Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 3 by Lindsay Dong.
The exoskeleton of the pinching side of claws with denticles and of the outer side without them on the coconut crab, Birgus latro, which is a rare organism, were studied using a materials science approach. The mechanical resistance of three claws of different sizes was investigated along the exoskeleton thickness from the outer surface to the inner surface, and the results were compared, including the contribution of the microstructure and chemical compositions. Mechanical properties, hardness (H) and stiffness (Er), were probed through nanoindentation tests.
- biomineralization
- coconut crab
- cuticle
- denticle
- nanoindentation
- microstructure
- crustacean
- hardness
- stiffness
1. Introduction
Organisms have a tissue structure that is adaptive to the environment. Understanding the diverse mechanisms by which organisms achieve exceptionally high mechanical properties may enable the development of unique, biologically inspired materials [1][2][3][4][5][6][7][8]. In fact, bio-inspired materials with excellent functional and mechanical properties have been reported [9][10][11][12][13][14]. The exoskeleton that covers the outside of the arthropod body is very hard, and its hardness is associated with calcification and tissue structure [15][16][17][18][19]. The tissue structure in a mineralized cuticle is characterized by a twisted plywood pattern [20][21][22][23][24][25]. In recent studies, it was reported that the complicated structure of this pattern was characterized with 3D analysis [22], and the hardness increased as the stacking height of the twisted plywood structure decreased [25]. Wu et al. [26] showed structural design strategies for the optimized effective fracture energy depending on the pitch angles, fiber lengths, fracture energy of crack bridging, and twist angle distribution in composites with this plywood pattern structure. Furthermore, the fabrication techniques and the corresponding mechanical properties of Bouligand biomimetic cellulose nanocrystal nanocomposites have been reported [27]. Since the Bouligand structure is the additional complexity involved in creating a helical twist in the layer-by-layer stacking, it may have thus far received less attention. However, new technologies such as 3D analysis and 3D and 4D printing [28][29] have made it possible to manufacture complex structures. A detailed understanding of the complex tissue structure in organisms is becoming more important.
The exoskeleton of crustaceans protects the organisms from predatory attacks and water loss. Many decapod crustaceans have characteristically large claws that are used to capture prey, crush a wide variety of rigid foods, and protect themselves from predators. Furthermore, in some species, the claws are used in mating, and their presence/absence may be crucial in mating success [30][31]. Hence, the claws have superior mechanical properties and strong calcification as compared to the carapace and legs [18][25][32][33][34].
The coconut crab, Birgus latro, [35] has an extraordinary pinching force as compared to other decapod crustaceans. Surprisingly, it has a pinching force of more than 90 times its body weight [36], and exerts the strongest force of almost all terrestrial animals with body weight near 1 kg [22]. Usually, the left claw of the coconut crab is larger than the right claw. The claw is composed of two articulating parts: the dactyl part is movable (often referred to as the “movable finger”), and the pollex part is fixed (the “fixed finger”), as shown in Figure 1. The fixed finger is the side that supports the force when pinched and is important for maintaining the function of the claw. In the claw fingers, there is an inner part that pinches prey (pinching side) and an outer part (outer side). The pinching side displays toothlike denticles, which come into direct contact with predators and prey. The denticles of the fixed finger in general are larger than these of the movable finger. These denticles have little or no epi- or exocuticle in this region in various crabs: Cancer borealis, Callinectes sapidus, Chionoecetes opilio, Paralomis birsteini, Paralithodes camtschaticus, and Scylla serrata [37][38]. Nevertheless, the hardness was 2.5 to 10 times higher in the denticle than in the endocuticle. The denticles serve as the points of contact in the capture of prey and defense against predators. Therefore, the