Biogenic silver nanoparticles are environmentally safer compared to particles obtained by chemical or physical methods due to the absence of toxic compounds in the technological process, gentle synthesis conditions and the possibility of utilizing the biomass used in their production. Biogenic silver nanoparticles are capable of self-assembly, including that on various surfaces, and there are mechanisms for controlling their morphology and size. Approaches and methods for obtaining biogenic silver nanoparticles using various parts of plants, algae, fungi and microorganisms have been described.
- nanoparticles
- biogenic silver nanoparticles
- antimicrobial properties
- self-assembly of nanoparticles
- bio-fabrication
1. Introduction
Today, developments in the nanotechnology field draw great attention due to the use in many fields of science of various nanomaterials, including nanoparticles. Nanomaterials have deserved this interest because of their unique size, physico-chemical properties, and activity in biological systems. The unique properties of metal nanoparticles find application in biological labeling, drug delivery, diagnostics, imaging, probing, gene insertion, artificial implant production, and tissue engineering [1]. Recent advances in nanotechnology show that nanomaterials, in particular silver-based ones, can play a crucial role in biological, pharmaceutical and biomedical fields. The size of nanoparticles significantly affects their properties (electrical, magnetic, toxic, etc.), which determines the importance of the synthesis process for obtaining nanoparticles of a given shape and size (Figure 1). Nanoparticles of certain morphology can be synthesized by self-assembly of Ag ions on supramolecular supports, and applied in such areas as biochemistry, catalysis, biosensors, and microelectronics [2].
Among the variety of metal nanoparticles, silver nanoparticles (AgNPs) have found wide application in various fields due to their superior physical, chemical and biological characteristics. Research in this area is aimed at finding new simplified methods for obtaining nanomaterials and their use in various fields of human activity. Biomimetic technologies are environmentally friendly and economically viable for the synthesis of materials [3][4]. Three main components are involved in the production of nanoparticles using biological methods: a solvent medium, an environmentally friendly reducing agent, and a non-toxic stabilizing agent [4][5]. The synthesis of nanoparticles using parts of plants, algae, fungi, and microorganisms has an advantage over chemical synthesis because the latter can produce toxic compounds adsorbed on a nanostructured surface, hindering the use of chemically synthesized particles for medical purposes [5][3]. It was also found that biogenic objects secrete a large amount of proteins that contribute to the reduction of metal ions and allow the control over the morphology of the resulting particles. At the same time, an important issue is the disposal of nanomaterials after their intended use, since, for example, the release of silver nanoparticles (AgNPs) into the aquatic environment can pose a potential risk to humans and other organisms when their levels exceed safe permissible levels [6]. Currently, there are a number of examples of the successful application of reusable functional products containing silver nanoparticles. AgNPs immobilized on cotton cloth were used as a catalyst for the reduction of nitroaromatics [7]. It has been shown that the catalyst can be recycled up to 6 times without significantly reducing its catalytic efficiency. A new nanocatalyst based on fibrous nanosilica with a high surface area containing silver nanoparticles dispersed on microsphere fibers has also been successfully developed [8]. The synthesized nanocatalyst was easily recovered and reused for at least 10 cycles. Veisi also notes that the AgNPS-based nanocatalyst can be isolated from reaction solutions and processed without losing its high efficiency [9].
Thus, biogenic synthesis of nanoparticles is economically efficient and safe. However, there is no orderly compiled data on the self-assembly of biogenic silver nanoparticles. It is necessary to collect new data on biogenic AgNPs, in particular, to compare the properties of silver nanoparticles obtained by different methods. The entreviewy also considers recent works on the antibacterial and antifungal properties of biogenic silver nanoparticles.
