Li/S batteries possess exceptional specific energy and a standard open-circuit potential of 2.15 V [1][14]. The theoretical maximum specific energy of a Li/S battery is 2600 W h kg S−1 [2][15], assuming the sulfur is fully utilized. However, complete utilization is not technically feasible due to the sluggish reaction kinetics and the insulating properties of elemental sulfur [3][4][16,17]. In addition, the weight of other cell components such as current collectors, separator, electrolyte, and cell housing materials should be considered when estimating the actual specific energy of Li/S batteries. Considering practical cell design parameters, a more realistic projection estimates the specific energy of Li/S batteries at between 400 and 500 W h kg−1 [5][6][12,18]. Though still early in the research phase of development, this technology currently exceeds the peak specific energy of Li-ion cells by about 100 W h kg−1 [5][7][12,19]. Li/S batteries also show improved safety during operation under abuse conditions compared to LiBs. Nail penetration tests demonstrated no explosive energy release when punctured due to a solid insulating coating that forms from lithium polysulfide (Li-PS), thereby preventing an internal short circuit via the penetrator [8][20]. Furthermore, because of the chemical stability of the active materials in the completely discharged state, the cells can be stored indefinitely without experiencing damage and without hazard risk [9][21].
2.1. Sulfur Electrode Design Challenges
The major technical challenges of Li/S cells discussed in the introduction section are mainly associated with the sulfur electrode. Although the sulfur electrode can theoretically deliver a very high specific capacity of 1675 mA h gS
−1, the practical utilization of sulfur is still not satisfactory (generally <60%) and the long-term cyclability and power density are not yet compatible with those of Li-ion cells. The problematic Li-PS shuttle causes the loss of active sulfur and the deposition of Li
2S onto the lithium electrode, resulting in cell capacity degradation. Inhomogeneous reconfiguration of the sulfur electrode microstructure due to the uncontrolled Li-PS formation and the volume changes of sulfur particles is another concern for the design of sulfur electrodes. On the other hand, Li-PS is also considered essential for Li/S-cell operation because it provides the sulfur electrodes with a kinetically fast route for reaction with lithium ions
[10][141]. The solution-based pathway involving Li-PS can overcome the large energy barrier for the charge transfer reaction at the sulfur/electrolyte interface and Li-ion diffusion through solid sulfur particles. Ideally, sulfur electrodes should prohibit the Li-PS shuttle while allowing homogeneous Li-PS formation and deposition back to the sulfur electrode as sulfur or Li
2S.
The sulfur–carbon composite is the most popular active material design for improving the sulfur electrode’s capacity and cyclability by mitigating the Li-PS shuttle effect. Various carbonaceous materials such as porous carbons, 1D carbons (carbon nanotubes, carbon nanofibers), and 2D carbons (graphene and graphene oxide) have been investigated as sulfur host materials or functional additives for sulfur electrodes to utilize their excellent electronic conductivity and good mechanical strength. Some carbonaceous materials have high sulfur-philic properties, which can mitigate the Li-PS shuttle phenomenon, resulting in improved cyclability
[5][1][11][12][13][14][12,14,53,142,143,144]. Sulfur electrodes are manufactured by the slurry-based tape casting process like Li-ion cell electrodes, but the sulfur electrodes reported in research papers generally contain a large amount of carbon, at least 10–20 wt.% or sometimes even more than 50 wt.% (including the carbon in sulfur–carbon composite active material), to sufficiently mitigate the problems of the sulfur electrodes
[1][14].
