Fungal secondary metabolites (SMs) comprise a vast collection of compounds expendable for these organisms under laboratory conditions. They exhibit enormous chemical diversity, and usually belong to four major families: terpenoids, polyketides, non-ribosomal peptides, or a combination of the last two. Their functions are very diverse and are normally associated with a greater fitness of the producing fungi in their environment, which often compete with other microorganisms or interact with host plants. Many SMs have beneficial applications, e.g., as antibiotics or medical drugs, but others, known as mycotoxins, are harmful to health.
- polyketides
- PKS
- terpenoids
- non-ribosomal peptides
- NRP
- PKS–NRPS hybrid genes
- gene clusters
- pigments
- antibiotics
- mycotoxins
1. Introduction
The production of metabolites by fungi began to receive attention in the first half of the last century [1], and acquired special relevance after the discovery of penicillin, a metabolite produced by the fungus Penicillium, which started the era of antibiotics [2]. Today, one of the most characteristic traits of fungi is their enormous metabolic versatility, which is reflected in the richness of secondary metabolism in many species [3]. Secondary metabolites (SMs) can be defined as chemical compounds resulting from specific biosynthetic pathways, whose production is not necessary for normal growth and development of the fungus in the laboratory. However, they are present in numerous species, and therefore their persistence in evolution implies a competitive benefit in nature. This entry reviews the major SM families, summarizes the genetic basis and regulatory mechanisms involved in their production, and provides with selected examples a general overview of their chemical diversity, possible roles in fungal life, and biological effects and applications in human life.
2. Chemical Families of SMs
The number of SMs identified in fungi is large [4], and it is presumed that only a minority of all those that exist in nature are currently known. The best known SMs usually belong to four chemical families: polyketides (PKs), terpenoids, non-ribosomal peptides (NRPs), and hybrid non-ribosomal peptide/polyketides (NRP/PKs). SMs are synthesized using substrates from primary metabolism, among which acetyl-CoA stands out as the precursor of polyketides and terpenoids. Each SM biosynthetic pathway starts with a characteristic type of enzyme, and it is completed with the activity of specific tailoring enzymes, introducing additional modifications to the molecules.
2.1. Polyketides
PKs constitute the most abundant and diverse SM family. Their biochemical pathways begin with the addition of acetyl-CoA units to form different structures, followed by a broad diversity of chemical reactions [5,6]. All polyketide biosynthetic pathways are initiated by a characteristic enzyme, known as polyketide synthetase (PKS), initially discovered in bacteria. According to their structures and mechanisms, PKSs are classified into three classes, known as types I, II, and III [7]. Type I PKSs are giant multifunctional enzymes structurally related to fatty acid synthetases [8]. They usually have in common a set of conserved domains that always include three basic ones: acyltransferase (AT), which recognizes the monomer that will be used in the synthesis; ketosynthase (KS), which joins it to the elongating polyketide chain; and acyl carrier protein (ACP), which has a prosthetic group of phosphopantetheine that serves as a covalent binding site for the intermediate formed in the synthesis. These are accompanied by other optional domains. The keto groups formed in the elongating process can be reduced by ketoreductase (KR), dehydratase (DH), or enol reductase (ER) domains to produce different modifications depending on the specific PK in question. There are other possible domains, e.g., methyl transferase (MT), or condensation/heterocyclization (HC), that can introduce additional changes.
PKSs can be iterative or non-iterative. In iterative PKSs the macro-enzyme functions as an extension module that elongates the product in successive reactive cycles. These PKSs can be reducing or non-reducing depending on the presence of the reducing KR, DH, and ER domains. Non-iterative PKSs are usually multimodular, with each module having its own domain combination responsible for a complete elongation cycle, and with a final module with a releasing thioesterase (TE) domain [9]. The products resulting from the PKS activity are modified by other enzymes, giving rise to the vast chemical diversity that characterizes this family. Some well-known examples of PKs synthesized by type I PKSs are described in Table 1 and Figure 1. In some cases, two different PKSs participate in the synthesis of the same compound, as occurs with zearalenone [10].