2. Synthesis and Self-Assembly of Biogenic Silver Nanoparticles
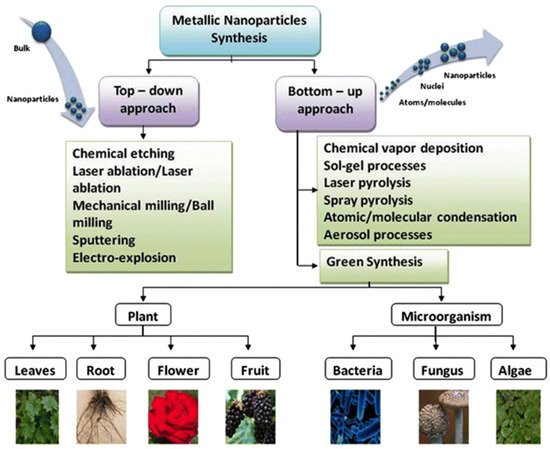
The properties of AgNPs depend on their size, shape, and morphology [10]. Different ways can be used for the synthesis of silver nanoparticles, namely chemical, physical and biological synthesis. Chemical method for AgNPs synthesis in solution requires reducing agents and a stabilizing agent. Physical synthesis of AgNPs includes the evaporation-condensation method and the laser ablation method [11]. Physicochemical synthesis, as a rule, is more laborious and hazardous than biological synthesis of AgNPs, which has emerged as an alternative approach and has a number of advantages [12]. Biogenic nanoparticles are safer and more environmentally friendly, since the synthesis process occurs at normal temperature and ambient pressure [13]. Additionally, the biomass used for nanoparticle synthesis is easily handled and utilized [14,15][14][15]. Thus, biological methods can be advantageous over physical and chemical methods of synthesis, such as thermal evaporation, ultrathin films method, lithography technique, diffusion-flame synthesis [16], sol—gel process, electrodeposition, chemical vapor deposition [17], chemical solution deposition, hydrolysis [10], catalytic method and coprecipitation method. Biogenic silver nanoparticles obtained using various parts of plants, fungi, microorganisms and algae have excellent self-assembly properties and exhibit much the same properties as AgNPs synthesized by chemical and physical methods.
The question of whether there are significant differences in shape, size, and polydispersity between silver nanoparticles obtained by chemical and biological methods seems to be quite interesting. A number of authors claim that the size of biogenic AgNPs is within 20–25 nm [33,34][18][19]. In the work of Spagnoletti it is indicated that the average size of biogenic silver nanoparticles (40 nm) was larger than that of chemically synthesized particles (20–30 nm) [35][20]. He noted that, with both types of nanoparticles being spherical, chemical AgNPs were more uniform in shape and highly dispersed, while biologically produced AgNPs formed agglomerates. In another work, sizes within 35 ± 10 nm, a spherical shape, and uniformity with a polydispersity index of 0.337 for biologically synthesized silver nanoparticles were shown [36][21]. Such fragmentary data from different authors do not give a general picture and sometimes contradict each other, which may mean that the entire set of synthesis conditions is important for these characteristics, and that multiple factors can influence the results. These assumptions are confirmed by some studies comparing AgNPs synthesized under different conditions; for example, it was shown that at pH 6, the average size of biogenic particles was 42.4 nm, while at pH 11, the particles had an average size of 21.4 nm and were monodisperse [37][22]. The pH of the solution affects not only the size, but also the shape and polydispersity of the obtained AgNPs [38][23]. Nanoparticles formed at pH 3 have different morphologies, such as rods, triangles, spheres, and other irregular shapes, while nanoparticles obtained at pH 5 to 7 have a predominantly spherical shape with a relatively uniform size distribution. At pH 9, a mixture of spherical and elongated nanoparticles is formed [38][23]. In addition to the acidity of the medium, other factors such as temperature and reaction time can be manipulated in biological synthesis. The effect of three different pH values 5.0, 7.0 and 9.0 at different temperatures and reaction times was observed when manipulating the silver nanoarchitecture [39][24]. Time has played an important role in the synthesis of spherical, penta/hexagonal and rectangular nanoparticles, along with pH and temperature. The particle size also increased from homogeneous nanoparticles with a size of 2–5 nm at 24 h (pH 7, 30 °C) to particles with a size of up to 80 nm after 72 h of incubation. The same results were obtained at pH 5 under all different reaction conditions, however, at pH 9, with an increase in the time interval from 24 to 72 h, the shape of silver nanoparticles changed from spherical to mixed, consisting of spherical, triangular and rectangular particles at 40 °C [39][24]. Thus, it turned out that there is not a single factor that controls the conversion of biogenic silver nanoparticles into different shapes and sizes, it is a unique balance of various interacting physical parameters. In chemical synthesis, the architecture of silver nanoparticles can be controlled using reducing agents such as poly (N-vinylpyrrolidone), polyacrylic acid, and capping with several organic solvents [39][24]. There have also been successful attempts to obtain various shapes and sizes of silver nanoparticles by chemical synthesis by varying the reaction time and stirring time. Thus, there is certain variability in the shape, size, and polydispersity of silver nanoparticles synthesized by both chemical and biological methods.
