Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sulaiman Abdulsalam and Version 2 by Vivi Li.
Plant nematodes (PPNs) have been documented as economically important pests of yam in different parts of the world with Pratylenchus spp. and Meloidogyne spp. being the most widespread and destructive pests in Asia, causing significant yield losses.
- yam
- Pratylenchus coffeae
- Meloidogyne spp.
- Merlinius brevidens
- morphology
- molecular
1. Introduction
Yam belongs to the genus Dioscorea (family; Dioscoreaceae) consisting of about 644 species with underground tubers [1]. The three most common cultivated species include D. rotundata, D. alata, and D. cayenensis [2], while D. esculenta, D. nummularia, D. bulbifera, D. pentaphylla, D. transversa, D. japonica, D. trifida, D. dumetorum, and D. opposita are also of economic importance. Dioscorea opposita is the only species cultivated in temperate areas and is commonly grown in China and Japan [3]. Yam is an important staple food crop in tropical and subtropical areas of Africa, Asia, South America, the Pacific, and the Caribbean region [4][5][4,5]. It is the fourth most important tuber crop after potato, cassava, and sweet potato globally, with West Africa accounting for about 92% (67 MT) of the total output annually [6]. Though China’s yam output was not included in the FAO statistics, it is currently a significant and isolated center of yam domestication [7]. In China, approximately 5 to 6 MT of yam are produced every year through the planting of diverse species in most yam-producing areas such as Jiangsu and Shandong provinces [7][8][7,8].
In China, consumption of yam is on the increase due to the recent belief that, nutritionally, its reserves of micronutrients, minerals, and proteins can be used to address potential deficiencies [7][9][7,9]. Additionally, its production brings in income to the country [5][7][5,7]. However, yam is susceptible to numerous diseases, particularly bacterial dry rot (Corynebacterium spp.), anthracnose (Colletotrichum spp.), viruses, and nematodes [4][10][4,10]. It is highly susceptible to plant-parasitic nematodes (PPN) especially the yam nematode (Scutellonema bradys), root-lesion nematodes (RLN; Pratylenchus spp.), and root-knot nematodes (RKN; Meloidogyne spp.). Plant-parasitic nematodes interacts with other plant pathogens, resulting in increased damage caused by other diseases and affecting global food supplies [4][5][4,5]. Yam infected with RKN has been reported to show galls and “crazy root” syndrome on tubers, altering tuber appearance and reducing tuber quality. Pratylenchus spp. and S. bradys also contribute to dry rot disease and tuber surface cracking, which in turn influence the production and quality of tuber [4][11][12][4,11,12]. Additionally, tubers with nematode symptoms are less appealing and so have poor market value [4][11][12][4,11,12]. The association of yam to PPN has been reported in China with RKN responsible for the loss of several cultivars [4][5][4,5]. About 24–80% yield reduction in yam in China is associated with RKN (M. arenaria), while about 30–100% is attributed to RLN (P. coffeae) [4].
Stunt nematodes of the genus Merlinius Siddiqi, 1970 include 34 nominal species [13]. These nematodes are migratory ectoparasites of a wide range of plants globally and are ubiquitous in agricultural settings. The accurate identification of these stunt nematodes from both rhizosphere soil and root samples is critical for successful disease management [13][14][15][13,14,15]. The majority of species attack and feed on plant roots and underground plant parts, resulting in low to moderate yield losses in several crops, including rice (Oryza sativa L.), wheat (Triticum spp.), maize (Zea mays), and potato (Solanum tuberosum L.) [13][15][13,15]. Allen [14] first described a species of this genus, Merlinius brevidens, as a major pathogen of grasses in the USA and several other countries [13][15][13,15]. Although this species is not among the species considered most important to yam (Dioscorea spp.), some of them can become destruction agents when populations exceed economic threshold levels. [13][16][13,16]. They are mostly recognized for the damage they caused to grasses and cereals through feeding on epidermal cells, resulting in root and foliage stunting, leaf yellowing, defoliation, and wilting [16]. Morphological identification of Merlinius brevidens is characterized by the absence of areolated lateral fields; six longitudinal incisures; female tail sub-cylindrical (c’ = 2.4–4.6) with rounded terminus; and robust stylet [17][18][17,18]. Males are rarely seen and unlikely to be required for reproduction. However, the substantial intraspecific diversity of key diagnostic features makes morphological identification of this genus challenging, and its evolutionary relationships with other genera in the same family are complex, making it problematic and contentious [16][17][18][16,17,18]. To elucidate the identity and evolutionary relationships of the species in this genus and related genera, morphological and molecular analyses are required [13][17][18][13,17,18].