denticles are expected to be abrasion-resistant, and may have excellent mechanical properties in the claw. Furthermore, these denticles are present in various sizes and are irregularly arranged [38], as shown in Figure 1, and these characteristics could possibly change with growth. However, there are few studies on the tissue and mechanical properties of denticles in the exoskeleton. Here, we prepared three left claws of the coconut crabs with different body weights and quantified their hardness, stiffness, tissue structure, and chemical compositions along the exoskeleton thickness from the outer surface to the inner surface in the claw cross section of the pinching side with the denticles of each fixed finger and the outer side without them.
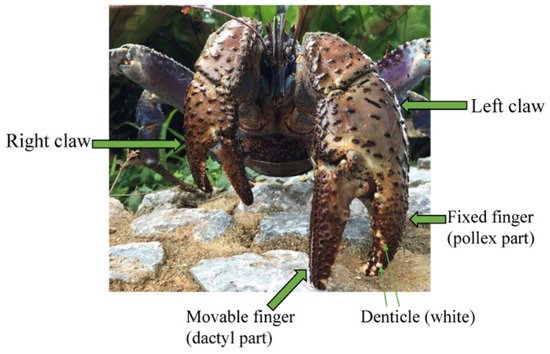
Figure 1. Photograph of coconut crab with robust/mighty claws.
2. Specimen Preparation
Coconut crabs were captured at Motobu town on northern Okinawa Island, located in southwestern Japan. As part of an ecological survey of wild coconut crabs inhabiting this area, their body weight (BW) and size (ThL: thoracic length; CL: claw length; CH: claw height) were recorded, and only the left claw was collected for the sample. Here, the three claws shown in Figure 2 were prepared as follows. All three coconut crabs were male, and their BWs were 1650 g (B1650 specimen), 610 g (B610 specimen), and 300 g (B300 specimen). The claws were frozen, transported from Okinawa Churashima Foundation to National Institute for Materials Science in Tsukuba for analysis, and stored at −18 ℃ prior to analysis. The claws were thawed under running water, and then the fixed finger was cut using a saw. The fixed fingers were cut to fit within the dimensions of a 40 mm diameter mounting cup used in the embedding process. Embedding cups were filled with epoxy (Struers, EPOFIX resin), and the three specimens were left to cure at room temperature for approximately 12 h. The specimens were placed under vacuum for 10 min immediately after the addition of epoxy to further ensure penetration of the epoxy into the specimen voids. Then the specimens were ground with 320/600/800 grit SiC papers; subsequently polished with a 9, 3, and 1 μm diamond suspension; and finally polished with a 0.05 μm alumina suspension. After polishing, cross-sectional micrographs of the three specimens were obtained using an optical microscope (OM). Subsequently, the specimens were coated with about 2 nm of osmium in order to characterize the microstructure and chemical compositions. After this was completed, the specimens were repolished in order to remove osmium coating and subjected to nanoindentation tests. The specimen thickness shaved by repolishing was approximately 0.3 mm.
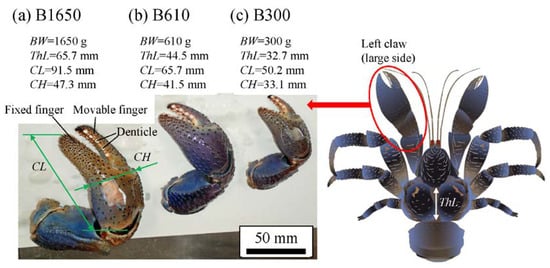
Figure 2. Photographs of the left claws of coconut crabs with different body weights of (a) 1650 g, (b) 610 g, and (c) 300 g used in the present study. Here, BW denotes the body weight, ThL the thoracic length, CL the claw length, and CH the claw height.
3. Cross-Sectional Images
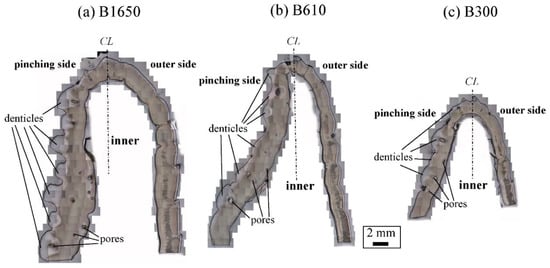
Figure 3 shows optical micrographs of a cross section of the fixed finger. In these micrographs, the pinching side with the denticles is on the left, and the outer side without denticles is on the right. On the outer side, the thickness of the exoskeleton was not perfectly constant, and narrow areas were observed in some places. As can be seen in Figure S1 in the Supplementary Materials, these narrow areas were associated with sensory hairs formed from the inner side through relatively thick pore canals. The sensory hairs could also be observed on the claws shown in Figure 2, and were mainly found on claws and legs. Interestingly, in Figure 3 and Figure S1, some pores were observed on the pinching side, and these pores were located within the denticles or within the endocuticle layer below the denticles. The exocuticle layer, endocuticle layer, and denticles were clearly observed in all specimens. The results of measuring the ratio of the exocuticle thickness to the exoskeleton thickness on the outer side, as well as the ratio of the denticle thickness to the exoskeleton thickness on the pinching side, are displayed in Figure S2 in the Supplementary Materials. The results are summarized in Figure 4, in which data for the male coconut crabs (340 g and 1070 g) inhabiting northern Okinawa Island we have measured so far are included.

