High-resolution episcopic microscopy (HREM) is a three-dimensional (3D) episcopic imaging modality based on the acquisition of two-dimensional (2D) images from the cut surface of a block of tissue embedded in resin. Such images, acquired serially through the entire length/depth of the tissue block, are aligned and stacked for 3D reconstruction. HREM has proven to be specifically advantageous when integrated in correlative multimodal imaging (CMI) pipelines. CMI creates a composite and zoomable view of exactly the same specimen and region of interest by (sequentially) correlating two or more modalities. CMI combines complementary modalities to gain holistic structural, functional, and chemical information of the entire sample and place molecular details into their overall spatiotemporal multiscale context. HREM has an advantage over in vivo 3D imaging techniques on account of better histomorphologic resolution while simultaneously providing volume data. HREM also has certain advantages over ex vivo light microscopy modalities. The latter can provide better cellular resolution but usually covers a limited area or volume of tissue, with limited 3D structural context.
1. Introduction
High-resolution episcopic microscopy (HREM) is a 3D episcopic imaging modality that is capable of generating volume data from whole embryos and isolated tissue specimens (biopsies). HREM is essentially an innovative adaptation of routine histology and light microscopy techniques which have been described in detail previously
[1] (pp. I.2.g-1–I.2.g-9)
[2][3][2,3]. As in routine histology, fixed samples are dehydrated in increasing concentrations of ethanol or methanol. Then, samples are infiltrated with and embedded in plastic methacrylate resin, which is coloured with eosin B. The polymerised resin blocks are then physically sectioned. After each section, an image is captured directly from the freshly exposed block surface using a fluorescence microscope equipped with a GFP or YFP filter-set. The preceding eosin staining provides unspecific contrast of the embedded tissues and ensures that only structures on the surface of the block are visualised. Both the optics set-up and the sample retain the same position during this procedure, and thus the images can be reconstructed to obtain three-dimensional data of the sample
[1] (pp. I.2.g-1–I.2.g-9)
[3][4][5][6][3,4,5,6].
Correlated multimodal imaging (CMI) represents a combinatorial approach of multiple sequential or parallel in vivo and ex vivo imaging and analysing modalities on the same tissue specimen. CMI is capable of providing structural and functional information on a tissue sample that is visualised at different lateral resolutions and penetration depths across relevant scales. This essentially involves the application of two or more complementary modalities, which in combination, provide a more informative and composite view of normal and abnormal features of the tissue specimen
[7][8][9][7,8,9]. Prominent examples of CMI approaches that combine two modalities include:
-
Combinations of radiology (including computed tomography (CT) and magnetic resonance imaging (MRI)) and pathology (including histopathology) provide valuable clinical diagnostic and preclinical information for better patient care or further biomedical discovery
[10][11][10,11].
-
Combinations of light microscopy and electron microscopy (EM) have been in vogue for diagnostic and research applications. In clinical and some preclinical settings, such combinations are well established in the forms of histopathology (HP) and ultrastructural pathology. In recent times, the combined application of HP, immunohistochemistry, and electron microscopy (scanning and transmission) is central to the characterisation of pulmonary lesions in fatal cases of COVID-19
[12]. In biological research, the combination of these two microscopic modalities is designated as correlative light and electron microscopy (CLEM)
[13][14][13,14].
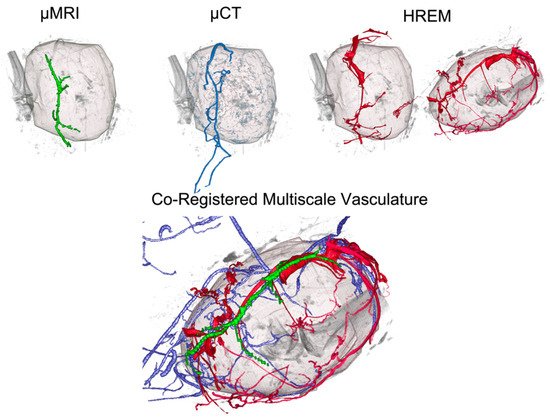
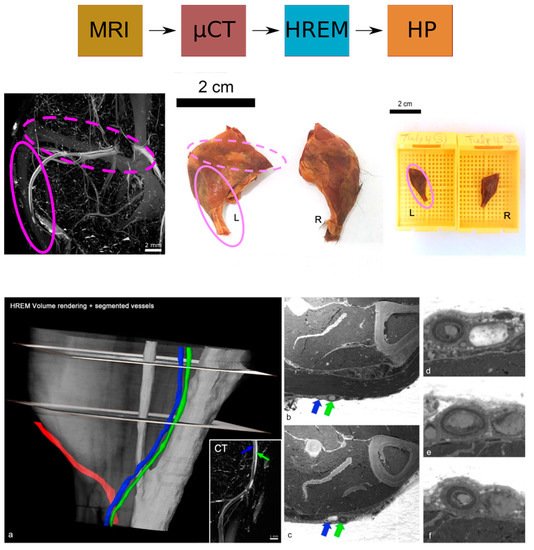
More recently, there have been CMI efforts to combine micro-magnetic resonance imaging (micro-MRI), micro-computed tomography (micro-CT), micro-Positron emission tomography (microPET), HREM, and HP, to evaluate murine tumour vasculature
[15] and murine non-neoplastic vascular lesions
[16] across scales. These efforts highlighted the benefits and challenges of such multimodal efforts. As expected, micro-MRI and micro-CT provided good in vivo anatomic resolution with lower tissue sensitivity, whereas micro-PET was highly sensitive but had poor spatial resolution. HREM and HP achieved the highest spatial histomorphologic resolution, but required irreversible ex vivo processing (trimming, embedding, and sectioning)
[1] (pp. I.2.g-1–I.2.g-9, I.2.i-1–I.2.i-13)
[15][16][15,16]. Despite some of the disadvantages, the combination of in vivo imaging followed by ex vivo processing for sequential or parallel HREM and HP can provide distinct advantages in the characterisation of sub-macroscopic and micro-anatomic morphologic defects
[1] (pp. I.2.g-1–I.2.g-9, I.2.i-1–I.2.i-13)
[15][16][15,16].
