Electrophysiological procedures are mainly performed using fluoroscopy, exposing both healthcare staff and patients to a non-negligible dose of radiation. To date, simple ablation procedures have often been approached with zero fluoroscopy. In complex ablation procedures, such as atrial fibrillation (AF) ablation, zero fluoroscopy is still challenging mainly because of transseptal puncture. We report a workflow to perform a complete zero-fluoroscopy AF ablation using a 3D electro-anatomical mapping system, intracardiac echocardiography and a novel steerable guiding sheath visible on the mapping system. We describe two cases, one with paroxysmal AF and the other with persistent AF during which this novel workflow was successfully applied with complete zero-fluoroscopy exposure and achieving pulmonary vein isolation.
- atrial fibrillation ablation
- zero fluoroscopy
- steerable sheath
- intracardiac echo
1. State of the Art
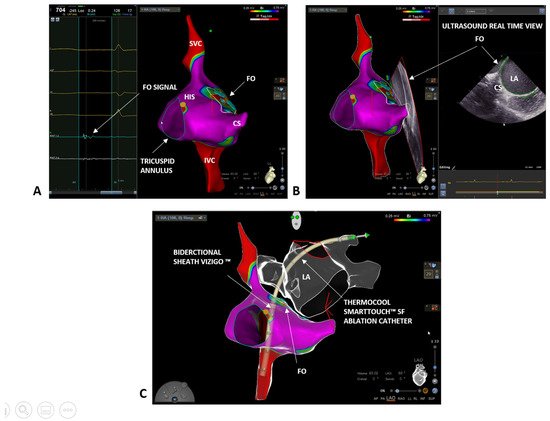
2. Case 1: Paroxysmal Atrial Fibrillation
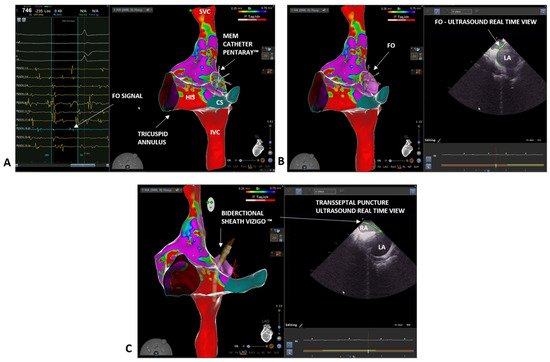
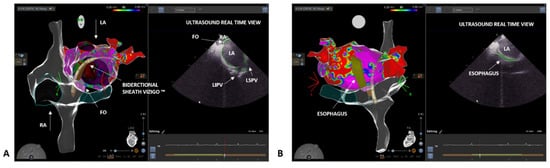
3. Case 2: Persistent Atrial Fibrillation
References
- Heidbuchel, H.; Wittkampf, F.H.M.; Vano, E.; Ernst, S.; Schilling, R.; Picano, E.; Mont, L.; Jais, P.; de Bono, J.; Piorkowski, C.; et al. Practical Ways to Reduce Radiation Dose for Patients and Staff during Device Implantations and Electrophysiological Procedures. Europace 2014, 16, 946–964.
- Gaita, F.; Guerra, P.G.; Battaglia, A.; Anselmino, M. The Dream of Near-Zero X-Rays Ablation Comes True. Eur. Heart J. 2016, 37, 2749–2755.
- Limacher, M.C.; Douglas, P.S.; Germano, G.; Laskey, W.K.; Lindsay, B.D.; McKetty, M.H.; Moore, M.E.; Park, J.K.; Prigent, F.M.; Walsh, M.N. ACC Expert Consensus Document. Radiation Safety in the Practice of Cardiology. American College of Cardiology. J. Am. Coll. Cardiol. 1998, 31, 892–913.
- Pani, A.; Giuseppina, B.; Bonanno, C.; Bongiorni, M.G.; Bottoni, N.; Brambilla, R.; de Ceglia, S.; Bella, P.D.; de Vito, G.; Malaspina, D.; et al. Predictors of Zero X-Ray Ablation for Supraventricular Tachycardias in a Nationwide Multicenter Experience. Circ. Arrhythmia Electrophysiol. 2018, 11, e005592.
- Jan, M.; Žižek, D.; Kalinšek, T.P.; Kuhelj, D.; Trunk, P.; Kolar, T.; Kšela, J.; Rauber, M.; Yazici, M. Minimising Radiation Exposure in Catheter Ablation of Ventricular Arrhythmias. BMC Cardiovasc. Disord. 2021, 21, 306.
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498.
- Romero, J.; Patel, K.; Briceno, D.; Alviz, I.; Tarantino, N.; Rocca, D.G.D.; Natale, V.; Zhang, X.-D.; di Biase, L. Fluoroless Atrial Fibrillation Catheter Ablation: Technique and Clinical Outcomes. Card. Electrophysiol. Clin. 2020, 12, 233–245.
- Malagù, M.; Vitali, F.; Marchini, F.; Fiorio, A.; Sirugo, P.; Mele, D.; Brieda, A.; Balla, C.; Bertini, M. Ablation of Atrioventricular Nodal Re-Entrant Tachycardia Combining Irrigated Flexible-Tip Catheters and Three-Dimensional Electroanatomic Mapping: Long-Term Outcomes. J. Cardiovasc. Dev. Dis. 2021, 8, 61.
- Scaglione, M.; Ebrille, E.; di Clemente, F.; Gaita, F.; Bradfield, J.S. Catheter Ablation of Atrial Fibrillation without Radiation Exposure Using A 3D Mapping System. J. Atr. Fibrillation 2015, 7, 56–62.
- Falasconi, G.; Penela, D.; Soto-Iglesias, D.; Jáuregui, B.; Chauca, A.; Antonio, R.S.; Ordoñez, A.; Teres, C.; Carreño, J.M.; Scherer, C.; et al. A standardized stepwise zero-fluoroscopy approach with transesophageal echocardiography guidance for atrial fibrillation ablation. J. Interv. Card. Electrophysiol. 2021. epub ahead of print.
- Tahin, T.; Riba, A.; Nemeth, B.; Arvai, F.; Lupkovics, G.; Szeplaki, G.; Geller, L. Implementation of a zero fluoroscopic workflow using a simplified intracardiac echocardiography guided method for catheter ablation of atrial fibrillation, including repeat procedures. BMC Cardiovasc. Disord. 2021, 21, 407.
- Sommer, P.; Bertagnolli, L.; Kircher, S.; Arya, A.; Bollmann, A.; Richter, S.; Rolf, S.; Hindricks, G. Safety profile of near-zero fluoroscopy atrial fibrillation ablation with non-fluoroscopic catheter visualization: Experience from 1000 consecutive procedures. EP Eur. 2018, 20, 1952–1958.
- Troisi, F.; Quadrini, F.; di Monaco, A.; Vitulano, N.; Caruso, R.; Guida, P.; Langialonga, T.; Grimaldi, M. Electroanatomic Guidance versus Conventional Fluoroscopy during Transseptal Puncture for Atrial Fibrillation Ablation. J. Cardiovasc. Electrophysiol. 2020, 31, 2607–2613.
- Phlips, T.; Taghji, P.; Haddad, M.E.; Wolf, M.; Knecht, S.; Vandekerckhove, Y.; Tavernier, R.; Duytschaever, M. Improving Procedural and One-Year Outcome after Contact Force-Guided Pulmonary Vein Isolation: The Role of Interlesion Distance, Ablation Index, and Contact Force Variability in the ‘CLOSE’-Protocol. EP Eur. 2018, 20, f419–f427.