Silver nanoparticles obtained by different methods are attracting more and more attention also due to their ability to self-assemble. In the process of self-assembly, nanoparticles or other discrete objects spontaneously self-organize into ordered structures through direct and/or indirect interactions. For example, particles of uniform size can be assembled into spatially ordered structures, and the type of organization of nanoparticles and the structure of the resulting array depend on the synthesis conditions, particle diameters, as well as the nature of external influences on the structure [41][25]. Self-assembly processes are regulated by the balance of entropic and enthalpic effects and thus are temperature dependent [42][26]. Arrays of metal nanoparticles are formed through electrostatic and capillary interactions. For controlled self-assembly of nanoparticles, substrates or templates can be used to define the geometry of the system [43][27]. Various forms of silver nanoparticles (cubic, spherical, porous) can be synthesized from a solution of silver nitrate by self-assembly of anionic surfactants and neutral polymers in the presence of ultrasonic radiation [44][28].
The formed nanoparticles were stable in colloidal solutions and showed good bactericidal activity against pathogenic microorganisms. Biochemical changes in cyanobacteria Spirulina platensis and Nostoc linckia during the synthesis of AgNPs were studied to determine the optimal conditions for nanoparticle formation without biomass degradation [71][29].
3. Antibacterial and Antifungal Properties of Chemically Synthesized and Biogenic Silver Nanoparticles
Since the number of available antibacterial drugs is limited, the importance of finding new antibacterial agents or cofactors enhancing the effectiveness of existing drugs increases [81][30]. One of the most promising directions for the development of antibacterial agents is the use of nanotechnology products [82][31], in particular, metal nanoparticles which have become one of the most promising options for overcoming microbial resistance and combating multidrug-resistant microorganisms ( Figure 42 ) [72][32]. By now, the antibacterial activity of nanoparticles from Ag, ZnO, CuO, MgO, Si, MoO 33, TiO2 2 and CaO has been confirmed [83,84][33][34]. Cadmium oxide nanoparticles also exhibited increased antimicrobial activity [85][35] while platinum nanoparticles demonstrated not only an antimicrobial activity, but were effective against cancer cells and fungi [86][36].
Among all metal nanoparticles, silver nanoparticles are ones of the most important due to their use as antimicrobial agents in nanomedicine [87][37], in groundwater treatment [88][38], for the manufacture of surgical masks [89][39], the development of wound dressings and textile fabrics for combustiology [90][40]. The advantage of silver nanoparticles in comparison with metallic silver or its salts is the slow and controlled release of silver ions from the nanoparticle, which provides a prolonged antibacterial effect. Microbes have a much lower ability of developing resistance to silver nanoparticles in comparison with antibiotics [91][41].
In turn, biogenic metal nanoparticles have shown effectiveness against drug-resistant microorganisms, both when used alone and in combination with antibiotics. Thus, biogenic silver nanoparticles synthesized using soil bacterium Pseudomonas putida were active against clinical isolates of Staphylococcus aureus , Escherichia coli , Bacillus cereus , Pseudomonas aeruginosa , and Helicobacter pylori [92][42]. The antibacterial effect was achieved by the nanoparticle penetration through the cell membrane, which caused the excretion of intracellular metabolites, leading to significant damage to the bacterial cell. The synthesized nanoparticles exhibited a noticeable antibacterial effect even at very low concentrations, and the growth of bacteria was inversely related to the dose used. Silver nanoparticles synthesized using an aqueous extract of the blue-green alga Spirulina platensis , showed high antibacterial activity against Staphylococcus sciuri and Pseudomonas aeruginosa with an inhibition zone increasing linearly with an increase in concentration of nanoparticles [93][43]. Biogenic colloidal silver nanoparticles synthesized with the extract of Mentha pulegium L. as a reducing agent demonstrated antibacterial and antifungal properties and, in addition, were cytotoxic for Hela and MCF-7 cancer cells [94][44].