Figure 3. Optical micrographs of a cross section of the fixed finger of (a) B1650, (b) B610, and (c) B300 after polishing.
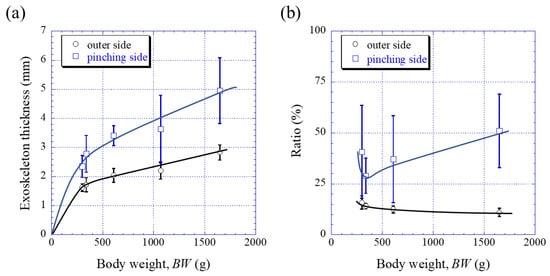

Figure 4. Variations in (a) the exoskeleton thickness on the outer side and on the pinching side with body weight (BW) and in (b) the ratio (=exocuticle thickness/exoskeleton thickness) on the outer side, as well as the ratio (=denticle thickness/exoskeleton thickness) on the pinching side with BW. Here, error bars represent standard deviations. Data were based on the measurement results shown in Figure S2 in the Supplementary Materials.
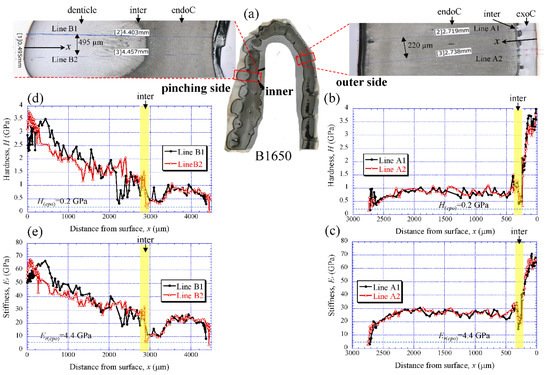
4. Hardness and Stiffness
Figure 5a shows the areas of the nanoindentation measurement of the B1650 specimen, and Figure 5b–e show the lateral distribution of mechanical resistance: the hardness (H) and the reduced stiffness (Er). Note that x refers to the distance from the outer surface of each side. Both the H and the Er revealed pronounced gradients between the surface and the inner side. On the outer side (Figure 5b,c), the H was the range of 3 to 4 GPa in the exocuticle layer, and decreased abruptly toward the intermediate layer. The value was 0.3–0.5 GPa. Subsequently, the H increased to 1.5 GPa and was in the range of 0.7 to 1.0 GPa in the endocuticle layer. The Er also showed the same change through the exoskeleton thickness. The value was in the range of 60 to 70 GPa in the exocuticle layer, decreased to 14–18 GPa in the intermediate layer and subsequently increased to 34 GPa, and was 22–30 GPa in the endocuticle layer. On the other hand, on the pinching side with the denticles (Figure 5d,e), although the two lines (B1 and B2) showed slightly different changes near the surface, the distributions of the H and the Er showed basically the same trends as the distributions on the outer side. They gradually decreased in the region of the denticle, decreased abruptly in the intermediate layer and subsequently increased, and become constant in the exocuticle layer. Surprisingly, the minimum values in the intermediate layer corresponded to the mechanical resistance of the cold epoxy resin used as the embedding material. These characteristics of mechanical resistance seen in the B1650 specimen were also obtained in the B610 and the B300 specimens.

Figure 5. (a) Areas where nanoindentation tests were conducted in the B1650 specimen. Distributions of (b) hardness, H, and (c) stiffness, Er, with distance from the surface, x, on the outer side; and of (d) H and (e) Er with x on the pinching side. Here, H(epo) and Er(epo) denote the hardness and stiffness of cold epoxy resin, respectively.
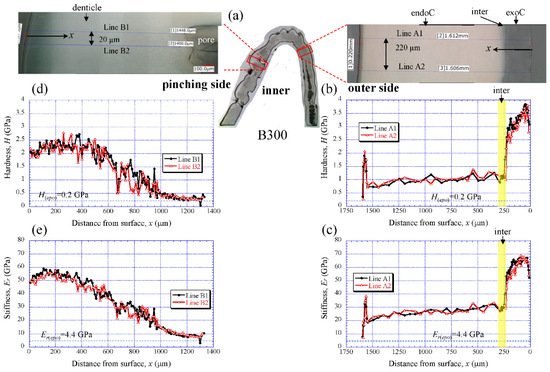
Figure 6 shows the results for the B300 specimen. On the outer side (Figure 6b,c), the H steadily increased from the surface, and the peak value occurred at about 50 µm in the exocuticle layer. Subsequently, it gradually decreased, decreased abruptly in the intermediate layer, and then slightly decreased in the endocuticle layer. The Er also exhibited the same distributions as the H. The maximum values of H and Er in the exocuticle layer and the values in the endocuticle layer were almost the same as in the B1650 specimen shown in Figure 5b,c. The H and Er of the pinching side shown in Figure 6d,e steadily increased from the surface and then gradually decrease in the denticles. The H and Er of near the surface of the denticle in B300 were found to be smaller than those in B1650, as shown in Figure 5d,e.