Plant-parasitic nematodes infecting yam must be identified accurately and promptly to develop appropriate management methods that will reduce losses. This is particularly important for yam due to the diversity of nematode species found in different yam-growing regions across the world [4][11][19][4,11,19]. Accurate nematode identification enables the differentiation of regulated and non-controlled nematode pests, as well as the exclusion of species under quarantine or regulatory measures. The prevalence and geographical distribution of PPNs in Asia were reviewed by Bridge et al. [11] who reported that eight species (P. coffeae, M. incognita, M. arenaria, M. hapla, Radopholus similis, Paratrichodorus porosus, Rotylenchulus reniformis, and Helicotylenchus dihystera) were found damaging yam. In China, only three species of PPNs (P. coffeae, M. incognita, and M. arenaria) have been reported in association with yam [4]. However, many other species were not reported and verified with appropriate molecular analysis. The current availability of molecular methods may aid in the identification of nematodes by providing tools for species differentiation. Therefore, ribosomal DNA sequences from partial 18S, ITS regions, and the D2-D3 expansion segments of the 28S, as well as mitochondrial DNA (mtDNA) sequences, have proven to be useful diagnostic tools for the characterization and establishment of phylogenetic relationships within PPNs, particularly when morphological features may cause doubt in interpretation [14][15][14,15]. Integrative taxonomy, which combines molecular methods with morphology and morphometry methods to diagnose the identified species, is critical for accurate nematode identification.
2. Survey
The 110 bulk soil samples and 48 tubers obtained from nine locations in Jiangxi and Shandong provinces of China yielded 16 species (four species were found in tubers) of PPNs (Table 12 and Table 23). Data pooled for all farming communities showed that Pratylenchus (depicted by the species P. coffeae) had the highest MPD, in both tuber (MPDs of 415 individuals/5 g) and soil (MPD = 291 indiv./100 mL) samples, followed by Meloidogyne (M. incognita, M. hispanica, and M. ethiopica), and Rotylenchulus (depicted by the species R. reniformis) (Figure 1, Figure 2 and Figure 3). Merlinius was the fourth in soil samples, according to MPDs (Figure 1), and the most commonly recorded genus with ectoparasitic feeding behavior. The least prominent genus in terms of MPDs in tubers was Ditylenchus and in soil Hirschmanniella mucronata (Figure 1 and Figure 2).
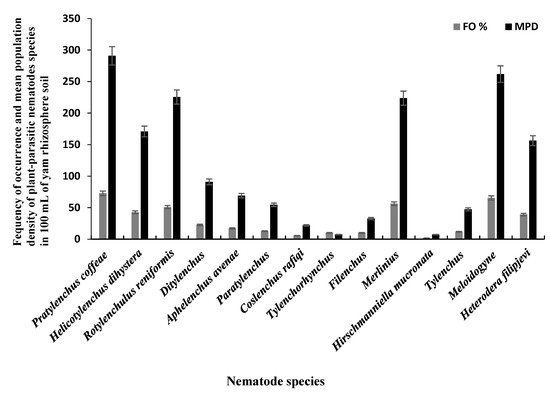

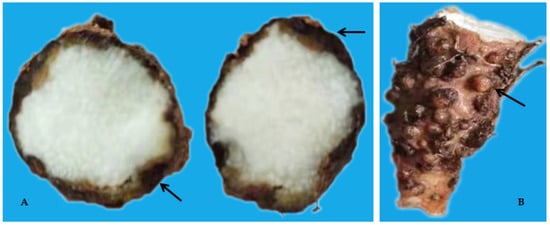

Figure 1. Mean population densities (MPD) and frequencies of occurrence (FO) of plant-parasitic nematodes species recovered from yam rhizosphere in Jiangxi and Shandong provinces of south-east China.

Figure 2. Mean population densities (MPD), and frequencies of occurrence (FO) of plant-parasitic nematodes species recovered from roots/tubers of yam in nine localities of Jiangxi and Shandong provinces across south-east China during 2020/2021.

Figure 3. Dry rot (A) and Galls (B) on yam tubers caused by Pratylenchus coffeae and Meloidogyne spp.
Table 12. Mean population densities (MPD), and frequencies of occurrence (FO) of plant-parasitic nematodes species isolated from 100 mL of rhizosphere soil sampled from yam plants in nine locations of Jiangxi and Shandong provinces across south-east China during 2020/2021.
| Province | |||||
|---|---|---|---|---|---|
| Jiangxi (n = 65) | Shandong (n = 45) | ||||
| MPD | FO (%) | MPD | FO (%) | ||
| - | |||||
| - | |||||
n = number of samples, - = no nematodes present.