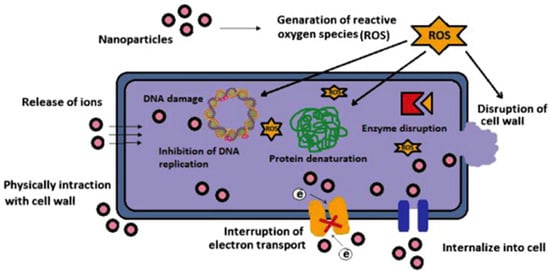
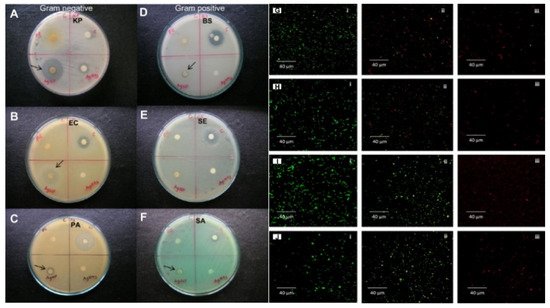
Silver nanoparticles are more effective when combined with antibiotics. The combination of commercial antibiotics and silver nanoparticles synthesized by reducing silver nitrate with an aqueous leaf extract of Epiphyllum oxypetalum (DC.) Haworth with was more active against Propionibacterium acne , Pseudomonas aeruginosa , and Klebsiella pneumoniae than the nanoparticles alone [60][45]. Silver nanoparticles are effective at low concentrations (mg/L) as antimicrobial agents against both gram-positive and gram-negative bacteria ( Figure 53 ) [95][46], selectively affect bacterial membranes [96][47], and are not cytotoxic for eukaryotic cells, including human erythrocytes [97][48]. The exact mechanism of action of colloidal silver solutions has not been clarified: the antibacterial activity of silver nanoparticles can be associated with the release of Ag+ ions or can be property of the silver nanoparticles themselves [98,99][49][50]. There are four main possible mechanisms of the antibacterial activity of colloidal silver solutions: the formation of free radicals (for example, reactive oxygen species) as a result of redox reactions, adhesion of silver NPs to the bacterial cell membrane and its destabilization, intercalation of silver nanoparticles between DNA bases with subsequent inhibition of DNA replication and transcription; and the destabilization of ribosomes, which inhibits protein synthesis.
4. Comparative Efficacy and Toxicity of Silver Nanoparticles Obtained by Different Methods of Synthesis
Several comparative analyses of therapeutic potentials of biogenic and chemically synthesized silver nanoparticles were carried out. In the study of antileishmanial activity, biogenic silver nanoparticles turned out to be more effective and significantly reduced the pathogenicity of parasites, in contrast to chemically synthesized AgNPs [120][51].
Silver nanoparticles synthesized using a leaf extract of medicinal plant Nathophodytes foetida exhibited a strong cytotoxic effect on human leukemic cells (K562), along with chemically synthesized nanoparticles [121][52]. The nanoparticles biosynthesized by Fusarium oxysporum and Azadirachta indica were polydisperse, 10–40 nm in size, and the chemically synthesized ones were monodisperse, 5 nm in size [122][53]. Antimicrobial analysis showed that the biogenic nanoparticles had better antibacterial properties against E. coli and S. aureus, probably because of higher protein capping of biogenic nanoparticles facilitating their entry into bacterial cells [122][53]. Other authors explained higher antibacterial activity of biogenic AgNPs by synergistic antibacterial effect of the nanoparticles and plant extract metabolites, adsorbed on them [123][54]. In their work, biogenic silver nanoparticles synthesized using DaturaDatura stramonium stramonium L. leaf extract had a spherical shape and a narrow size range, and demonstrated high antibacterial and DNA-cleaving activity, as well as antioxidant properties. Chemically synthesized AgNPs were smaller but did not possess antioxidant properties and had weaker antibacterial and DNA-cleaving activity [123][54]. When a plant culture used for biogenic nanoparticle synthesis had little or no antimicrobial activity, size differences could be responsible for better performance of green-AgNPs compared to the chemical AgNP [124][55]. Thus, silver nanoparticles synthesized by Elettaria cardamomum leaf extract demonstrated lower minimal inhibitory concentration when tested on a number of fungal phytopathogens, in comparison with chemical AgNPs. The fungicidal effect of both AgNPs types further increased in combination with fungicides (carbendazim, mancozeb, and thiram) [124][55].