Due to the plethora of potentially beneficial imaging combinations, CMI has been used to tackle a variety of research questions and will continue to broaden the accessible biomedical information significantly. In this rentry, researchersview, we highlight HREM as versatile imaging technology that helps bridge preclinical and biological imaging and integrate in vivo dynamics with ex vivo high resolution in a variety of model organisms.
Advantages and Limitations of HREM in a Multimodal Context
The strengths and limitations of HREM are represented in
Table 1 in the context of other imaging modalities that, to
researcheour
s knowledge, HREM has been combined with. As can be seen in
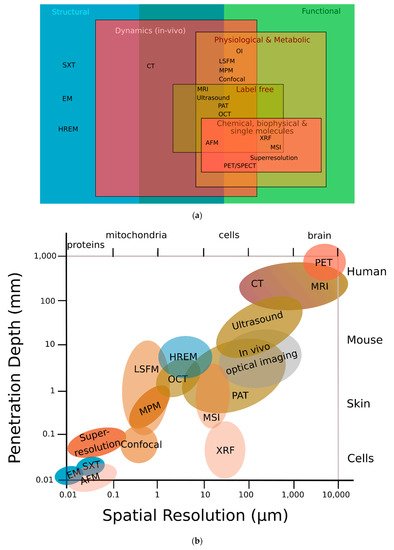
Figure 1a,b, HREM assesses structural information, occupies a resolution niche that is not accessible to any other imaging modality, and connects preclinical imaging (such as micro-MRI, micro-CT, or small-animal ultrasound (US)) with biological microscopy (such as advanced fluorescence, electron, or atomic force microscopy)
[7].
Figure 1. HREM can provide both high image resolution and high sample penetration depths. (
a) Classification of different modalities according to their function; HREM offers structural information that most other modalities cannot. (
b) HREM lies in the middle-field concerning both penetration depth and spatial resolution. Adapted with permission from
[13]. (AFM) atomic force microscopy; (CT) computed tomography; (EM) electron microscopy; (HREM) high-resolution episcopic microscopy; (LSFM) light sheet fluorescence microscopy; (MPM) multiphoton microscopy; (MRI) magnetic resonance imaging; (MSI) mass spectrometry imaging; (OCT) optical coherence tomography; (OI) optical interferometry; (PAT) photoacoustic tomography; (PET) positron emission tomography; (SPECT) single-photon emission computed tomography; (SXT) soft X-ray tomography; (XRF) X-ray fluorescence.
Table 1. Different modalities that have previously been combined in CMI studies in combination with HREM, and their imaging parameters, advantages, and limitations
[1][7][17][18][1,7,17,18]. (CT) computed tomography; (HP) histopathology; (HREM) high-resolution episcopic microscopy; (MRI) magnetic resonance imaging; (OCT) optical coherence tomography; (PAT) photoacoustic tomography; (PET) positron emission tomography; (US) ultrasound.
