Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Lingzhen Ye and Version 2 by Peter Tang.
Beer is one of the oldest and also most widely consumed alcoholic beverages. It is a kind of colloid solution with complex composition and weak stability. The haze formation in beer is a serious quality problem, as it primarily affects the shelf life and flavor of beer. Hazes are caused by suspended insoluble particles of colloidal or larger size that can be perceived visually or by instruments.
- beer
- haze
- haze-active proteins
- barley
- molecular breeding
- genes
1. Introduction
Beer is one of the oldest and also most widely consumed alcoholic beverages. It is a kind of colloid solution with complex composition and weak stability. The haze formation in beer is a serious quality problem, as it primarily affects the shelf life and flavor of beer. Hazes are caused by suspended insoluble particles of colloidal or larger size that can be perceived visually or by instruments.
Beer haze can be divided into biological and non-biological ones. The biological haze can be reduced or avoided, as it is caused by wild bacteria or yeast due to poor hygiene during beer processing and storage (Figure 1). In contrast, the non-biological haze is difficult to deal with, is caused by the large molecular substances in beer, such as dextrin, β-glucan, proteins, and polyphenols, etc. [1][2][3][1,2,3]. In the process of storage and transportation, due to the light irradiation and vibration, the large molecular substances undergo changes such as combination and agglutination, resulting in the formation of turbidity. Therefore, the clarity stage of beer is temporary, whereas the final stage of beer is turbidity.
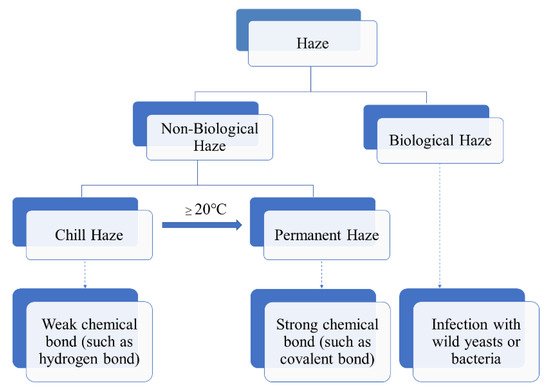
Figure 1.
Scheme of haze formation in beer.
According to the European Brewery Convention (EBC), the non-biological haze was classified into two types: chill and permanent. The chill haze forms when the beer is chilled to 0 °C, but re-dissolves when the beer is warmed to 20 °C or more (Figure 1). The particle sizes existed in beer with chill haze range from 0.1 to 1.0 μm in diameter. While permanent haze is present in beer even at 20 °C or higher temperature, with particles ranging from 1 to 10 μm in diameter [4]. The small particles are more likely to be intermediate precursors for the larger haze particles [5]. It is generally believed that chill haze results from weak chemical bond (such as hydrogen bond) interaction between large molecular substances (such as proteins and polyphenols) in beer. While permanent haze is considered to be the result of strong chemical bonds (such as covalent bonds) interaction between the large molecular substances (Figure 1).
2. The Methodologies for Measurements of Haze in Beer
In the past, the turbidity of fluid (such as beer) was measured using a transparent container with a viewing panel attached to it, which could be viewed through the fluid. At present, some standardized analysis methods are used, including optical, microscopic, and enzymatic methods as well as particle size analysis. Moreover, these methods can also be used in combination [6][7]. Recently, Raman spectroscopy, especially TI-RMS (Turbidity Identification Raman Micro-Spectroscopy), was used to identify and differentiate turbidity particles in a complex solution such as beer. Although the analysis itself is fast and simple, the cost of the equipment is quite high; therefore, this evolving method is particularly recommended for large laboratories [7][8].
In general, the forced aging method is applied to predict the shelf life of beer by many beer companies. In detail, the turbidity of beer was tested after being stored for a specific time under certain conditions. The treatment condition and time were determined by the experimental purpose [4][8][4,9]. For example, Jongberg et al., used a forced aging method to investigate the haze formation in commercial beer [9][10]. The procedure is as follows: beer was exposed to aging treatment by heat/chill cycles of 60 °C for 48 h, followed by 0 °C for 24 h, according to the Analytica-EBC method 9.30 with slight modifications. Two levels were set as medium (samples subjected to five aging cycles) and high (samples subjected to ten forced aging cycles). However, the method is relatively time-consuming. A more rapid method, called the alcohol-chill test, is widely used. The procedures were as follows: 5% pure ethanol was added into beer sample and carefully mixed, frozen at −8 °C for 40 min, then measured by a turbimeter [10][11][11,12].
