Nanocarriers enhance the dissolution and bioavailability of drugs and facilitate their targeting effect. Taking the potential toxicity into consideration, the incorporation of natural “green” materials, derived from plants, in the nanocarriers fabrication, improve their safety and biocompatibility. These green components can be used as mechanical platforms, targeting ligands or can play be involved in the synthesis of nanoparticles.
- green nanotechnology
- nanocarriers
- plant extracts
1. Introduction
Nanotechnology is an interdisciplinary field of science concerned with the processing of matter at atomic/molecular level [1]. Nanotechnology is applicable in many areas such as the chemical industry, pharmaceutical industry, optics, electronics, energy science and biomedical sciences [2]. The size of the nanoparticles (NPs) is comparable to the size of proteins and various intracellular macromolecules, which allows them to take advantage of cellular machinery to assist in the drug delivery [3]. Additionally, in comparison to bulky materials, NPs have large surface to volume ratio allowing for chemical modifications to tune their properties [4]. NPs can be used as nanocarriers to encapsulate drugs or biomolecules inside their structures and/or absorb them on their surfaces. There are different types of nanocarriers including polymeric NPs, liposomes, micelles, dendrimer, hydrogel, mesoporous, 0, 1 and 2-D materials [5]. These types are broadly divided into organic, inorganic and hybrid nanocarriers [6]. Several types of payloads can be delivered using NPs such as conventional drugs, polypeptides, proteins, vaccines, nucleic acids, genes, etc. [7].
The commonly used conventional chemotherapies include alkylating agents, antitumour antibiotics (e.g., epirubicin, doxorubicin (DOX)), antimetabolites (e.g., 5-fluorouracil (5-FU), methotrexate, gemcitabine), topoisomerase inhibitors, and mitotic inhibitors (e.g., paclitaxel and docetaxel) [8]. Chemotherapy drug delivery for local and metastatic tumours is associated with various drawbacks. Such problems may include high toxicity in normal cells, lack of tumour target selectivity, high volume drug distribution and rapid drug clearance [9]. Nanocarriers can improve the safety and efficiency of drugs by increasing their water solubility and stability, enhance their circulation time, improve their uptake by targeted cancer cells or prevent their enzyme degradation [10]. The current reports on the use of nanocarriers for drug delivery focus on: (1) the choice of suitable carrier materials to achieve high drug encapsulation rate and controlled and targeted release speed; (2) improvement of targeting ability via surface functionalization; (3) augmentation of drug biological activity with using carrier materials of similar activity; (4) formulating responsive nanocarriers that are able to release the loaded drugs at designated sites in a response to the local environment (e.g., pH-response release and response to enzymatic degradation of nanocarriers, etc.); (5) performing in vitro and in vivo assays to compare the biological activity between the loaded drugs and their free forms and to assess the safety of the nanocarriers and their stability. The use of nanocarriers inside the body will allow them to interact with blood components and vessels, normal tissues, etc., meaning that they can influence human health and therefore it is important to consider the safety of the components included in the synthesis of these nanomaterials. Despite advancements in nanocarriers, their transformation in medical applications remains insufficient. This mainly is due to their lack of biodegradation, instability in circulation, poor bioavailability, long-term potential toxicity, and inadequate tissue distribution.
Therefore, the overall aim of including green components in the fabrication of NPs as nanocarriers is to decrease toxicity to the body, to have an environmentally benign industry process and to increase affordability. The green synthesis may be facilitated by plants extracts and microbes or their isolated biomolecules [11]. The green based nanocarriers are mainly synthesized by the bottom-up approach within three fundamental conditions of synthesis. These conditions are based on the selection of a green non-toxic solvent, coupled by a good reducing agent, and thirdly incorporating an efficient stabilization material.
2. Plant Extracts-Based Nanocarriers for anticancer therapy
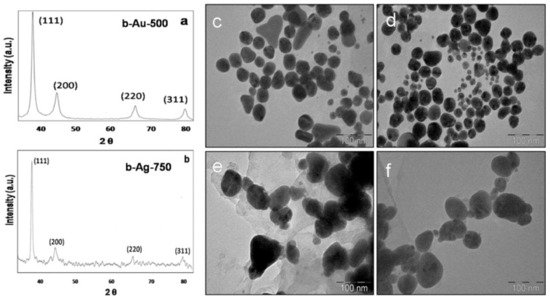
References
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: a review. Environ. Chem. Lett. 2020, 19, 355–374, doi:10.1007/S10311-020-01074-X.Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2020, 19, 355–374.
- Raliya, R.; Chadha, T.S.; Hadad, K.; Biswas, P. Perspective on nanoparticle technology for biomedical use. Curr. Pharm. Des. 2016, 22, 2481–2490, doi:10.2174/1381612822666160307151409.Raliya, R.; Chadha, T.S.; Hadad, K.; Biswas, P. Perspective on nanoparticle technology for biomedical use. Curr. Pharm. Des. 2016, 22, 2481–2490.