| Modality |
Contrast |
Penetration (mm) |
Lateral Resolution (µm) |
VOI |
Advantages |
Limitations |
| micro-MRI |
Emitted RF signal after nuclear spin excitation |
>500 |
≤100 |
whole organism |
|
- -
-
non-ionising radiation
- -
-
excellent soft tissue contrast
- -
-
biochemical information (spectroscopy)
|
|
|
- -
-
expensive equipment
- -
-
high maintenance costs
|
|
| micro-US |
Acoustic impedance
between tissue interfaces; detection of echoes from moving particles |
<150 |
30–800 |
whole organism |
|
- -
-
high temporal and spatial resolution
- -
-
portable instrumentation
- -
-
cost-efficient
|
|
|
- -
-
limited tissue penetration
- -
-
poor contrast
- -
-
difficult to quantitate
|
|
| PAT |
Acoustic waves generated by optical absorption of tissue chromophores |
~10 |
~40 |
10 × 10 mm | 2 |
|
- -
-
in vivo
- -
-
penetration depth
- -
-
endogenous and exogenous contrast
|
|
|
- -
-
resolution
- -
-
speed
- -
-
structural contrast
|
|
| OCT |
Optical scattering based on refractive index changes;
motion contrast due to blood flow (OCT angiography) |
~1–2 |
1–10 (diffraction limited) |
10 × 10 mm | 2 |
|
- -
-
in vivo
- -
-
fast
- -
-
non-invasive
- -
-
label-free
- -
-
morphology
- -
-
quantitative blood flow
|
|
|
- -
-
limited molecular information
- -
-
reduced sub-cellular resolution
- -
-
minimum blood flow required
|
|
| micro-PET |
Photon emission after positron annihilation |
>500 |
1000–2000 |
whole organism |
|
- -
-
high sensitivity
- -
-
fully quantitative
- -
-
broad range of applications (imaging agent dependent)
- -
-
dynamic measurements
|
|
|
- -
-
use of radioactive agents
- -
-
highly specialised equipment and staff required
- -
-
high costs
|
|
| micro-CT |
Differential X-ray attenuation of tissues related to their density |
>500 |
≤100 |
whole organism |
|
- -
-
excellent bone imaging
|
|
|
- -
-
radiation dose
- -
-
low soft-tissue contrast (use of contrast agents)
|
|
| HREM |
Light scattering based on unspecific eosin staining |
Sample size up to 12 mm in thickness |
>1 |
8 mm × 8 mm × 12 mm |
|
- -
-
digital volumes in histologic quality at high resolution
|
|
|
- -
-
whole-mount contrasting of specimens
- -
-
time-consuming (fixation and acquisition of 3D volume)
- -
-
ex vivo, no dynamics, structural data only
|
|
| HP |
Light scattering
(various staining methods impart colour and contrast to cellular and tissue components) |
<0.1 |
>500 |
up to 1 mm | 3 |
|
- -
-
evaluation of overall tissue features at low costs
- -
-
excellent cellular detail at light microscopic resolution
- -
-
spatial contextual correlation of microscopic morphology
|
|
|
- -
-
2D and static
- -
-
detailed evaluation (especially of abnormal features in lesions) requires additional expertise
|
|
In imaging, penetration depth comes at the expense of lateral resolution, which restricts the scope of 3D imaging of small animals at micrometre resolution. HREM covers the mesoscopic imaging range, which refers to techniques that allow the 3D visualisation of large samples at the millimetre to centimetre scale. HREM allows the combination of a large field of view and the ability to image thick tissues of several millimetres in thickness with a high-micrometre resolution. Thus, fine structures can be analysed in the context of the overall morphology of surrounding tissues or even whole organisms. The large penetration depth of HREM is achieved by physical sectioning, which, as a downside, limits its application to dead, sacrificed samples. Since the probed volume is sectioned, images are captured from the block surface and the whole 3D sample is reconstructed virtually. There is no need for clearing the sample, as, for example, is the case in light sheet microscopy, which is best suited to optically homogeneous and relatively transparent samples. In addition, while light sheet microscopy can achieve higher resolution than HREM for similar probed volumes, the higher resolution in light sheet microscopy comes with a narrower field of view that would need to be tiled across large specimens.
2. State-of-the-Art
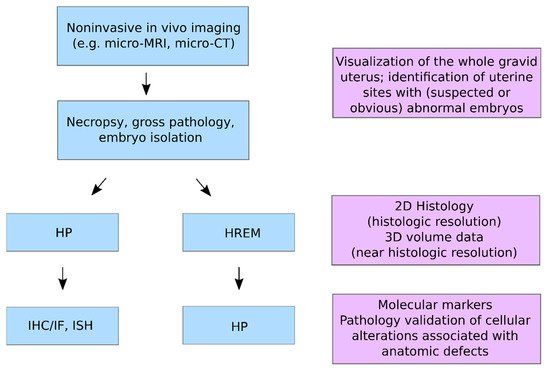
HREM is compatible with a wide range of fixatives and various contrast agents, and tissue processing for HREM does not require special chemicals except for resin embedding and contrasting with eosin. Therefore, HREM can be easily combined with almost all upstream and downstream techniques in multimodal imaging pipelines. It proved to be the method of choice to correlatively reconstruct tumour capillaries and murine vasculature of sufficient contrast and quality at micrometre resolution in selected volumes of interest (VOIs), and to visualise minute anatomical structures and volume displays of mouse, chick, and zebrafish embryos (such as heart malformations) in combination with optical coherence tomography (OCT), photoacoustic tomography (PAT), micro-US, micro-CT, and micro-MRI. HREM was shown to be compatible with contrast agent-enhanced CT and micro-MRI and with the fixation, staining, and dehydration media used after tumour and embryo removal for ex vivo micro-CT or HP. Even though HREM is destructive to the tissue, it can be combined and correlated with HP by collecting physical sections for subsequent examination.