Figure 6. (a) Areas where nanoindentation tests were conducted in the B300 specimen. Distributions of (b) hardness, H, and (c) stiffness, Er, with distance from the surface, x, on the outer side; and of (d) H and (e) Er with x on the pinching side. Here, H(epo) and Er(epo) denote the hardness and stiffness of cold epoxy resin, respectively.
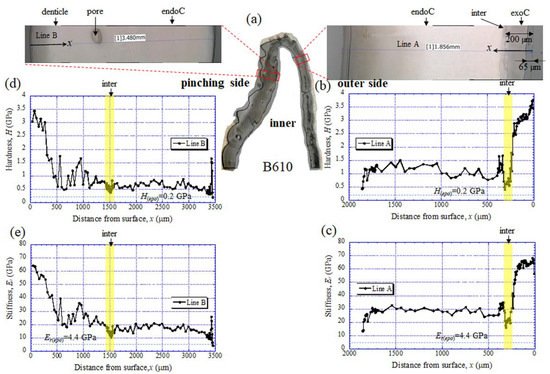
The results for the B610 specimen are shown in Figure 7 and Figure 8. In this specimen, only one line was measured on each side, and instead a nanoindentation test was also performed at seven characteristic areas, from A to G. These areas of the claw tip were similarly observed in the other two specimens, as shown in Figure 3, Figures S1 and S2. The distributions and values shown in Figure 7b–e were fairly close to those of the B1650 specimen shown in Figure 5b–e. Figure 8 shows the results at seven areas, from A to G. A and B corresponded to the center areas in the exocuticle and endocuticle layers, respectively. C and D were the areas of the hump observed in Figure 8; the humps were seen near the top in all specimens, and they corresponded to the many black protrusions seen in the claw surface in Figure 1 and Figure 2. E was the area of distinctly different tissues below the hump. F and G on the pinching side were areas near the surface of the denticle and at the center of the denticle thickness. The H and Er in areas A and B and in areas F and G were consistent with the results for the outer side and the pinching side shown in Figure 7. The H and Er in area C were the same as in area D; these values were much smaller than in areas F and G in the denticle and area B in the endocuticle. That is, the hump was not the denticle. The many black protrusions seen on the claw surface and the claw top in coconut crabs may have been keratin or chitin, a nonmineralized area [39]. This point will be examined through detailed tissue analysis and component analysis in the future. The values in area E corresponded to those in area B.

Figure 7. (a) Areas where nanoindentation tests were conducted in the B610 specimen. Distributions of (b) hardness, H, and (c) stiffness, Er, with distance from the surface, x, on the outer side; and of (d) H and (e) Er with x on the pinching side. Here, H(epo) and Er(epo) denote the hardness and stiffness of cold epoxy resin, respectively.
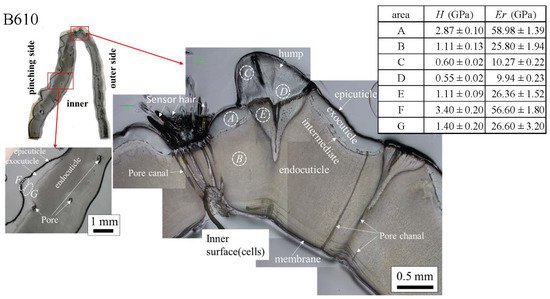

Figure 8. Hardness, H, and stiffness, Er, at seven characteristic areas (A to G) in the B610 specimen obtained from nanoindentation tests. The tests were conducted five times for each area.
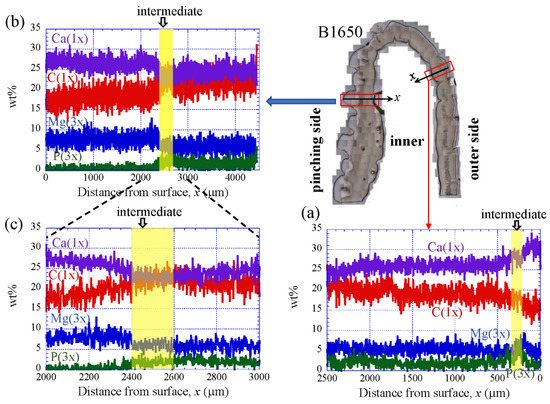
5. Chemical Compositions
Figure 9 shows the line-scanning profiles of calcium (Ca), magnesium (Mg), phosphorus (P), and carbon (C) through the thickness of the outer side and pinching side in the B1650 specimen measured by EDS. Note that the weight % of Mg and P were expanded by a factor of 3 relative to those of Ca and C. The line scans revealed decreasing Ca concentrations from the surface to the specimen interior, regardless of which side, and a concomitant increase in C throughout the same region. Mg concentrations decreased slightly in the intermediate layer, while P concentrations increased. In the denticle, Ca concentrations were slightly lower than those in the exocuticle on the outer side, P concentrations were almost zero, and Mg concentrations were high as compared to the exocuticle and endocuticle. Mg concentrations in the endocuticle on the pinching side were the same as those in the endocuticle on the outer side. Similar trends were seen in the B300 specimen (Figure 10) and the B610 specimen (Figure 11). On the pinching side of the B300 specimen, since a large pore existed in the exoskeleton thickness, the inorganic components of Ca, Mg, and P became zero, and the organic components of C and O changed significantly in that region due to the effect of resin. The P concentration increased as it approached to the pore, while the Mg concentration decreased. These changes related to P and Mg were also seen in aggregations of small pores in the denticle of the B610 specimen, as shown in Figure 11. In all specimens, the Ca concentration in the exocuticle on the outer side was in the range of 28 to 33 wt %, and that in the denticle on the pinching side was in the range of 24 to 28 wt % regardless of BW. In short, no calcification in the exocuticle and denticle was strongly changed by BW.