Moreover, biosynthesized silver nanoparticles have a higher biocompatibility compared to chemically synthesized AgNPs [125][56]. The leaf extract of MillettiaMillettia pinnata pinnata (L.) Panigrahi was used as a reducing agent for the biosynthesis of silver nanoparticles. Capping of biogenic AgNPs by polyphenolic compounds present in the leaf extract provided high antioxidant activity of the nanoparticles and significantly reduced their toxicity compared to chemically synthesized AgNPs [126][57]. The influence of biogenic and chemically synthesized silver nanoparticles on the structure of benthic bacterial communities was studied. Thermoleophilia bacteria were resistant to both forms of AgNPs, while Koribacteraceae bacteria were sensitive [127][58]. A comparative toxicity study of chemically and biologically synthesized silver nanoparticles towards the plant SolanumSolanum lycopersicum lycopersicum L. showed that biogenic AgNPs were less toxic than the chemically synthesized ones [128][59]. Begum et al. studied the effect of biogenic and chemically synthesized silver nanoparticles on the biomass accumulation of callus cultures of Fagonia indica Burm. Biogenic AgNPs, were more biocompatible, producing larger biomass of Fagonia indica Burm. [129][60]. In studies with Bacillus subtilis cultures, biogenic AgNPs synthesized using the Allium cepa L. extract were significantly less toxic to normal human gut microbiota than chemically synthesized ones [130][61]. The effect of various concentrations of biologically and chemically synthesized silver nanoparticles was studied in freshwater fish Oreochromis niloticus L. A higher level of expression of the heat shock protein (HSP70) was observed in all tissues of fish exposed to chemically synthesized silver nanoparticles, compared with biologically synthesized ones [131][62].
References
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262.
- Padnya, P.; Gorbachuk, V.; Stoikov, I. The role of calixarenes and pillararenes in the design of silver nanoparticles: Self-assembly and application. Int. J. Mol. Sci. 2020, 21, 1425.
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84.
- Kalishwaralal, K.; Deepak, V.; Pandian, S.R.K.; Nellaiah, H.; Sangiliyandi, G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008, 62, 4411–4413.
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32.
- Murthy, H.C.A.; Zeleke, T.D.; Ravikumar, C.R.; Kumar, M.R.A.; Nagaswarupa, H.P. Electrochemical properties of biogenic silver nanoparticles synthesized using Hagenia abyssinica (Brace) JF. Gmel. medicinal plant leaf extract. Mater. Res. Express 2020, 7, 055016.
- Feng, W.; Huang, T.; Gao, L.; Yang, X.; Deng, W.; Zhou, R.; Liu, H. Textile-supported silver nanoparticles as a highly efficient and recyclable heterogeneous catalyst for nitroaromatic reduction at room temperature. RSC Adv. 2018, 8, 6288–6292.
- Dong, Z.; Le, X.; Li, X.; Zhang, W.; Dong, C.; Ma, J. Silver nanoparticles immobilized on fibrous nanosilica as highly efficient and recyclable heterogeneous catalyst for reduction of 4-nitrophenol and 2-nitroaniline. Appl. Catal. B Environ. 2014, 158-159, 129–135.
- Veisi, H.; Razeghi, S.; Mohammadi, P.; Hemmati, S. Silver nanoparticles decorated on thiol-modified magnetite nanoparticles (Fe3O4/SiO2-Pr-S-Ag) as a recyclable nanocatalyst for degradation of organic dyes. Mater. Sci. Eng. C 2019, 97, 624–631.