The large quantity of porous carbons often severely affects the slurry quality because carbons tend to aggregate with each other or absorb too much solvent in a slurry, making the slurry less flowable or inhomogeneous. In addition, the extremely low powder density of these carbons (e.g., Super P: 0.16 g cm−3, Ketjen black: 0.1 g cm−3) tends to increase the sulfur electrode’s thickness compared to that of the Li-ion-cell positive electrode. Casting a poorly prepared slurry often results in inhomogeneous thickness distribution, pinholes, or even delamination during the drying process. Increasing the binder content may help mitigate the mechanical damage, but it increases the ‘dead weight’ of the sulfur electrode. These issues will become more formidable as higher sulfur mass loading is required to achieve high specific energy. Therefore, more systematic design strategies are needed to design high-mass-loading sulfur electrodes. To develop thick and large-area sulfur electrodes for high energy Li/S pouch cells, functional materials or electrode fabrication parameters previously evaluated in small laboratory-scale research will need to be revisited and reoptimized.
For Li/S pouch cells to succeed in the electric transportation battery market, we anticipate that a high specific energy of >300 W h kg
−1 is required. Design calculation of the obtainable specific energy (W h kg
−1) for a Li/S pouch cell is helpful to predict the essential design parameters of Li/S pouch cells. In particular, we evaluated the effect of sulfur mass loading, sulfur content, E/S ratio, and sulfur utilization (equivalent to the specific capacity of the sulfur electrode) on the obtainable specific energy of Li/S pouch cells. The passive weight values of pouch cell components listed in
Table 1 were used for the design calculations.
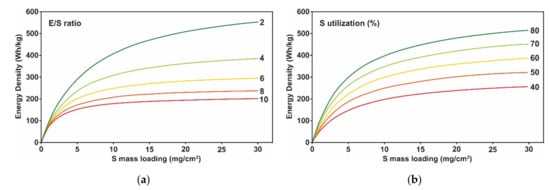
Figure 4a shows the specific energy of the Li/S pouch cell for various sulfur mass loadings and E/S ratios. The results indicate that a specific energy of >300 W h kg
−1 requires a high sulfur mass loading of >~6 mg cm
−2 and a low E/S ratio of ≤4 while achieving >70% sulfur utilization (1172.5 mA h gS
−1). A Li/S pouch cell with an E/S ratio of higher than six is unlikely to show the desired specific energy, no matter what the sulfur mass loading, unless significantly higher sulfur utilization (80–90%) is achieved.
Figure 4b shows the variation of the obtainable specific energy for various sulfur utilization and sulfur mass loading values at a fixed E/S ratio of 3. The results (
Figure 4b) indicate that sulfur utilization plays a critical role in achieving a high specific energy of >300 W h kg
−1. The 50% utilization is still mandated even with a very high sulfur loading of 20 mg cm
−2 and a low E/S ratio of 3. According to the design calculations shown in
Figure 4a,b, targeting the following electrode fabrication parameters is reasonable for delivering a specific energy of 300 W h kg
−1: a sulfur loading of ≥6 mg cm
−2, a sulfur utilization of ≥70%, and an E/S ratio of ≤4. The design parameters become more challenging for a higher specific energy of 400 W h kg
−1: a sulfur loading of ≥10 mg cm
−2, a sulfur utilization of ≥80%, and an E/S ratio of ≤3. Unfortunately, a low E/S ratio of ≤4 often results in low sulfur utilization
[15][145] or poor cyclability
[16][146] as it results in a slow electrochemical process at the sulfur electrode (e.g., low Li-PS solubility, electrolyte decomposition)
[17][18][147,148].
Figure 4. Design calculation of Lithium-Sulfur (Li/S) pouch cell specific energy (a) Obtainable specific energy for various sulfur mass loading and electrolyte/sulfur (E/S) ratios. Sulfur content and sulfur utilization were fixed to 80% and 70%, respectively. (b) Obtainable specific energy for various sulfur loading and sulfur utilization. E/S ratio and sulfur content were fixed to 3 and 80%, respectively.