Table 1. A selection of fungal secondary metabolites. Five representative examples are indicated for each SM family.
|
Chemical Family |
Metabolite |
Function/Activity |
Representative Producing Genera |
Reference |
|
Polyketides (PKs) |
Fumonisin B1 |
Mycotoxin |
Fusarium |
[11] |
|
Lovastatin |
HMG-CoA reductase inhibitor |
Aspergillus |
[12] |
|
|
Aflatoxin |
Mycotoxin |
Aspergillus |
[13] |
|
|
Bikaverin |
Antibiotic (protozoa) |
Fusarium |
[14] |
|
|
Zearalenone |
Mycotoxin (estrogenic) |
Fusarium |
[10] |
|
|
Non ribosomal peptides (NRPs) |
Enniatin B |
Mycotoxin (cytotoxic) |
Fusarium |
[15] |
|
Cyclosporine A |
Immunosuppressant |
Tolypocladium |
[16] |
|
|
Ergotamine |
Ergot alkaloid |
Claviceps |
[17] |
|
|
Penicillin G |
Antibiotic (bacteria) |
Penicillium |
[18] |
|
|
Apicidin |
Histone deacetylase inhibitor |
Fusarium |
[19] |
|
|
Hybrid NRP/PKs |
Equisetin |
Antibiotic (bacteria) |
Fusarium |
[20,21] |
|
Fusarin C |
Mycotoxin |
Fusarium |
[22] |
|
|
Cytochalasin |
Actin inhibitor |
Penicillium, Chaetomium |
[23] |
|
|
Cyclopiazonic acid |
Mycotoxin |
Aspergillus, Penicillium |
[24] |
|
|
Ochratoxin A |
Mycotoxin |
Aspergillus, Penicillium |
[25,26] |
|
|
Terpenoids |
Gibberellic acid (GA3) |
Plant hormone |
Fusarium |
[27,28] |
|
Deoxynivalenol |
Mycotoxin |
Fusarium |
[29] |
|
|
Neurosporaxanthin |
Carotenoid pigment |
Neurospora, Fusarium |
[30,31] |
|
|
Austinol |
Unknown |
Aspergillus |
[32] |
|
|
Helvolic acid |
Antibiotic (bacteria) |
Aspergillus |
[33] |
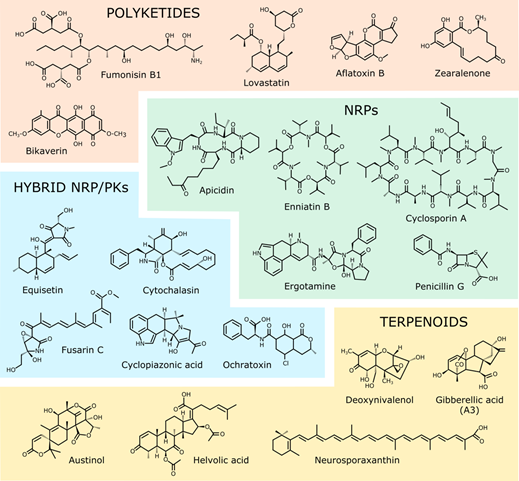
Figure 1. Chemical structures of the representative SMs from the four major families described in Table 1.
Type II PKSs are large multienzymatic complexes, combining a set of catalytic activities that act iteratively and that frequently produce aromatic compounds [34]. This class of enzymes is typically found in prokaryotes, and genome analyses indicate that they are absent in fungi. Type III PKSs are homodimeric enzymes of less diverse function. They are mainly known for their participation in chalcone biosynthesis in plants [35], where they play a defensive role. Type III PKSs differ from type I PKSs in that they are able to interact directly with acyl CoA substrates, whereas in type I this substrate is provided by an acyl transporter protein. Type III PKSs are found in fungal genomes, although they are much less abundant than type I.
Type II PKSs are large multienzymatic complexes, combining a set of catalytic activities that act iteratively and that frequently produce aromatic compounds [34]. This class of enzymes is typically found in prokaryotes, and genome analyses indicate that they are absent in fungi. Type III PKSs are homodimeric enzymes of less diverse function. They are mainly known for their participation in chalcone biosynthesis in plants [35], where they play a defensive role. Type III PKSs differ from type I PKSs in that they are able to interact directly with acyl CoA substrates, whereas in type I this substrate is provided by an acyl transporter protein. Type III PKSs are found in fungal genomes, although they are much less abundant than type I.
PKs are usually soluble molecules with well-defined chemical structures. However, there are exceptions. Melanins are polymeric pigments with indole and benzene rings, and their chemical nature is still under study due to their lack of solubility [36]. They can be produced by two alternative pathways, one of them, the most frequent in fungi, initiated by a type I PKS [37].