Figure 9. Line-scanning profiles with distance from the surface of the B1650 claw cross-sectional plane measured by energy-dispersive X-ray spectroscopy (EDS): (a) outer side; (b,c) pinching side. Here, the weight percentages of magnesium (Mg) and phosphorus (P) were expanded by a factor of 3 relative to those of calcium (Ca) and carbon (C).
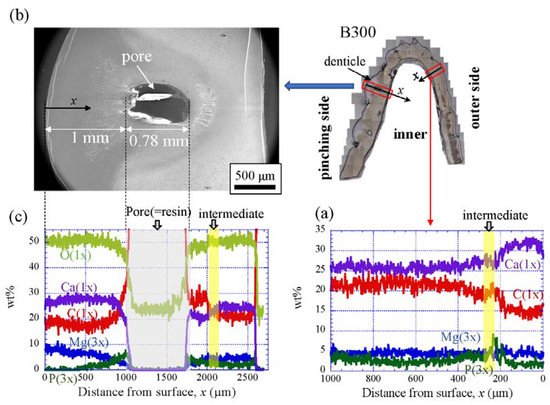

Figure 10. Line-scanning profiles with distance from the surface of the B300 claw cross-sectional plane measured by EDS: (a) outer side; (b) SEM micrographs of the denticle on the pinching side; (c) line-scanning profiles of its area. Here, the weight percentages of magnesium (Mg) and phosphorus (P) were expanded by a factor of 3 relative to those of calcium (Ca), carbon (C), and oxygen (O).
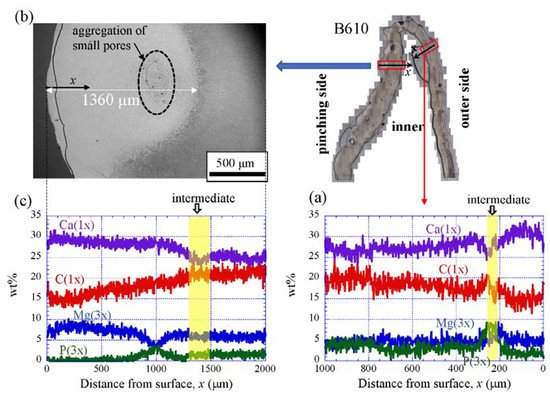

Figure 11. Line-scanning profiles with distance from the surface of the B610 claw cross-sectional plane measured by EDS: (a) outer side; (b) SEM micrographs of the denticle on the pinching side; (c) line-scanning profiles of its area. Here, the weight percentages of magnesium (Mg) and phosphorus (P) were expanded by a factor of 3 relative to those of calcium (Ca), carbon (C), and oxygen (O).
6. Microstructures
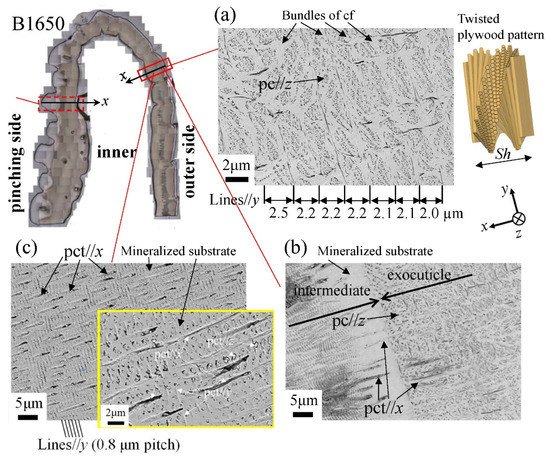
Figure 12 shows SEM micrographs at three layers on the outer side of the B1650 specimen. The tissue structure remarkably exhibited a morphological change in the intermediate layer. In the exocuticle layer, the lamella thickness (Lines//y) observed in Figure 12a corresponded to the stacking height, Sh, in a twisted-plywood-patterned structure rotated 180° around an axis normal to the surface, which was characteristic of the cuticle of arthropods [15][20][22]. The Sh tended to decrease as it approached the surface [25]. On the other hand, the endocuticle layer shown in Figure 12c was a tissue structure in which thick pore canal tubules (pct//x) parallel to the x-direction and thin pct (pct//y, pct//z) in two directions normal to it intersected in the mineralized matrix [22]. The tissue structure in the outer side was the same as that in the other two specimens: the B300 specimen (Figure 13) and the B610 specimen. This was consistent with the results of a coconut crab of 1070 g reported earlier [22][25].