A low E/S ratio of 3–4 with reasonable specific capacity (1000–1200 mA h gS
−1) has recently been reported via chemical or morphological modification of sulfur electrodes
[19][20][21][22][149,150,151,152]. Although the capacity retentions were not sufficient for commercialization, the authors’ accomplishments in successfully reducing the E/S ratio emphasize the importance of the sulfur electrode design. However, in most research studies, the electrolyte amount was adjusted to achieve the target E/S ratio, regardless of the electrode’s design parameters such as porosity and sulfur content. In principle, however, the pore volume and the electrode surface area generally determine the required amount of the liquid electrolyte, since the pores of the sulfur electrode are supposed to be filled by the liquid electrolyte to form a continuous ionic percolation network and an adequate electrochemical interface. Li/S cells suffer from either electrolyte shortage or excess if the electrolyte amount is determined without considering these parameters.
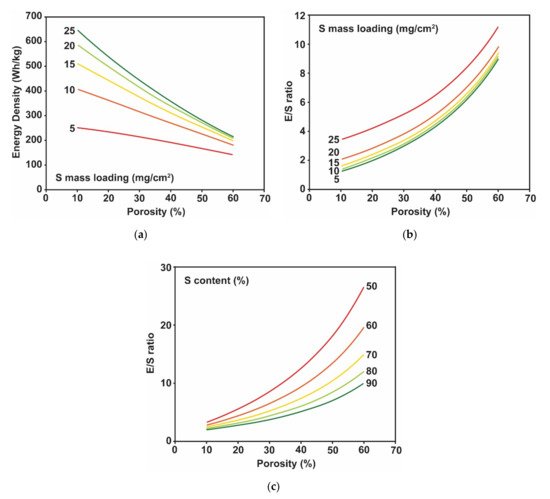
For this reason, we performed additional design calculations for the Li/S pouch cell to reflect the correlation between porosity, sulfur content, and the E/S ratio. We assumed that the required electrolyte volume was the same as the total pore volume of the sulfur electrode and a separator, with no excess added. The design calculation results shown in Figure 5a,b indicate that the porosity of the sulfur electrode plays a critical role in determining the obtainable specific energy because the E/S ratio increases as the porosity of the electrode increases. We previously stated that a sulfur mass loading of >6 mg cm−2 would provide an opportunity to achieve 300 W h kg−1 when the E/S ratio is ≤4 and sulfur utilization is <70% (Figure 4a). However, according to the design calculation, the low E/S ratio of ≤4 is unlikely to be achievable with a sulfur mass loading of 6 mg/cm2 and sulfur content of 90%, unless the sulfur electrode has an extremely low electrode porosity of 10%. Based on the calculation results, we anticipate that porosity of about 3–40% with a sulfur mass loading of >10 mg cm−2 would offer an excellent opportunity to achieve a high specific energy of >300 W h/kg.
Figure 5. Design calculation results (a) Obtainable specific energy of a Li/S pouch cell for various porosity of sulfur electrode and sulfur mass loading. Sulfur content and sulfur utilization were assumed as 90% and 70%, respectively. (b) Variation in the E/S ratio depending on the sulfur electrode porosity and sulfur mass loading. The required electrolyte amount corresponds to the pore volume of the sulfur electrode and separator. The sulfur content was assumed as 90%. (c) Variation in the E/S ratio depending on the sulfur electrode porosity and sulfur content. Sulfur mass loading used for the calculation was 10 mg cm−2.
The effect of electrode porosity raises another critical question about the influence of the sulfur content on the E/S ratio. The design calculations for the Li/S pouch cells shown in
Figure 4 result in similar calculated specific energies of 311 W h kg
−1 at a sulfur content of 50% and 349 W h kg
−1 at a sulfur content of 90%, respectively (sulfur mass loading of 10 mg cm
−2, sulfur utilization of 70%, and an E/S ratio of 3). However, lowering the sulfur content of the sulfur electrode to add more carbon additives while maintaining sulfur mass loading significantly increases the thickness of the sulfur electrode. In other words, the total pore volume of the sulfur electrode is larger when the sulfur content is lowered, implying a higher E/S ratio for the Li/S cells. The design calculation results (
Figure 5c) clearly show that the sulfur content affects the E/S ratio dramatically. According to the design calculation results, an E/S ratio of <~4 for a sulfur mass loading of 10 mg cm
−2 is only achievable when the sulfur content is higher than 80% and the porosity is lower than 30% for the sulfur electrode. The calculated specific energies of the Li/S pouch cells (sulfur mass loading of 10 mg cm
−2 and sulfur utilization of 70%) for sulfur content values of 50% and 90% are 184.6 and 314.2 W h kg
−1, respectively, which is obviously different from the calculated specific energy without consideration of porosity. The design calculation results emphasize that a high-sulfur-mass-loading electrode with a large quantity of carbon additive will not offer high specific energy even if high sulfur utilization is achieved. However, increasing the sulfur content can lower the sulfur utilization due to poor electronic conductivity
[17][147]. Thus, adjusting the sulfur content must be considered thoroughly. Of course, the effect of electrode porosity can be mitigated if a carbon additive with a higher powder density is used or the powder density of carbon is increased by incorporating sulfur into the pores of the carbon.