2.2. Non-Ribosomal Peptides
Non-ribosomal peptides (NRPs) are low-molecular-weight peptides with extensive chemical variety [38]. As their name indicates, they are synthesized by a mechanism unrelated to protein synthesis in the ribosome. In addition to their smaller sizes compared to most proteins, they differ from these in their structures, which are frequently cyclical, and in the participation of atypical amino acids, such as hydroxylated or methylated variants, or their D forms. Unlike proteins, which undergo modifications only after their synthesis, NRPs undergo chemical changes in their amino acids during their formation or by other enzymes after they have been released.
Like PKs, NRPs are produced by gigantic multi-enzyme complexes known as NRP synthetases (NRPSs). They are usually organized as modules, each consisting of several catalytic domains that function in a coordinated fashion [39]. A basic module includes an adenylation (A) and a thiolation domain (T). Selection and activation of the substrate is carried out by the A domain. The substrate is then transferred to the T domain, where it is covalently linked by a thiodiester bond into a phosphopantetheine unit. Each module contributes one aminoacyl or aryl residue to be used in the formation of the final NRP. Other enzymatic domains optionally present in the modules introduce modifications in the residues, which can lead to their epimerization (E) or methylation (M), or to other chemical changes, while the condensation domains (C) catalyze the union by peptide bonds of substrates linked to the adjacent phosphopantetheine. Reactions proceed until the full NRP is generated. The NRP’s release is often accompanied by a cycling reaction carried out by a thioesterase domain located at the carbon end of the multienzyme complex. In many cases, the NRP undergoes new chemical modifications by other enzymes, including oxidation, halogenation, or glycosylation reactions, among others, thereby increasing the chemical diversity of the resulting NRPs.
A database of NRPs called Norine [40] is available to researchers, and at the time this article was written it included 1740 peptides formed by 544 different monomers. The number of monomers in each NRP varies from 2 to 26 [41]. Due to their numerous applications, different strategies have been used to improve the biotechnological production of many NRPs [38,42,43]. Some representative examples of fungal NRPs are described in Table 1 and Figure 1.
2.3. Hybrid Non-Ribosomal Peptide/Polyketides
Some SMs are the result of the joint action of PKS and NRPS [44]. Both enzymatic complexes can be encoded by independent genes participating in the same biosynthetic pathway. This is the case in the ochratoxin A biosynthetic clusters in Aspergillus and Penicillium sp. [25]. Other examples are the PKS and NRPS genes involved in the synthesis of the lipopeptides fusaristatin A in Fusarium graminearum and W493 A and B in Fusarium pseudograminearum [45].
The similarities between the modular structures of type I PKS and NRPS have facilitated the evolution of chimeric genes, which combine PKS and NRPS modules [46]. Examples are found with the two orientations in their amino-carboxyl distribution, PKS–NRPS and NRPS–PKS [47]. In these large multifunctional complexes, usually consisting of a single NRPS module with one or more PKS modules, the substrates are passed between the different modules to introduce the corresponding PK or NRP extension to the molecule. This new combinatorial possibility provides an additional source of diversity to the resulting compounds [48]. Examples of PKS–NRPS hybrid products are shown in Table 1 and Figure 1.
2.4. Terpenoids
Terpenoids are a vast family of chemicals derived from a molecule of five carbon atoms (5-C), isopentenyl diphosphate (IPP) [49]. This compound can be produced by two different biosynthetic pathways: the deoxyxylulose 5-phosphate pathway, and the mevalonate pathway [50]. The latter is the only one that has been identified so far in fungi. The name of this pathway comes from the synthesis of IPP from mevalonate, a compound produced by hydroxymethyl-glutaryl CoA reductase. The dephosphorylation of IPP gives isoprene, a volatile molecule abundant in nature due to its emission by plants. In turn, many low-molecular-weight terpenoids produced by fungi are volatile, giving rise to the concept of “volatome”, as the one described in Aspergillus fumigatus [51].
