via probes fixed on conducting channels [73][150]. This type of biosensor is recently used extensively in creating assays for SARS-CoV-2 detection using spike membrane protein] [74][151]. An example of this method is a graphene FET-based biosensor that can detect COVID-19 related viruses through its spike proteins in only 120 s, also in another way by employing spike protein-specific antibodies, with a 0.2 pM detection limit for the assay [75][152].
An electronic biosensor device based on FET was developed for the detection of COVID-19 related viruses in clinical samples. The sensor was prepared by covering graphene sheets used in the FET with a unique antibody to the spike protein of SARS-CoV-2. The three determinants of the device’s performance are using a cultured virus, antigen protein, and nasopharyngeal swab specimens from infected patients. The resulting device is a super-sensitive immunological diagnostic method for COVID-19 with minimum sample preparations [76][71].
2.2.3. Piezoelectric Biosensors
Piezoelectric biosensors are a collection of analytical devices operated based on recording affinity interaction. A piezoelectric platform or piezoelectric crystal is known as a sensor part that works on the basis of oscillations change that results from the mass tied on the piezoelectric crystal surface [77][153].
Piezoelectric biosensors technology is commonly used to detect hormones, cells, bacteria, viruses, and to study a wide range of interactions on a biomolecular level. This tech offers immediate and unlabelled transduction with high susceptibility, simplicity, and velocity [78][79][154,155]. In diagnosing SARS, the piezoelectric biosensor was utilized to measure a type of coronavirus using a sputum sample. To get the antibodies to stick to the piezoelectric crystals, the experiment bound the SARS-CoV horse polyclonal antibody from protein A to the surface. Changes in mass from the crystal, due to viral binding, recorded a shift in frequency [80][156]. COVID-19 samples are being directly detected by the piezoelectric microcantilever biosensor, which was built without requiring further processing. The biosensor functions as a transducer, and it is coated with an antibody that is relevant to the substance being detected. Because of the mass change caused by the SARS-CoV-2 antigens’ spike proteins, the microcantilever’s surface would experience surface stress and exhibit a quantifiable tip deflection and floating voltage [81][157]. Compared to other biosensors, piezoelectric biosensors seem to display an enhanced level of performance [82][83][158,159]. There is still more study to be carried out before the technology can be applied; while the piezoelectric sensors can detect viral frequency changes, they can also detect them using output voltage directly. On top of that, piezoelectric energy-harvesting devices are anticipated to be used in the IoT, where it is possible to detect viruses by monitoring mechanical vibration [84][160].
2.2.4. Optical Biosensors
High sensitivity and selectivity are benefits of optical biosensors. They can provide precise detection based on a variety of signals, including absorption, refraction, reflection, dispersion, infrared, polarisation, chemiluminescence, fluorescence, phosphorescence, and so on [85][161]. In the industry and publications, there are various types of optical biosensors, including fiber-optic biosensors, such as the optrode biosensor and the evanescent wave biosensor, as well as time-resolved fluorescence, the resonant mirror biosensor, interferometric biosensors, and surface plasmon resonance biosensors. They are capable of detecting various biomolecules on both medical and biological specimens, with an impressive window of use [86][87][162,163].
Four types of optical biosensors (fluorescence, surface plasmon resonance, localized surface plasmon coupled fluorescence (LSPCF), and fiber optic) will be thoroughly described in this section.
Starting with the up to date known largest group of sensors Fluorescence-based optical biosensors, its popularity is credited to the accessibility of countless fluorescent probes, high quality and fitting optical instruments [88][164]. This biosensor is characterized by having a variety of intensity, lifetime, energy, transfer and quantum yield that offers opportunities for further exploration [89][165].
A detection stripe assay based on fluorescent immunochromatographic was built to detect N proteins in a duration of 10 min using samples of nasopharyngeal aspirate and urine with a 68–98% sensitivity rate [90][166]. Another rapid and quantitative approach of anti-COVID-19 IgG antibody based on the fluorescence biosensor optofluidic POC testing was built. It is an easily handled portable system that is suited for instantaneous results for detecting anti-COVID-19 IgG antibodies in samples. Given perfect conditions, the testing duration can be brought down to 25 min with a detection limit of 12.5 ng/mL that surely meets the diagnostic requirements [91][167].
Another auspicious platform for pathogens, as well as COVID-19 N gene detection biosensors, is SPR-based biosensors, owing to their characteristic of label-free real-time sensing [74][151]. An additional label-free SPR-based pioneering aptasensore was developed for COVID-19 N gene detection through thiol-modified niobium carbide MXene quantum dots (Nb2C SH QDs). The resulting tool had a low limit of detection (LOD) of 4.9 pg·mL−1. Thus, it exhibited exceptional selectivity in the existence of different respiratory viruses in human bio serum [92][168].