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865.
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406.
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14.
- Punjabi, K.; Choudhary, P.; Samant, L.; Mukhejee, S.; Vaidya, S.; Chowdhary, A. Biosynthesis of gold nanoparticles: A review. In Metal Nanoparticles in Microbiology; Rai, M., Duran, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 37–74.
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; Alothman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ.–Sci. 2019, 31, 257–269.
- Parashar, V.; Parashar, R.; Sharma, B.; Pandey, A.C. Parthenium leaf extract mediated synthesis of silver nanoparticles: A novel approach towards weed utilization. Dig. J. Nanomater. Biostructures 2009, 4, 45–50.
- Govindaraju, K.; Kiruthiga, V.; Kumar, G.; Singaravelu, G. Extracellular Synthesis of Silver Nanoparticles by a Marine Alga, Sargassum Wightii Grevilli and Their Antibacterial Effects. J. Nanosci. Nanotechnol. 2009, 9, 5497–5501.
- Hu, D.; Ogawa, K.; Kajiyama, M.; Enomae, T. Characterization of self-assembled silver nanoparticle ink based on nanoemulsion method. R. Soc. Open Sci. 2020, 7, 200296.
- Sunkar, S.; Nachiyar, V.; Namasivayam, S.K.R. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymu. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959.
- Velgosova, O.; Čižmárová, E.; Málek, J.; Kavuličova, J. Effect of storage conditions on long-term stability of Ag nano-particles formed via green synthesis. Int. J. Miner. Metall. Mater. 2017, 24, 1177–1182.
- Spagnoletti, F.N.; Kronberg, F.; Spedalieri, C.; Munarriz, E.; Giacometti, R. Protein corona on biogenic silver nanoparti-cles provides higher stability and protects cells from toxicity in comparison to chemical nanoparticles. J. Environ. Manag. 2021, 297, 113434.
- Ottoni, C.; Neto, M.L.; Léo, P.; Ortolan, B.; Barbieri, E.; De Souza, A. Environmental impact of biogenic silver nanoparticles in soil and aquatic organisms. Chemosphere 2020, 239, 124698.
- Amaladhas, T.P.; Sivagami, S.; Devi, T.A.; Ananthi, N.; Velammal, S.P. Biogenic synthesis of silver nanoparticles by leaf extract of Cassia angustifolia. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 045006.
- Rajput, S.; Werezuk, R.; Lange, R.M.; McDermott, M. Fungal Isolate Optimized for Biogenesis of Silver Nanoparticles with Enhanced Colloidal Stability. Langmuir 2016, 32, 8688–8697.
- Kumari, M.; Pandey, S.; Giri, V.P.; Bhattacharya, A.; Shukla, R.; Mishra, A. Tailoring shape and size of biogenic silver nano-particles to enhance antimicrobial efficacy against MDR bacteria. Microb. Pathog. 2017, 105, 346–355.
- Grzybowski, B.A.; Wilmer, C.E.; Kim, J.; Browne, K.P.; Bishop, K.J.M. Self-assembly: From crystals to cells. Soft Matter 2009, 5, 1110–1128.
- Boal, A.K.; Ilhan, F.; DeRouchey, J.; Thurn-Albrecht, T.; Russell, T.P.; Rotello, V.M. Self-assembly of nanoparticles into structured spherical and network aggregates. Nature 2000, 404, 746–748.
- Long, N.V.; Ohtaki, M.; Yuasa, M.; Yoshida, S.; Kuragaki, T.; Thi, C.M.; Nogami, M. Synthesis and Self-Assembly of Gold Nanoparticles by Chemically Modified Polyol Methods under Experimental Control. J. Nanomater. 2013, 2013, 793125.
- Moghimi-Rad, J.; Isfahani, T.D.; Hadi, I.; Ghalamdaran, S.; Sabbaghzadeh, J.; Sharif, M. Shape-controlled synthesis of silver particles by surfactant self-assembly under ultrasound radiation. Appl. Nanosci. 2011, 1, 27–35.