The synthesis of all terpenoids begins with the fusion of IPP with dimethylallyl diphosphate (DMAPP), an IPP isomer [52]. The result of this reaction is 10-C geranyl diphosphate (GPP), precursor of monoterpenoids. New additions of IPP yield 15-C farnesyl diphosphate (FPP), the origin of sesquiterpenoids, and 20-C geranylgeranyl diphosphate, which gives rise to diterpenoids. The union of two FPP molecules leads to triterpenoids, and the union of two GGPP to tetraterpenoids. In general, the responsible enzymes are known as terpenoid synthases [53]. Terpenoids may derive from any of these branches, resulting in a large chemical diversity [54,55]. Thus, to cite some well-known terpenoid families that are also mentioned below, gibberellins are diterpenoids, trichothecenes are sesquiterpenoids, and carotenoids are tetraterpenoids.
Once the initial terpenoid skeleton has been synthesized, different enzymes introduce diverse chemical modifications until the mature SM is generated. Among the terpenoid synthases that intervene on linear chains there is a characteristic type, known as terpenoid cyclases, with recognizable biochemical and structural features [56]. These enzymes are largely responsible for the enormous diversity of terpenoids found in nature, including many fungal SMs. In some cases, a molecule of non-terpenoid origin is added to the structure, giving rise to the meroterpenoids [57].
3. Genetic Organization and Regulation of SM Genes
The genes responsible for SM synthesis are usually clustered in the fungal genomes sharing the same regulation [58]. Typical SM gene clusters contain a key pathway gene, coding for a PKS, a NRPS, or a terpene cyclase, which is often easily identified by its larger size. They also contain genes for other modifying enzymes frequently belonging to easily recognizable families, e.g., P450 monooxygenases [59], and in some cases genes for permeases involved in their excretion. SM biosynthetic pathways can be controlled by specific regulatory genes [60] frequently belonging to the Zn cluster family and normally included in the corresponding cluster. As a prototypical example, in F. fujikuroi the transcription factor Bik5 is necessary for the synthesis of bikaverin, supported in this case by a second regulatory protein Bik4 [61]. In other cases, regulatory genes are not found in the cluster, as occurs with the gibberellin cluster in the same species [27] or in penicillin and cephalosporin clusters in other fungi [62].
Due to the interest of SM production, much attention has been devoted to its regulatory mechanisms. SM clusters are regulated by a diversity of environmental cues and are controlled by different regulatory proteins, which are frequently involved in more general regulatory networks [63,64]. External signals controlling SM biosynthesis include the availability of nitrogen or carbon sources, pH, or light, mediated by global regulation systems that act simultaneously on different SM clusters as well as on other metabolic processes [65]. Regulation of SM production by nitrogen is very frequent [66], and different proteins participate in it, among which AreA-like proteins play a pivotal role. Carbon source availability affects many metabolic processes [67], including SM production [68], that usually involve a catabolite repressor of the CreA family. SM regulation by pH is usually controlled by proteins of the PacC family, with examples in different fungi [69]. Another general regulator of special interest is LaeA [70], which is associated with light regulation and development control with Velvet proteins forming a complex [71,72]. It has recently been observed that the main regulator by light in F. fujikuroi, WcoA, positively or negatively controls different SM clusters in this fungus [73]. This double positive/negative role on different pathways is frequent in these global regulatory systems, even when responding to the same signal. For example, AreA mutation results in derepression of gibberellin biosynthesis in F. fujikuroi [74], but repression of fumonisin production in F. verticillioides [75]. Examples of mutations on genes affecting fungal SM production—in some cases encoding enzymes, as found for glutamine synthetase in F. fujikuroi [76]—have been abundantly described in the literature.
SM clusters may be in any chromosomal region, but in many cases they are found in subtelomeric regions [77]. Such locations may be associated with epigenetic silencing in the form of heterochromatin. Supporting this, some regulatory proteins modulate SM production at the level of chromatin structure by histone modifications [78], a conclusion reinforced by the effects of mutations of genes involved in such modifications, such as that for the methyltransferase Kmt6 in Fusarium sp. [79,80]. This level of regulation provides a selective advantage to the genomic organization as clusters since it allows the simultaneous inactivation of all the genes of a pathway through heterochromatinization. This is the way through which LaeA acts in the Velvet complex, but other regulatory proteins, such as AreA, also participate in this control mechanism [81]. Regulation occurs also at the level of cell compartmentalization: e.g., there are regulatory systems for controlling the enzymes of each metabolic pathway and their localization in the appropriate cellular compartments, such as peroxisomes, vacuoles, endoplasmic reticulum and Golgi, or cytosol [82].