Many methodologies have been developed to determine the amount of haze active proteins in beer [12][13][13,14]. Currently, the method of tannic acid distribution droplets is most widely used, which is based on adding a fixed amount of tannin to an examined sample [14][15][15,16]. Due to the affinity between tannin and HA proteins, the turbidity formed during the aging process of beer. While the aging process of beer can be predicted by adding different concentrations of tannins. This method is characterized by the determination of tannin-related HA proteins. In addition, the saturated ammonium sulfate precipitation method is also used to determine the content of HA proteins [12][16][13,17].
3. The Reasons for Haze Formation in Beer
The stability of beer haze is primarily affected by the properties of malt barley, which is used as the main raw material for brewing. If the carbohydrates in malt are not sufficiently broken down during mashing, the long-chain dextrins cannot be used by yeast. After fermentation, dextrins will cause turbidity, as the solubility of dextrins is quite low in alcohol beverages [17][18]. Moreover, β-glucan in wort may increase turbidity due to its larger molecular weight [18][19][19,20]. Similarly, arabinoxylan is also a chemical compound causing beer turbidity through its connection with relevant proteins [20][21][21,22]. However, among the factors causing non-biological haze formation, the interaction between haze-active proteins and polyphenols is the most well-known [4][12][22][4,13,23].
3.1. Protein–Polyphenol Haze
The model for haze formation of protein–polyphenol interaction has been described (Figure 2) [22][23]. According to the model, a haze active HA protein is conceptualized as having multiple binding sites with HA polyphenols, while a HA polyphenol has relatively fewer ends binding to HA protein, thus forming protein-polyphenol polymers with strong light scattering ability [23][24]. Meanwhile, beer haze stability depends on the proportion of HA proteins and polyphenols.
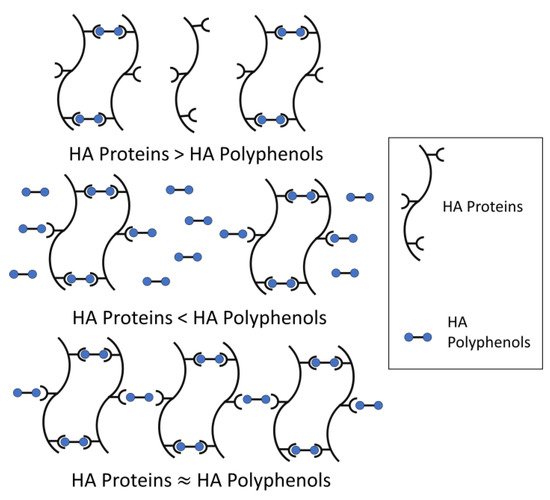

When HA proteins are relatively excessive, most HA polyphenols form dimers with HA proteins, and only a small amount of HA polyphenol binds to protein-polyphenol polymerization to produce a large cross-linking. Therefore, the formed particles are too small to form turbidity. Similarly, when HA polyphenols are relatively excessive, HA protein cannot provide enough binding sites for cross-linking and cannot form turbidity easily. Only in conditions where the total concentration of polyphenol ends is roughly equal to the number of binding sites in proteins, will turbidity be formed because of the development of such a large network, corresponding to large colloidal particles and maximum light scattering (Figure 2). Incipiently, the weak binding force between HA protein-polyphenol polymers is easy to re-break, which can be used to explain the formation of chill haze. Therefore, the initial reaction is more like hydrogen and/or hydrophobic bonding than covalent bonding, because most haze is caused by chilling partially or totally dissolves when a hazy beer is warmed. When these protein-polyphenol polymers are no longer broken, the reversible haze will become permanent [4].
3.2. Haze Active Proteins
Beer contains large amounts of barley proteins which have been hydrolyzed and chemically modified during malting and brewing processes.
During beer filtration, silica gel is always used to remove HA proteins to reduce haze formation. The silica eluate (SE) proteins are mainly composed of hordein, being proline-rich and glutamate-rich [20][24][21,34].
Currently, two-dimensional electrophoresis (2-DE) combined with mass spectrometry (MS) analysis has been widely used to study the protein composition in beer and beer turbidity. Obviously, the components of total proteins and HA proteins in beer are quite complex. However, the isobaric tags for relative and absolute quantification (iTRAQ) method, which is widely used in protein analysis of barley seedling, grain, and malt [25][26][39,40], is very limited in beer protein study. Therefore, a high-throughput method of protein identification should be applied in future studies.