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in therapeutics: a focus on nanoparticles as a drug delivery system. Futur. Med. 2012, 7, 1253–1271, doi:10.2217/NNM.12.87.Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Future Med. 2012, 7, 1253–1271.
- Ray, P.C. Size and Shape Dependent Second Order Nonlinear Optical Properties of Nanomaterials and Their Application in Biological and Chemical Sensing. Chem. Rev. 2010, 110, 5332–5365, doi:10.1021/CR900335Q.Ray, P.C. Size and Shape Dependent Second Order Nonlinear Optical Properties of Nanomaterials and Their Application in Biological and Chemical Sensing. Chem. Rev. 2010, 110, 5332–5365.
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018 31 2018, 3, 7, doi:10.1038/s41392-017-0004-3.Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7.
- Din, F. ud; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomedicine 2017, 12, 7291–7309, doi:10.2147/IJN.S146315.Ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309.
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662, doi:10.1016/J.ADDR.2008.09.001.Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662.
- Sak, K. Chemotherapy and Dietary Phytochemical Agents. Chemother. Res. Pract. 2012, 2012, 282570, doi:10.1155/2012/282570.Sak, K. Chemotherapy and Dietary Phytochemical Agents. Chemother. Res. Pract. 2012, 2012, 282570.
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomedicine 2021, 16, 1313–1330, doi:10.2147/IJN.S289443.Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomed. 2021, 16, 1313–1330.
- Gupta, P.; Garcia, E.; Sarkar, A.; Kapoor, S.; Rafiq, K.; Chand, H.; Jayant, R. Nanoparticle Based Treatment for Cardiovascular Diseases. Cardiovasc. Hematol. Disord. Drug Targets 2019, 19, 33–44, doi:10.2174/1871529X18666180508113253.Gupta, P.; Garcia, E.; Sarkar, A.; Kapoor, S.; Rafiq, K.; Chand, H.; Jayant, R. Nanoparticle Based Treatment for Cardiovascular Diseases. Cardiovasc. Hematol. Disord. Drug Targets 2019, 19, 33–44.
- Lateef, A.; Ojo, S.A.; Elegbede, J.A. The emerging roles of arthropods and their metabolites in the green synthesis of metallic nanoparticles. Nanotechnol. Rev. 2016, 5, 601–622, doi:10.1515/NTREV-2016-0049.Lateef, A.; Ojo, S.A.; Elegbede, J.A. The emerging roles of arthropods and their metabolites in the green synthesis of metallic nanoparticles. Nanotechnol. Rev. 2016, 5, 601–622.
- Kumari, A.; Kumar, V.; Yadav, S.K. Plant Extract Synthesized PLA Nanoparticles for Controlled and Sustained Release of Quercetin: A Green Approach. PLoS One 2012, 7, e41230, doi:10.1371/JOURNAL.PONE.0041230.Kumari, A.; Kumar, V.; Yadav, S.K. Plant Extract Synthesized PLA Nanoparticles for Controlled and Sustained Release of Quercetin: A Green Approach. PLoS ONE 2012, 7, e41230.
- Kumar, S.; Lather, V.; Pandita, D. A facile green approach to prepare core-shell hybrid PLGA nanoparticles for resveratrol delivery. Int. J. Biol. Macromol. 2016, 84, 380–384, doi:10.1016/J.IJBIOMAC.2015.12.036.Kumar, S.; Lather, V.; Pandita, D. A facile green approach to prepare core-shell hybrid PLGA nanoparticles for resveratrol delivery. Int. J. Biol. Macromol. 2016, 84, 380–384.
- Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 2012, 23, 455103, doi:10.1088/0957-4484/23/45/455103.Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 2012, 23, 455103.
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309, doi:10.1016/J.MSEC.2015.04.048.Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309.
- Mukherjee, S.; Sau, S.; Madhuri, D.; Bollu, V.; Madhusudana, K.; Sreedhar, B.; Banerjee, R.; Patra, C. Green Synthesis and Characterization of Monodispersed Gold Nanoparticles: Toxicity Study, Delivery of Doxorubicin and Its Bio-Distribution in Mouse Model. J. Biomed. Nanotechnol. 2016, 12, 165–181, doi:10.1166/JBN.2016.2141.Mukherjee, S.; Sau, S.; Madhuri, D.; Bollu, V.; Madhusudana, K.; Sreedhar, B.; Banerjee, R.; Patra, C. Green Synthesis and Characterization of Monodispersed Gold Nanoparticles: Toxicity Study, Delivery of Doxorubicin and Its Bio-Distribution in Mouse Model. J. Biomed. Nanotechnol. 2016, 12, 165–181.