2.1. HP and HREM: Mutual Complementarity
HP is well established in several non-clinical settings including translational biomedical research, model organism phenotyping, preclinical therapeutic discovery, and preclinical safety assessment. In these settings, HP is integral to study specific data generation and validation
[1] (pp. I.2.i-1–I.2.i-13)
[19][20][21][22][23][19,20,21,22,23]. HREM has been most impactful in the phenotyping of embryos and characterisation of structural developmental anomalies. HREM has also found some application in the three-dimensional visualisation of structures in tissues such as human skin and human liver, and in the characterisation of aberrant tumour vasculature and vessel wall lesions in mouse models
[1] (pp. I.2.g-1–I.2.g-9)
[15][16][15,16]. HREM is not typically featured in routine diagnostics, therapeutic discovery, or safety assessment
[1] (pp. I.2.i-1–I.2.i-13)
[19][20][21][22][23][19,20,21,22,23].
Founded on the same principles of histology, HREM and HP are modalities that generate valuable morphologic data at relatively low costs
[1] (pp. I.2.g-1–I.2.g-9, I.2.i-1–I.2.i-13)
[6][19][21][22][6,19,21,22].
HP is based on the evaluation of tissue sections on glass slides, typically by expert pathologists, followed by the acquisition of 2D images at different magnifications to represent specific features of interest observed during evaluation. HREM on the other hand is based on the acquisition of images from the cut surface of the block (rather than the section itself) through the entire thickness of the embedded tissue. The use of the lower magnification objectives incorporates a greater area of the tissue for serial imaging and 3D reconstruction but provides lower cellular detail than available in HP images.
HREM and HP focus on imaging different surfaces of the embedded tissue (block surface versus tissue section) while following the same fundamental principles of histology and light microscopy. They are closely related and mutually complementary modalities, which—when properly combined—can provide histomorphologic information with better 2D cellular detail and 3D spatial context
[1] (pp. I.2.g-1–I.2.g-9)
[3][4][5][6][15][16][3,4,5,6,15,16]. The similarities and differences between certain aspects of the two modalities are summarised in
Table 2.
Table 2.
Comparison of selected aspects of HP and HREM.
| |
HP | 1 |
HREM | 2 |
| Fixation |
Predominantly aldehyde-based fixatives
(10% formaldehyde,
4% paraformaldehyde, Bouin’s fluid) |
Predominantly aldehyde-based fixatives
(10% formaldehyde,
4% paraformaldehyde, Bouin’s fluid) |
Processing
(infiltration) |
Automated or manual processing
Paraffin (most common)
Resin, agar, gelatine, celloidin (alternative, less common) |
Manual processing
Resin (JB-4) |
| Embedding |
Paraffin
Resin |
Resin (JB-4) |
| Sectioning |
Manual rotary microtomy
Automated microtomy |
Automated microtomy |
| Sectioning |
Single or multiple sections at specific planes of the embedded tissue for most routine diagnostic cases and discovery projects; serial sections are reserved for specialised analyses |
Serial sections (at specific intervals through the entire thickness of the block) |
| Section thickness |
1 µm to 5 µm |
1 µm to 3 µm |
| Staining |
Tissue sections are placed on glass slides and then stained |
Tissues are stained during infiltration (prior to embedding) |
| Stains |
Several histochemical stains including Hematoxylin and Eosin (H&E), Periodic Acid Schiff (PAS) and Luxol Fast Blue (LFB) |
Eosin |
| Imaging |
Light microscopy, multiple objectives and magnifications |
Light microscopy, single objective and magnification (selected at the start of sectioning) |
| Imaging surface |
Tissue section on glass slide |
Cut surface of resin block |
| Visualisation and resolution |
2D; higher histomorphologic and cellular resolution with better discernment of specific lesions | 3 |
2D and (virtual/reconstructed) 3D; broader spatial resolution and architectural overview with lower cellular resolution (than HP) | 3 |
| Spatial contextual analysis of molecular (protein and nucleic acid) markers |
More options for immunostaining and in situ hybridisation on paraffin embedded sections |
Fewer options for immunostaining and in situ hybridisation on JB-4 resin embedded sections |