In addition to the above-mentioned methods of reverse genetics (e.g., proteomics), a positive genetics strategy (e.g., QTL analysis) is also used to figure out the most important genes or proteins limiting beer shelf life [11][12]. There was a wide difference in the tannin-related HA proteins and chill haze stability among barley genotypes. The HA proteins and colloidal stability of beer are controlled by many loci. Furthermore, barley α-amylase/trypsin inhibitor CMb and CMd (BATI-CMb and BATI-CMd) were identified as two key genes controlling the chill haze stability of beer [11][15][12,16].
It is generally accepted that HA proteins have a higher level of proline [23][24]. The proline composition of proteins adsorbed onto silica gel was approximately 20 mol%, while those of BDAI-1, CMb, and CMe were lower than 10 mol%. Hence it is speculated that BDAI-1, CMb and CMe are not predominant haze active proteins, but they are initiation or growth factors in the formation of colloidal haze [27][33]. It may be concluded that HA proteins may consist of proline-poor proteins, such as BTI-CMe, BATI-CMd, and BATI-CMb, and proline-rich proteins such as hordeins.
3.3. Haze Active Polyphenols
Polyphenols in beer derive from both hops and malt. In detail, approximately 80% is originated from malt, while only 20% from hop-derived polyphenols [28][29][44,45]. It is well known that polyphenols play important roles in brewing [30][46]. Polyphenols are important flavor substances in beer, being closely associated with flavor and taste of beer. Polyphenols also greatly affect the colloid stability of beer, which can extend the shelf life of beer by acting as free radical scavengers and reducing agents [31][32][47,48]. For haze formation, the polyphenols are the potential substances that maintain the appropriate contents in beer. In this way, they ensure both the colloid stability and flavor requirement of beer. The most commonly found phenolic compounds in beer were catechin, epicatechin, Ferulic acid, p-coumaric acid, and vanillic acid [28][44]. These phenolic compounds can be easily transformed into highly flavor-active volatile, such as phenols 4-vinyl guaiacol and 4-vinyl phenol, which reduce beer flavor stability [30][33][34][46,49,50].
According to the molecular weight (MW), polyphenols in beer can be divided into tannic compounds (MW: 500~3000 U) and non-tannic compounds (MW < 500 U or MW > 3000 U). Phenolic compounds with a molecular weight less than 500 U mainly include phenolic acids, flavonols, flavanols, etc. Due to the excellent reducibility, these compounds were beneficial to the stability of beer. However, once these compounds are oxidized and polymerized, they will become the basis of beer turbidity. It was suggested that the complexed flavanols formed the bulk of the polyphenols in beer. McMurrough claimed that the low levels of such materials cause the problem of haze [35][51]. Polyphenols with molecular weight between 500 U and 3000 U can be simply called tannins, which are responsible for beer flavor and the main components of HA polyphenols. Polyphenols with molecular weights above 3000 U tend to precipitate easily, so most of them will be removed during brewing.
Because polyphenols are highly attractive to proteins containing proline residues [36][37][52,53]. They are major factors causing haze formation in beer, wine, and fruit juices [38][26]. The monomer polyphenols in beer tend to oxidize and polymerize, so that they have more ends that can bind to HA proteins, thus forming large HA polymers. The monomer polyphenols in beer mainly include flavonols and flavanols, with MW lower than 500 U. It has been found that the concentration of phenolic compounds with MW < 500 U was reduced gradually, as they were converted to tannins during beer storage [35][51]. Forced aging of beer experiments have shown significant losses of gallic acid, salicylic acid, hydroxy-phenyl lactic acid, chlorogenic acid, epicatechin, vanillic acid, ferulic acid, pyrocatechuic acid, and luteolin during the forced aging. It was supposed that these phenolic compounds took part in the colloidal changes, proposedly upon polymerization into tannins [9][39][10,54]. It has been reported that proanthocyanidins, one of the flavanol compounds, were important for the haze stability of beer. The haze stability of beer made from proanthocyanidins (PA)-free malt barley is better than that made from PA-containing barley [40][55].
4. The Passways for Preventing or Reducing Haze Development
According to the causes of beer haze formation, at present three strategies can be used to prevent or reduce haze development in beer: reducing HA polyphenols, reducing HA proteins, and removing both HA polyphenols and HA proteins at a certain proportion. These strategies can be implemented both in raw materials and during beer processing. To lengthen the shelf life of beer, the manufacturers now generally perform stabilizing treatments in beer processing. However, these treatments will increase the cost of beer production and also deteriorate some flavor due to reduced relevant polyphenols and proteins [41][56]. On the other hand, developing the malt barley cultivars with lower HA proteins and/or HA polyphenol is the most efficient way to control colloidal haze formation in beer.