- Kumar, C.S.; Raja, M.D.; Sundar, D.S.; Gover Antoniraj, M.; Ruckmani, K. Hyaluronic acid co-functionalized gold nanoparticle complex for the targeted delivery of metformin in the treatment of liver cancer (HepG2 cells). Carbohydr. Polym. 2015, 128, 63–74, doi:10.1016/J.CARBPOL.2015.04.010.Kumar, C.S.; Raja, M.D.; Sundar, D.S.; Gover Antoniraj, M.; Ruckmani, K. Hyaluronic acid co-functionalized gold nanoparticle complex for the targeted delivery of metformin in the treatment of liver cancer (HepG2 cells). Carbohydr. Polym. 2015, 128, 63–74.
- Ganeshkumar, M.; Sathishkumar, M.; Ponrasu, T.; Dinesh, M.G.; Suguna, L. Spontaneous ultra fast synthesis of gold nanoparticles using Punica granatum for cancer targeted drug delivery. Colloids Surfaces B Biointerfaces 2013, 106, 208–216, doi:10.1016/J.COLSURFB.2013.01.035.Ganeshkumar, M.; Sathishkumar, M.; Ponrasu, T.; Dinesh, M.G.; Suguna, L. Spontaneous ultra fast synthesis of gold nanoparticles using Punica granatum for cancer targeted drug delivery. Colloids Surf. B Biointerfaces 2013, 106, 208–216.
- Chinnaiyan, S.; Soloman, A.; Perumal, R.; Gopinath, A.; Balaraman, M. 5 Fluorouracil-loaded biosynthesised gold nanoparticles for the in vitro treatment of human pancreatic cancer cell. IET nanobiotechnology 2019, 13, 824–828, doi:10.1049/IET-NBT.2019.0007.Chinnaiyan, S.; Soloman, A.; Perumal, R.; Gopinath, A.; Balaraman, M. 5 Fluorouracil-loaded biosynthesised gold nanoparticles for the in vitro treatment of human pancreatic cancer cell. IET Nanobiotechnol. 2019, 13, 824–828.
- Sadalage, P.S.; Patil, R. V.; Havaldar, D. V.; Gavade, S.S.; Santos, A.C.; Pawar, K.D. Optimally biosynthesized, PEGylated gold nanoparticles functionalized with quercetin and camptothecin enhance potential anti-inflammatory, anti-cancer and anti-angiogenic activities. J. Nanobiotechnology 2021, 19, 84, doi:10.1186/S12951-021-00836-1.Sadalage, P.S.; Patil, R.V.; Havaldar, D.V.; Gavade, S.S.; Santos, A.C.; Pawar, K.D. Optimally biosynthesized, PEGylated gold nanoparticles functionalized with quercetin and camptothecin enhance potential anti-inflammatory, anti-cancer and anti-angiogenic activities. J. Nanobiotechnol. 2021, 19, 84.
- Lee, K.X.; Shameli, K.; Mohamad, S.E.; Yew, Y.P.; Isa, E.D.M.; Yap, H.-Y.; Lim, W.L.; Teow, S.-Y. Bio-Mediated Synthesis and Characterisation of Silver Nanocarrier, and Its Potent Anticancer Action. Nanomaterials 2019, 9, 1432, doi:10.3390/NANO9101423.Lee, K.X.; Shameli, K.; Mohamad, S.E.; Yew, Y.P.; Isa, E.D.M.; Yap, H.-Y.; Lim, W.L.; Teow, S.-Y. Bio-Mediated Synthesis and Characterisation of Silver Nanocarrier, and Its Potent Anticancer Action. Nanomaterials 2019, 9, 1432.
- Cai, W.; Guo, M.; Weng, X.; Zhang, W.; Owens, G.; Chen, Z. Modified green synthesis of Fe3O4@SiO2 nanoparticles for pH responsive drug release. Mater. Sci. Eng. C 2020, 112, 110900, doi:10.1016/J.MSEC.2020.110900.Cai, W.; Guo, M.; Weng, X.; Zhang, W.; Owens, G.; Chen, Z. Modified green synthesis of nanoparticles for pH responsive drug release. Mater. Sci. Eng. C 2020, 112, 110900.
- Akbarian, M.; Mahjoub, S.; Elahi, S.; Zabihi, E.; Tashakkorian, H. Green synthesis, formulation and biological evaluation of a novel ZnO nanocarrier loaded with paclitaxel as drug delivery system on MCF-7 cell line. Colloids Surfaces B Biointerfaces 2020, 186, 110686, doi:10.1016/J.COLSURFB.2019.110686.Akbarian, M.; Mahjoub, S.; Elahi, S.; Zabihi, E.; Tashakkorian, H. Green synthesis, formulation and biological evaluation of a novel ZnO nanocarrier loaded with paclitaxel as drug delivery system on MCF-7 cell line. Colloids Surf. B Biointerfaces 2020, 186, 110686.