4. Biological Functions
As already stated, a distinctive feature of SMs is that they are dispensable for the fungus under controlled growth conditions, so that mutants unable to produce them are not affected in their viability in the laboratory. However, the persistence of these biosynthetic processes in fungi imply adaptive advantages in their natural environment. In some cases the functions are obvious and in other cases they are less clear. Some illustrative examples of known SM functions are mentioned below. Melanin protects against UV radiation, facilitating the survival of the fungus under strong sun exposure. However, it also exerts protective effects against other sources of stress, such as oxidative or thermal stress, as well as mechanical damage [83]. SMs can fulfill more specific physiological functions. Siderophores are NRPs that act as high-affinity iron chelators, which are used by some fungi to scavenge environmental iron or to sequestrate internal reactive iron [84]. Fusarubin and 5-deoxybostrycoidin-based melanin provide dark pigmentation to perithecia in F. fujikuroi [85] and F. graminearum [86], respectively. Other SMs are used for more than one purpose. β-carotene is useful as a protective agent against oxidative stress [87], but it is also used as a source of derivatives with more specific functions. In mucormycotina fungi, such as B. trispora or P. blakesleeanus, β‑carotene is cleaved to produce sexual hormones called trisporic acids, which are needed to carry out the sexual cycle [88,89]. However, in F. fujikuroi [30] and Ustilago maydis [90] the same carotene is cleaved to produce retinal, the prosthetic group of rhodopsins.
Many secondary metabolites play roles in the interactions of fungi with other organisms, both in terms of competition and pathogenesis [91]. There are many examples where fungi produce antibiotics to avoid competition. For example, Beauveria bassiana produces the polyketide oosporein to limit bacterial growth in the parasitized insect [92]. It is well known that the interaction between fungi and plants, either mutualistic or pathogenic, involves the simultaneous production of molecular signals from the interacting species [93,94]. Moreover, some SM gene clusters are expressed in the host plant but not in others. The F. fujikuroi FUB1 gene, coding for a PKS for the synthesis of the toxin fusaric acid, is expressed when infecting its host but not when infecting other plants [95]. The participation of SMs in pathogenesis is not easily predictable. In Pyricularia oryzae, the causative agent of rice blast disease, melanins are required for pathogenesis, but no role is apparently played by tenuazonic acid, a hybrid NRP/PKS mycotoxin, by nectriapyrones, polyketide compounds with antibacterial activity, or by pyriculols, phytotoxic polyketide compounds [96]. The dependence on specific needs in their ecological niches explains why many fungal SM clusters are not expressed under laboratory conditions. However, their functions may be investigated by activating them in a targeted way [97,98].
The relation of SMs with pathogenesis has been investigated by analyzing the virulence of mutants with alterations in their syntheses. As an example, mutants of fusaric acid production in F. oxysporum revealed that this SM contributes importantly to virulence in tomato plants and in immunocompromised mice [99]. Another interesting example was provided by a study of the role of gibberellins in F. fujikuroi, which are assumed to participate in the infection of rice because of the characteristic over-elongation of the seedlings for the “bakanae” disease caused by this fungus. Infection of rice with a non-producing F. fujikuroi mutant showed a lack of over-elongation in the infected seedlings and a reduced cell invasion capacity of the hyphae in the plant [100].
5. Biological Properties and Applications
Many SMs possess useful biological properties or have biotechnological applications [101], while others are detrimental or have disadvantageous effects [102,103]. SMs useful for humans include a large diversity of antibiotics. In addition to the historical example of penicillin, there are numerous SMs with a very varied spectrum of antibiosis. Among them are other antibacterials, such as cephalosporin obtained from Acremonium chrysogenum [104], antifungals, such as griseofulvin produced by Penicillium or other fungi [105], or antiprotozoals, such as bikaverin synthesized by Fusarium species [14]. Other compounds have medical or pharmaceutical applications, such as immunosuppressant cyclosporin A, produced by Tolypocladium inflatum [16]; cholesterol-lowering statins, with lovastatin from Aspergillus terreus as the best known example [12]; vitamin-A precursor β‑carotene, industrially obtained from Blakeslea trispora [106]; and anticancer drugs, such as the indole alkaloid camptothecin and taxol, produced by the endophytic fungus Entrophospora infrequens [107] and Taxomyces andreanae [108], respectively. An outstanding case in biotechnological applications is the aforementioned gibberellins, growth-regulating plant hormones with agricultural applications, which are mostly represented by gibberellic acid obtained from F. fujikuroi [27].