Figure 12. Scanning electron microscope (SEM) micrographs at (a) x = 110 µm (the exocuticle layer), (b) x = 240 µm (the intermediate layer), and (c) x = 330 µm (the endocuticle layer) on the outer side of the B1650 specimen. Here, cf denotes chitin fibers in the mineral–protein matrix, pct denotes pore canal tubules, and pc denotes pore canals.
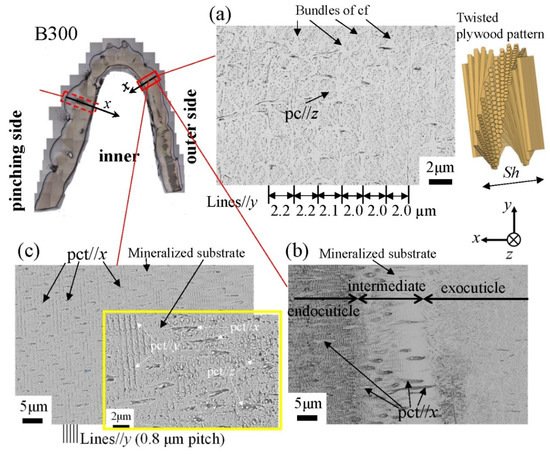

Figure 13. SEM micrographs at (a) x = 110 µm (the exocuticle layer), (b) x = 210 µm (the intermediate layer), and (c) x = 250 µm (the endocuticle layer) on the outer side of the B300 specimen. Here, cf denotes chitin fibers in the mineral–protein matrix, pct denotes pore canal tubules, and pc denotes pore canals.
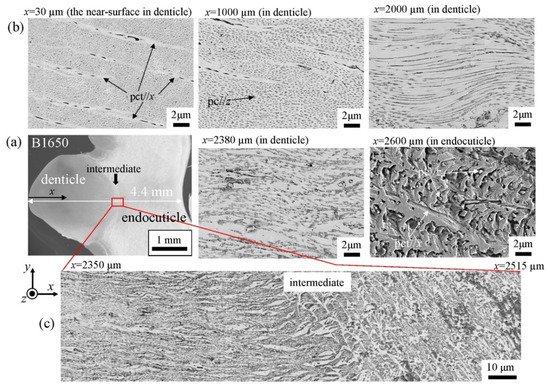
The microstructures on the pinching side of the B1650 specimen are shown in Figure 14. The tissue structure in the denticle was clearly different from that on the outer side. The white streaks (perhaps pct//x) parallel to the x-direction became wavy at x = 2000 µm and their density increased, while very small black dots parallel to the z-direction, pc//z, became larger. The low-magnification SEM (×3500) micrograph near the intermediate layer shown in Figure 14c revealed a distinct shift in tissue structure from the denticle to the endocuticle; i.e., the tissue flow changed from “parallel to the x-direction” to “parallel to the y-direction” in the intermediate layer. This meant that the tissue structure mainly aligned normally to the surface within the denticle. Figure 15 shows the microstructures on the pinching side in the B300 specimen. The tissue changes in the denticle exhibited basically the same characteristics. The denticle microstructures in the B610 specimen are shown in Figure 16. Curiously, a small amount of exocuticle layer was visible near the surface of the denticle. Its thickness covering the denticle was about 37 μm, forming a twisted-plywood-patterned structure of Sh ≈ 0.8–2.8 µm. This characteristic is also found in the red king crab, Paralithodes camtschaticus [37], of the same infraorder, Anomura, as the coconut crab. The microstructures within the endocuticle layer on the pinching side of all specimens are shown in Figure 17. The tissue structure corresponded to that in the endocuticle layer of the outer side.

Figure 14. SEM micrographs (a) from the denticle to the endocuticle on the pinching side of the B1650 specimen, (b) at the distance from the surface, x, and (c) near the intermediate layer.
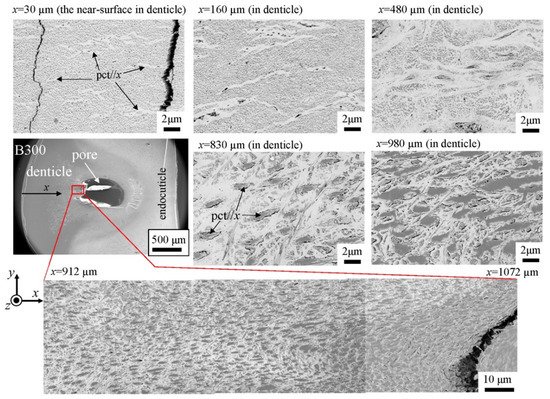

Figure 15. SEM micrographs from the denticle to the endocuticle on the pinching side of the B300 specimen.
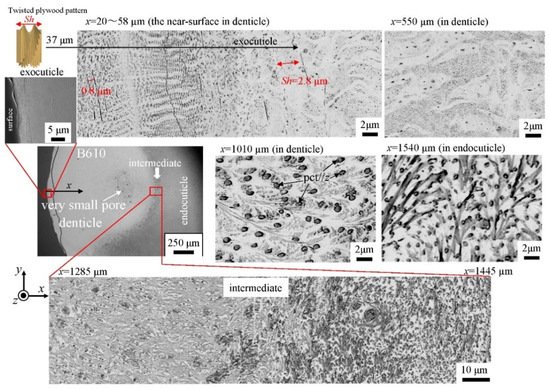