2.3. Electrolyte Design Challenges
Li/S cells generally employ liquid organic electrolytes consisting of ether-based solvents, LiTFSI as a lithium salt, and LiNO
3 as a functional additive. Tetraethylene glycol dimethyl ether (TEGDME) and a mixture of DOL and DME are the most widely used solvents among ether-based solvents. Ether-based solvents promote the Li-PS-based reaction route of sulfur electrodes, enabling a high sulfur utilization. Some other salts and solvents such as lithium perchlorate (LiClO
4), lithium trifluoromethanesulfonate (LiCF
3SO
3), LiPF
6, and carbonates-based solvents have also been investigated, but they are not as effective as the conventional electrolyte systems. As discussed above, the Li-PS shuttle effect easily occurs in the absence of an effective barrier. LiNO
3 is an effective functional additive that mitigates the shuttle effect by forming a passivation layer on the lithium metal electrode, but recent studies indicate that LiNO
3 in the liquid electrolyte can increase the consumption of the active sulfur as a result of SEI formation on the sulfur electrode
[28][158], or serve as a source of gas formation at approximately 40 °C
[29][159].
Organic liquid electrolytes with high salt concentrations have been investigated as an alternative solution to mitigate the Li-PS shuttle effect. Several reports suggested that the high Li-ion concentration in electrolytes affects PS solubility due to the dissolution equilibrium principle. The results showed that the higher the lithium salt concentration, the lower the Li-PS solubility; hence, the Li-PS shuttle can be prevented, improving the cyclability of Li/S cells
[30][160]. Similarly, adding ionic liquids to the ether-based electrolyte also improves the cyclability of sulfur electrodes by mitigating the Li-PS shuttle
[31][127] or forming a passivation surface layer on a lithium metal electrode
[32][161]. However, neither high-salt-concentration electrolytes nor ionic-liquid-containing electrolytes are feasible for commercialization because they often exhibit very high viscosity that causes a high voltage polarization or low power capability. In addition, LiTFSI and ionic liquids are still relatively expensive, so they are not economical options for Li/S cell manufacturing.
Solid electrolytes for solid-state Li/S cells have recently attracted attention because they can inhibit Li-PS formation and the shuttle effect due to the lack of liquid medium. In addition, the high mechanical strength of the solid electrolytes offers a better opportunity to suppress the internal cell shorting caused by lithium dendrite growth. Moreover, the wide thermal and electrochemical windows and non-flammable natures of the solid electrolytes can dramatically reduce the safety concerns regarding the Li/S cells. However, none of the research has demonstrated high-performance solid-state Li/S cells comparable to conventional Li/S cells. Unfortunately, solid-state Li/S cells have several technical problems at the lithium–solid electrolyte and the sulfur–solid electrolyte interfaces
[33][34][35][162,163,164]. Furthermore, the solid-state interfacial electrochemistry of the Li/S solid-state cells is not fully understood; thus, intensive fundamental research is certainly required. Some studies showed improved sulfur utilization and cyclability, but their testing conditions are far from those of practical operation conditions (e.g., too low discharge cut-off voltage, high operation temperature, or low current for testing).