In addition, the localized surface plasm coupling fluorescence (LSPCF) fiberoptic biosensor was developed to combine a sandwich immunoassay with the SARS coronavirus LSP detection technique. The SARS-CoV N protein can detect very low concentrations (~1 pg/mL) in serum from the LSPCF fiber-optic biosensor [93][169].
Biosensors that use fiber optics are another type of optical biosensor. A device made from fibers is used in the field of optical science to measure biological species (cells, proteins, DNA, and so on) [85][161]. To detect the COVID-19 N protein at the point of care, researchers created an easy-to-use plasmonic fiber optic absorbance biosensor (PFAB) that does not need washing. To perform the P-FAB test, the fiber-optic sensor probe is U-bent, and has high EWA (Evanescent Wave Absorbance) sensibility. The COVID-19 N-protein is measured as the light propagates by the U-bent sensor probe linked with the green LED and the photodetector. The P-FAB approach resulted in the lodging of ~2.5 ng/mL or less within 10 min of reading time by using a GOF fused silica/glass optical fiber (GOF) with citrate-capped AuNP labels (size ~40 nm) [94][170].
3. Coronavirus Detection Methods Analysis Based on Biosensor Usage
COVID-19 detection methods based on biosensor usage were thoroughly analyzed by comparing 11 research reported studies from the year 2004 up till now, including the detection of SARS-CoV, MERS-CoV and COVID-19. Table 2 analyzes and compares the development of four biosensor-based techniques with the aim of virus detection. Different materials were used in the biosensors such as PANI (Electrochemical), Gold (Electrochemical), Gold nanoparticles (Optical (P-FAB)), Graphene (Electrical (FET)), Crystal with quartz wafer (Piezoelectric), Nb2C-SH QD (Optical (SPR)) and Polymethyl methacrylate (Optical (LSPCF)). Detection of COVID-19 using electrochemical biosensor reported the lowest temperature of 4 °C and fastest detection time of 10–30 s using test samples of spiked saliva.
Table 2. Coronavirus detection methods analysis based on biosensor application.
| Reference | Publication Year | Coronavirus | Biosensor Detection Technique | Material | Target | Detection Time | Linear Range | Tested Sample | Limit of Detection | Temperatures | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [71] | [148] | 2 April 2021 | COVID-19 | Electrochemical | (PANI) | N gene | 1 h | 10 | −14 | to 10 | −9 | M | NR | 3.5 fM | 37 °C |
| [95] | [171] | 11 May 2020 | COVID-19 | Electrochemical | Gold | S protein | 10–30 s | 1 fM to 1 μM | Spiked saliva samples | 90 fM | 4 °C | ||||
| [96] | [172] | 27 February 2019 | MERS-CoV | Electrochemical | Gold | S protein | 20 min | 1 pg·mL | −1 | to 10 μg·mL | −1 | Spiked nasal samples | 0.4 and 1.0 pg·mL | −1 | RT |
| [76] | [71] | 15 April 2020 | COVID-19 | Electrical (FET) | Graphene | S protein | 4 h | NR | nasopharyngeal swab | 1.6 × 101 pfu/mL | NR | ||||
| [75] | [152] | 2020 | COVID-19 | Electrical (FET) | Graphene | S protein | 2 min | NR | Spiked spike protein solutions | 0.2 pM | NR | ||||
| [80] | [156] | 1 July 2004 | SARS-CoV | Piezoelectric | Crystal with quartz wafer | Antigen sputum | 1 h | 1–4 µg/µL | NR | 0.60 mg/mL | RT | ||||
| [90] | [166] | 13 March 2020 | COVID-19 | Optical (fluorescence) | Not Specified | N protein | 10 min | NR | Nasopharyngeal aspirate swabs and urine | Not Specified | NR | ||||
| [91] | [167] | 14 August 2021 | COVID-19 | Optical (fluorescence) | Not Specified | IgG | 25 min | NR | Human serum | 12.5 ng/mL | NR | ||||
| [92] | [168] | 11August 2021 | SARS-CoV-2 | Optical (SPR) | Nb2C-SH QD | N gene | NA | 0.05 to 100 ng·mL | −1 | Human serum | 4.9 pg·mL | −1 | NR | ||
| [93] | [169] | 17 July 2009 | SARS-CoV | Optical (LSPCF) | polymethyl methacrylate | N protein | 2 h | 0.1 pg/mL to 1 ng/mL | Human serum | ∼1 pg/mL | 37 °C | ||||
| [94] | [170] | 1 September 2021 | COVID-19 | Optical (P-FAB) | Gold nanoparticles | N protein | 10 min | 0.1 ng/mL and 100 ng/mL | PBS Buffer | ~2.5 ng/mL | NR |