- Cepoi, L.; Rudi, L.; Chiriac, T.; Valuta, A.; Zinicovscaia, I.; Duca, G.; Kirkesali, E.; Frontasyeva, M.; Culicov, O.; Pavlov, S.; et al. Biochemical changes in cyanobacteria during the synthesis of silver nanoparticles. Can. J. Microbiol. 2015, 61, 13–21.
- Duval, R.E.; Grare, M.; Demoré, B. Fight Against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria. Molecules 2019, 24, 3152.
- Arokiyaraj, S.; Arasu, M.V.; Vincent, S.; Oh, Y.-K.; Kim, K.H.; Choi, K.-C.; Choi, S.H.; Prakash, N.U. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: An in vitro study. Int. J. Nanomed. 2014, 9, 379–388.
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145.
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284.
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. New function of molybdenum trioxide nanoplates: Toxicity towards pathogenic bacteria through membrane stress. Colloids Surf. B Biointerfaces 2013, 112, 521–524.
- Andeani, J.K.; Mohsenzadeh, S. Phytosynthesis of Cadmium Oxide Nanoparticles from Achillea wilhelmsii Flowers. J. Chem. 2013, 2013, 147613.
- Salehi, B.; Mehrabian, S.; Ahmadi, M. Investigation of antibacterial effect of Cadmium Oxide nanoparticles on Staphylococcus Aureus bacteria. J. Nanobiotechnol. 2014, 12, 1–8.
- Babu, G.M.M.; Gunasekaran, P. Extracellular synthesis of crystalline silver nanoparticles and its characterization. Mater. Lett. 2013, 90, 162–164.
- Mpenyana-Monyatsi, L.; Mthombeni, N.H.; Onyango, M.S.; Momba, M.N.B. Cost-Effective Filter Materials Coated with Silver Nanoparticles for the Removal of Pathogenic Bacteria in Groundwater. Int. J. Environ. Res. Public Health 2012, 9, 244–271.
- Li, Y.; Leung, P.; Yao, L.; Song, Q.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63.
- Durán, N.; Marcato, P.D.; De Souza, G.I.H.; Alves, O.L.; Esposito, E. Antibacterial Effect of Silver Nanoparticles Produced by Fungal Process on Textile Fabrics and Their Effluent Treatment. J. Biomed. Nanotechnol. 2007, 3, 203–208.
- Hwang, I.-S.; Hwang, J.H.; Choi, H.; Kim, K.-J.; Lee, D.G. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J. Med. Microbiol. 2012, 61, 1719–1726.
- Gopinath, V.; Priyadarshini, S.; Loke, M.F.; Arunkumar, J.; Marsili, E.; Mubarak, D.A.; Velusamy, P.; Vadivelu, J. Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab. J. Chem. 2017, 10, 1107–1117.
- Suganya, K.U.; Govindaraju, K.; Kumar, G.; Dhas, S.; Karthick, V.; Singaravelu, G.; Elanchezhiyan, M. Size controlled biogenic silver nanoparticles as antibacterial agent against isolates from HIV infected patients. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 144, 266–272.
- Kelkawi, A.H.A.; Kajani, A.A.; Bordbar, A. Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity. IET Nanobiotechnol. 2017, 11, 370–376.
- Paralikar, P. Biogenic Synthesis of Silver Nanoparticles Using Leaves Extract of Epiphyllum Oxypetalum and its Antibacterial Activity. Austin J. Biotechnol. Bioeng. 2014, 1, 5. Available online: http://d.researchbib.com/f/enLKImqTyhpUIvoTymnTyhM2qlo3IjYzAioF9vnJ90MJAboz9fo2q5YJWco2IhM2yhMJIlnJ5aY2Eiq25fo2SxYaObpQ9znJkyCJM1oTk0MKu0Y2SdLaEvMF12ZF1cMQRjZmVhpTEz.pdf (accessed on 24 November 2021).
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the anti-bacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nano-Technol. Biol. Med. 2007, 3, 168–171.
- Gajjar, P.; Pettee, B.; Britt, D.W.; Huang, W.; Johnson, W.P.; Anderson, A.J. Antimicrobial activities of commercial nano-particles against an environmental soil microbe, Pseudomonas putida KT2440. J. Biol. Eng. 2009, 3, 9.