Table 23. Mean population densities (MPD) and frequencies of occurrence (FO %) of plant-parasitic nematodes species isolated from 5 g of yam roots/tubers from nine locations of Jiangxi and Shandong provinces of south-east China during 2020/2021.
| Province | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jiangxi (n = 28) | Shandong (n = 20) | ||||||||||||||
| MPD | FO (%) | MPD | FO (%) | ||||||||||||
| Nematode species | |||||||||||||||
| Nematode species | |||||||||||||||
| Pratylenchus coffeae | 302 | 75 | 276 | 69 | |||||||||||
| Pratylenchus coffeae | 607 | 93 | 145 | 35 | Meloidogyne species (M. incognita, M. ethiopica, M. hispanica) | 166 | 42 | 400 | 100 | ||||||
| Meloidogyne species (M. incognita, M. ethiopica, M. hispanica) | 264 | 54 | 325 | 80 | Rotylenchulus reniformis | 218 | 55 | 382 | 82 | ||||||
| Rotylenchulus reniformis | 115 | 21 | 110 | 25 | Helicotylenchus dihystera | 191 | 48 | 142 | 36 | ||||||
| Ditylenchus spp. | 96 | 18 | 35 | 10 | (4.8–5.9) | (4.5–5.3) | Merlinius brevidens | 117 | 29 | 231 | 58 | ||||
| b’ | 4.9 ± 0.3 (4.5–5.8) |
- | (4.7–5.8) | - | - | - | - | - | - | Aphelenchus avenae | 74 | 18 | 62 | 16 | |
| c | 13.2 ± 1.9 (10.1–16.3) |
(11.7–13.6) | (11.3–15.0) | (12.9–17.0) | (11.3–15.7) | (12–15) | (11–13) | 11.0–13.0 | (10–12) | Ditylenchus spp. | 92 | 23 | 89 | 22 | |
| c’ | 3.6 ± 0.7 (2.7–5.4) |
(3.8–4.9) | (2.8–4.9) | (2.9–3.2) | (2.4–3.2) | (2.4) | (2.4) | (2.9) | - | Tylenchorhyncus (T. annulatus, T. zeae) | 55 | 14 | 18 | 4 | |
| MB | 53.1 ± 4.6 (45.2–61.1) |
(46.2–54.3) | (46–57) | - | (41.1–46.5) | - | - | (41–51) | Filenchus vulgaris | 55 | 14 | 18 | 4 | ||
| Tylenchus spp. | 68 | 17 | 18 | 4 | |||||||||||
| Coslenchus spp. | 37 | 9 | |||||||||||||
| Paratylenchus spp. | 68 | 15 | 36 | 9 | |||||||||||
| Heterodera filipjevi | - | - | 382 | 96 | |||||||||||
| Hirschmanniella mucronata | 12 | 3 | |||||||||||||
n = number of samples.
Pooled soil data for all the fields sampled in each province show that P. coffeae and Meloidogyne spp. were the most prevalent PPNs in terms of mean population density (MPD) and frequency of occurrence (FO) (Table 12). The MPDs for P. coffeae ranged from 276 (Shandong) to 302 (Jiangxi), and this species was present in 69–75% of the fields sampled. Meloidogyne was present in 42–100% of the fields with MPDs ranging between 166 (Jiangxi) and 400 (Shandong). R. reniformis was usually third in dominance with MPDs ranging between 218 (Jiangxi) and 382 (Shandong) and occurred in 55–82% of the fields (Table 12). According to MPDs, the species with the least prevalence was H. mucronata with MD of 12 in Jiangxi and being present in 3% of the fields while in Shandong, Tylenchus, Filenchus, and Tylenchorhynchus were the least with MPDs of 18 and occurred in 4% of the farms (Table 12). In tubers, the MPDs for P. coffeae ranged between 145 (Shandong) and 607 (Jiangxi) with individuals identified in fields of each province in up to 35–93% of the samples (Table 23). For Meloidogyne, MPDs ranged between 264 (Jiangxi) and 325 (Shandong) individuals in up to 54–80% of the samples. Ditylenchus, had low MPDs (ranging between 35 for Shandong and 96 for Jiangxi), with individuals occurring in up to 10–18% of the samples (Table 23).