Frequently, secondary metabolites absorb visible light and have striking colors, ranging across all ranges of the spectrum: e.g., bikaverin and fusarubin have a reddish pigmentation [28]. In some cases, although it is not related to their biological function, different SMs are used commercially as pigments. Among them some carotenoids stand out, such as astaxanthin. This pigment, produced by the yeast Xhantophyllomyces dendrorhous [109] and some algae, is used in aquaculture as feed additive to provide an orange color to certain fish and crustaceans. Other well-known fungal pigments are polyketides produced by Monascus purpurea [110], a fungus used in rice fermentation since ancient times in Chinese and Japanese cuisine. These polyketides include monascorubramine as well as rubropunctatin and its derivatives, which are of various yellowish, orange, or reddish colors, and to which numerous healthy properties are attributed, such as anticancer, antidiabetic, or antiobesity properties.
Regardless of their real roles in nature, many SMs are toxic to humans, and their presence in plant foods, due to contamination by producing fungi before or after the harvest, constitutes an important public health problem [102,103,111]. These harmful SMs are known generically as mycotoxins. One specially studied for its high toxicity is aflatoxin B1, produced by various species of Aspergillus [112]. Its consumption is associated with a syndrome known as acute aflatoxicosis, as well as with liver cancer or other damaging effects [103]. Many well-known mycotoxins are produced by the Fusarium species [113], among them fumonisins, zearalenones, trichothecenes, and fusarins. Fumonisins inhibit sphingolipid metabolism and also have carcinogenic properties. A correlation between their consumption and esophageal cancer is well documented [102]. Trichothecenes inhibit protein synthesis and produce toxic syndromes in humans and animals. A well-known trichothecene is deoxynivalenol, which produces alimentary toxic aleukia, acute gastroenteritis, and growth impairment, among other effects [103]. Fusarins, especially fusarin C, are mutagenic in the Ames test, presumably due to their transformation into more toxic derivatives in the body [114]. Another mutagenic mycotoxin is ochratoxin A, which provokes renal cancer [102]. Zearalenones have lower toxicity, but produce an estrogenic syndrome in pigs, presumably due to their resemblance to this family of hormones [103].
An interesting consequence of the large metabolic diversity of fungi is that different species produce specific patterns of SMs, which allow for their use in taxonomic studies. The identification of fungal species based on the metabolites produced is known as chemotaxonomy [115]. This tool is especially relevant in the case of lichens, which are symbiotic associations between a fungus and a photoautotrophic partner, usually an alga. Lichens show an enormous capacity to produce SMs, which is mainly due to the fungal partner [116]. In many cases, these metabolites provide protection against the harmful effects of UV in their natural habitats [117]. The availability of powerful analytical techniques for metabolite identification allows for the creation of databases, which facilitate the assignment of lichens based on the metabolites detected [118].
6. Conclusions and Prospects
The production of secondary metabolites by fungi represents a vast field of research whose interest continues to grow, as evidenced by the large number of publications on the subject that appear every year. The chemical variety of secondary metabolites is impressive (see, e.g., [119,120]), of which this entry mentions only a few representative examples. The large number of investigated SM clusters, many of them with biochemical knowledge of the encoded enzymes, and the increasing number of fungal genomes whose sequences are available in the databases, have allowed an explosion of genetic mining work in many fungi [4,121]. This is facilitated by computer prediction programs, such as SMURF [122], antiSMASH [123], or MIDDAS-M [124], that allow for the identification of new clusters whose biosynthetic functions can only be tentatively assumed, pending experimental demonstration. A current research challenge in this field is the assignment of metabolites to new clusters that are being discovered, for which a combination of genetic, chemical, and biochemical methods is used [125–127]. Due to their dispensability, there are considerable differences in the presence of SM clusters not only between different genera, but also between different species of the same genus, with Aspergillus [128], Penicillium [129], and Fusarium [130] as outstanding examples of SM versatility. The identification of new metabolites is an exciting field due to its enormous biotechnological potential, since it is inferred that most of them remain to be discovered. In this regard, manipulation of SM pathways taking advantage of the combinatorics that allow the modular organization of PKS and NRPS enzymes is especially promising, and considerable progress has been made [131–133]. In conclusion, fungal secondary metabolism constitutes a field in continuous growth and development, and future discoveries should provide unprecedented possibilities in medicine or biotechnology.