- Krajewski, S.; Prucek, R.; Panacek, A.; Avci-Adali, A.; Nolte, A.; Straub, A.; Zboril, R.; Wendel, H.P.; Kvitek, L. He-mocompatibility evaluation of different silver nanoparticle concentrations employing a modified Chandler-loop in vitro assay on human blood. Acta Biomater. 2013, 9, 7460–7468.
- Yan, X.; He, B.; Liu, L.; Qu, B.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564.
- Haider, A.; Kang, I.-K. Preparation of Silver Nanoparticles and Their Industrial and Biomedical Applications: A Comprehensive Review. Adv. Mater. Sci. Eng. 2015, 2015, 165257.
- Ullah, I.; Cosar, G.; Abamor, E.S.; Bagirova, M.; Shinwari, Z.K.; Allahverdiyev, A.M. Comparative study on the antileishmanial activities of chemically and biologically synthesized silver nanoparticles (AgNPs). 3Biotech 2018, 2, 98.
- Datkhile, K.D.; Durgawale, P.P.; Patil, M.N. Biogenic silver nanoparticles are equally cytotoxic as chemically synthe-sized silver nanoparticles. Biomed. Pharmacol. J. 2017, 10, 337–344.
- Bawskar, M.; Deshmukh, S.; Bansod, S.; Gade, A.; Rai, M. Comparative analysis of biosynthesised and chemosynthesised silver nanoparticles with special reference to their antibacterial activity against pathogens. IET Nanobiotechnol. 2015, 9, 107–113.
- Mousavi-Khattat, M.; Keyhanfar, M.; Razmjou, A. A comparative study of stability, antioxidant, DNA cleavage and antibacterial activities of green and chemically synthesized silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1022–1031.
- Jamdagni, P.; Rana, J.S.; Khatri, P. Comparative study of antifungal effect of green and chemically synthesised silver nano-particles in combination with carbendazim, mancozeb, and thiram. IET Nanobiotechnol. 2018, 12, 1102–1107.
- Quinteros, M.A.; Bonilla, J.O.; Alborés, S.V.; Villegas, L.B.; Páez, P.L. Biogenic nanoparticles: Synthesis, stability and biocompatibility mediated by proteins of Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2019, 184, 110517.
- Priya, R.S.; Geetha, D.; Ramesh, P. Antioxidant activity of chemically synthesized AgNPs and biosynthesized Pongamia pinnata leaf extract mediated AgNPs—A comparative study. Ecotoxicol. Environ. Saf. 2016, 134, 308–318.
- Welz, P.J.; Khan, N.; Prins, A. The effect of biogenic and chemically manufactured silver nanoparticles on the benthic bacterial communities in river sediments. Sci. Total Environ. 2018, 644, 1380–1390.
- Girilal, M.; Fayaz, A.M.; Elumalai, L.; Sathiyaseelan, A.; Gandhiappan, J.; Kalaichelvan, P. Comparative Stress Physiology Analysis of Biologically and Chemically Synthesized Silver Nanoparticles on Solanum Lycopersicum L. Colloid Interface Sci. Commun. 2018, 24, 1–6.
- Begum, S.; Zahid, A.; Khan, T.; Khan, N.Z.; Ali, W. Comparative analysis of the effects of chemically and biologically synthesized silver nanoparticles on biomass accumulation and secondary metabolism in callus cultures of Fagonia indica. Physiol. Mol. Biol. Plants 2020, 8, 1739–1750.
- Tyagi, P.K.; Tyagi, S.; Verma, C.; Rajpa, A. Estimation of toxic effects of chemically and biologically synthesized silver nano-particles on human gut microflora containing Bacillus subtilis. J. Toxicol. Environ. Health Sci. 2013, 5, 172–177.
- Girilal, M.; Krishnakumar, V.; Poornima, P.; Fayaz, A.M.; Kalaichelvan, P.T. A comparative study on biologically and chemically synthesized silver nanoparticles induced Heat Shock Proteins on fresh water fish Oreochromis niloticus. Chemosphere 2015, 139, 461–468.