Figure 16. SEM micrographs from the denticle to the endocuticle on the pinching side of the B610 specimen.
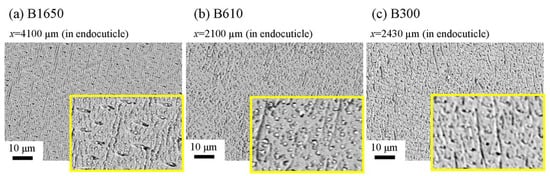

Figure 17. SEM micrographs of the endocuticle layer on the pinching side of (a) B1650, (b) B610, and (c) B300 specimens.
References
- Islam, M.K.; Hazell, P.J.; Escobedo, J.P.; Wang, H. Biomimetic armour design strategies for additive manufacturing: A review. Mater. Des. 2021, 205, 109730.
- Zhang, B.; Han, Q.; Zhang, J.; Han, Z.; Niu, S.; Ren, L. Advanced bio-inspired structural materials: Local properties determine overall performance. Mater. Today 2020, 41, 177–199.
- Vincent, J.F. Survival of the cheapest. Mater. Today 2002, 5–12, 28–41.
- Meyers, M.A.; Chen, P.Y.; Lin, A.Y.M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206.
- Fabritius, H.-O.; Sachs, C.; Triguero, P.R.; Raabe, D. Influence of structural principles on the mechanics of a biological fiber-based composite material with hierarchical organization: The exoskeleton of the lobster homarus americanus. Adv. Mater. 2009, 21, 391–400.
- Huang, W.; Restrepo, D.; Jung, J.-Y.; Su, F.Y.; Liu, Z.; Ritchie, R.O.; McKittrick, J.; Zavattieri, P.; Kisailus, D. Multiscale Toughening Mechanisms in Biological Materials and Bioinspired Designs. Adv. Mater. 2019, 31, 1561.
- Meyers, M.A.; Mckittrick, J.; Chen, P.-Y. Structural Biological Materials: Critical Mechanics-Materials Connections. Science 2013, 339, 773–779.
- Jiménez-Jiménez, C.; Manzano, M.; Vallet-Regí, M. Nanoparticles Coated with Cell Membranes for Biomedical Applications. Biology 2020, 9, 406.
- Amorim, L.; Santos, A.; Nunes, J.P.; Viana, J.C. Bioinspired approaches for toughening of fibre reinforced polymer composites. Mater. Des. 2021, 199, 109336.
- Yao, H.; Zheng, G.; Li, W.; McDowell, M.T.; Seh, Z.; Liu, N.; Lu, Z.; Cui, Y. Crab shells as sustainable templates from nature for nanostructured battery electrodes. Nano Lett. 2013, 13, 3385–3390.
- Cheng, L.; Thomas, A.; Glancey, J.L.; Karlsson, A.M. Mechanical behavior of bio-inspired laminated composites. Compos. Part A 2011, 42, 211–220.
- Pan, L.; Wang, F.; Cheng, Y.; Leow, W.R.; Zhang, Y.W.; Wang, M.; Cai, P.; Ji, B.; Li, D.; Chen, X. A supertough electro-tendon based on spider silk composites. Nat. Commun. 2020, 11, 1332.
- Launey, M.E.; Ritchie, R.O. On the fracture toughness of advanced materials. Adv. Mater. 2009, 21, 2103–2110.
- Sun, Y.; Tian, W.; Zhang, T.; Chen, P.; Li, M. Strength and toughness enhancement in 3d printing via bioinspired tool path. Mater. Des. 2020, 185, 108239.
- Raabe, D.; Sachs, C.; Romano, P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005, 53, 4281–4292.
- Wang, Y.; Li, X.; Li, J.; Qiu, F. Microstructure and mechanical properties of the dactylopodites of the chinese mitten crab (Eriocheir sinensis). Appl. Sci. 2018, 8, 674.
- Chen, P.Y.; Lin, A.Y.M.; Lin, Y.S.; Seki, Y.; Stokes, A.G.; Peyras, J.; Olevsky, E.A.; Meyers, M.A.; McKittrick, J. Structure and mechanical properties of selected biological materials. J. Mech. Behav. Biomech. Mater. 2008, 1, 208–226.
- Boßelmann, F.; Romano, P.; Fabritius, H.; Raabe, D.; Epple, M. The composition of the exoskeleton of two crustacea: The American lobster Homarus americanus and the edible crab Cancer pagurus. Thermochim. Acta 2007, 463, 65–68.
- Sayekti, P.R.; Cerniauskas, G.; Robert, C.; Retnoaji, B.; Alam, P. The impact behaviour of crab carapaces in relation to morphology. Materials 2020, 13, 3994.
- Bouligand, Y. Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell 1972, 4, 189–217.
- Yano, I.; Kobayashi, S. Calcification and age determination in crustacea—I. Possiblity of age determination in crabs on the basis of number of lamella in cuticles. Bull. Jpn. Soc. Sci. Fish 1969, 35, 34–42.
- Inoue, T.; Oka, S.; Hara, T. Three-dimensional microstructure of robust claw of coconut crab, one of the largest terrestrial crustaceans. Mater. Des. 2021, 206, 109765.
- Kellersztein, I.; Cohen, S.R.; Bar-On, B.; Wagner, H.D. The exoskeleton of scorpions’ pincers: Structure and micro-mechanical properties. Acta Biomater. 2019, 94, 565–573.
- Weaver, J.C.; Milliron, G.W.; Miserez, A.; Lutterodt, K.E.; Herrera, S.; Gallana, I.; Mershon, W.J.; Swanson, B.; Zavattieri, P.; DiMasi, E.; et al. The stomatopod dactyl club: A formidable damage-tolerant biological hammer. Science 2012, 336, 1275–1280.
- Inoue, T.; Hara, T.; Nakazato, K.; Oka, S. Superior mechanical resistance in the exoskeleton of the coconut crab, Birgus latro. Mater. Today Biol. 2021, 12, 100132.
- Wu, K.; Song, Z.; Zhang, S.; Ni, Y.; Cai, S.; Gong, X.; He, L.; Yu, S. Discontinuous fibrous Bouligand architecture enabling formidable fracture resistance with crack orientation insensitivity. Proc. Natl. Acad. Sci. USA 2020, 117, 15465–15472.
- Natarajan, B.; Gilman, J.W. Bioinspired Bouligand cellulose nanocrystal composites: A review of mechanical properties. Phil. Trans. R. Soc. A 2017, 376, 20170050.
- Demoly, F.; Dunn, M.L.; Wood, K.L.; Qi, H.J.; Andre, J. The status, barriers, challenges, and future in design for 4D printing. Mater. Des. 2021, 212, 110193.
- Duigou, A.L.; Correa, D.; Ueda, M.; Matsuzaki, R.; Castro, M. A review of 3D and 4D printing of natural fibre biocomposites. Mater. Des. 2020, 194, 108911.
- Zaklan, S.D. Review of the family Lithodidae (Crustacea: Anomura: Paguroidea): Distribution, biology, and fisheries. In Crabs in Cold Water Regions: Biology, Management, and Economics; Report No., AK-SG-02-01; Paul, A.J., Dawe, E.G., Elner, R., Jamieson, G.S., Kruse, G.H., Otto, R.S., Sainte-Marie, B., Shirley, T.C., Woodby, D., Eds.; University of Alaska Sea Grant: Anchorage, AK, USA, 2002; pp. 751–845.
- Lovrich, G.A.; Vinuesa, J.H.; Smith, B.D. Growth, maturity, and mating of male southern king crab (Lithodes santolla) in the Beagle Channel, Argentina. In Crabs in Cold Water Regions: Biology, Management, and Economics; Report No., AK-SG-02-01; Paul, A.J., Dawe, E.G., Elner, R., Jamieson, G.S., Kruse, G.H., Otto, R.S., Sainte-Marie, B., et al., Eds.; University of Alaska Sea Grant: Anchorage, AK, USA, 2002; pp. 147–187.
- Fabritius, H.O.; Karsten, E.S.; Balasundaram, K.; Hild, S.; Huemer, K.; Raabe, D. Correlation of structure, composition and local mechanical properties in the dorsal carapace of the edible crab Cancer pagurus. Z. Krist. Cryst. Mater. 2012, 227, 766–776.
- Chen, P.-Y.; Lin, A.Y.; McKittrick, J.; Meyers, M.A. Structure and mechanical properties of crab exoskeletons. Acta Biomater. 2008, 4, 587–596.
- Coffey, W.D.; Nardone, J.A.; Yarram, A.; Long, W.C.; Swiney, K.M.; Foy, R.J.; Dickinson, G.H. Ocean acidification leads to altered micromechanical properties of the mineralized cuticle in juvenile red and blue king crabs. J. Exp. Mar. Biol. Ecol. 2017, 495, 1–12.
- Drew, M.M.; Harzsch, S.; Stensmyr, M.; Erland, S.; Hansson, B.S. A review of the biology and ecology of the Robber Crab, Birgus latro (Linnaeus, 1767) (Anomura: Coenobitidae). Zool. Anz. 2010, 249, 45–67.
- Oka, S.; Tomita, T.; Miyamoto, K. A mighty claw: Pinching force of the coconut crab, the largest terrestrial crustacean. PLoS ONE 2016, 11, 0166108.
- Rosen, M.N.; Baran, K.A.; Sison, J.N.; Steffel, B.V.; Long, W.C.; Foy, R.J.; Smith, K.E.; Aronson, R.B.; Dickinson, G.H. Mechanical resistance in decapod claw denticles: Contribution of structure and composition. Acta Biomater. 2020, 110, 196–207.
- Waugh, D.A.; Feldmann, R.M.; Schroeder, A.M.; Mutel, M.H. Differential cuticle architecture and its preservation in fossil and extant Callinectes and Scylla claws. J. Crustacean Biol. 2006, 26, 271–282.
- Achrai, B.; Wagner, H.D. Micro-structure and mechanical properties of the turtle carapace as a biological composite shield. Acta Biomater. 2013, 9, 5890–5902.
More