In this study, we also provide a morphological and molecular characterization of Merlinius species while the other four prominent species (P. coffeae, Meloidogyne, R. reniformis, and H. dihystera), infesting yam (Dioscorea spp.) in Jiangxi and Shandong provinces of China were also verified with appropriate molecular analysis.
3. Morphological and Molecular Characterizations of Merlinius brevidens
3.1. Morphology of Merlinius brevidens (Allen, 1955) Siddiqi, 1970 = Geocenamus brevidens (Allen, 1955)
Female: Body shape slightly straight or open C-shaped when heat relaxed with the tapering tail region. The cuticle annulated, lateral field with six incisures. Cephalic region continuous, broadly rounded, 4.6–6.8 μm long and 10.7–12.9 μm wide; with 3–4 indistinct annuli. Stylet robust, with rounded basal sloping knobs, about 2.1–3.8 μm high and 4.4–6.5 μm wide (Figure 4). Median pharyngeal bulb rounded, 4.6–6.0 μm long and 9.0–11.0 μm wide, bearing refractive valve plates, well-developed. Isthmus slender, surrounded by a nerve ring; basal bulb is oval with a small, rounded cardia. Hemizonid prominent, located just anterior to the excretory pore. Excretory pore is anterior to the basal bulb 106.6–114.8 μm long from the end. Vulva transverse, with well-formed epiptygma, protecting the opening of the vulva. Vagina is straight, occupying the ca one-third (20–30%) of the corresponding body width. Didelphic reproductive system; ovaries with oocytes grouped in a single row; distally separated oviducts with rounded spermatheca, filled with sperm; uteri with thin walls as long as the width of the body. Tail subcylindrical with 18–22 annuli, and somewhat set off with bluntly rounded, almost smooth terminus; often acute to bulb-like. The tail hyaline region is conspicuous. Phasmids are distinct, near or slightly posterior to the middle of the tail. No males were found.
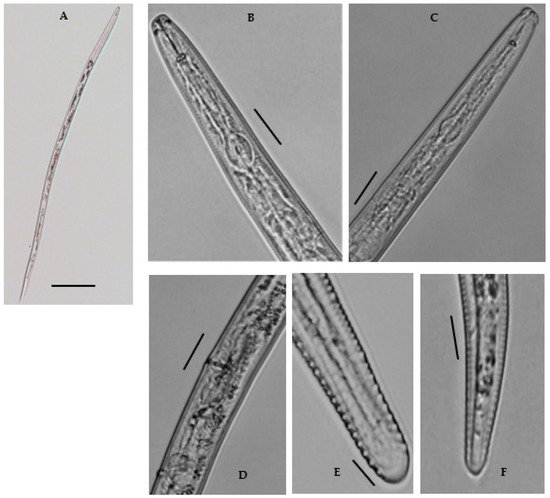

Figure 4. A Photomicrographs of female Merlinius brevidens: (A) entire female; (B,C) variation in head region; (D) reproductive region; (E,F) variation in tail region (Scale bars: (A) = 200 μm; (B–F) = 50 μm).
Remarks (n = 14): The current population analyzed as M. brevidens from this study morphologically correlates closely with the original description of M. brevidens by Allen [16], including the morphometric data described by Munawar et al. [20][27], but differing from both to stylet length (17.4–19.5 μm vs. 14–16 μm, 15–17.5 μm) (Table 34). These diagnostic characters also showed only some morphometric variation to populations described from different geographical regions, particularly in the pharynx length (107.2–124.6 μm vs. Greece isolate, 123–136 μm, and Iranian isolate, 118.5–141 μm) and stylet length (17.4–19.5 μm vs. Greece isolate, 13.0–16.0 μm, Iranian isolate, 16–16.5 μm, Canadian isolates, 15–17.5 μm, and the UK isolate, 14–15.5 μm). The other morphological features, such as lip and tail morphology, overall body habitus, and vulva appearance, were all in line with the original description. Males were mentioned in the initial description by Siddiqi [21][28], but no males were ever found in later reports. Males were scarce in M. brevidens, according to Geraert [22][29], and the studied population was equally devoid of males. Siddiqi [21][28] also observed tightly closed stylet knobs, an inconspicuous anus, and a cylindrical tail in the population isolated from India. In some of the studied populations, the anus was conspicuous, with rounded stylet knobs and a cylindrical tail with a broadly rounded, somewhat truncated terminus. The morphological variations of the studied population compared with the other isolates can be due to differences in their geographical origin.
Table 34. Comparative morphometrics of females from populations of Merlinius brevidens. All measurements are in micrometers (except n, ratio, and percentage) and in the form: mean ± standard deviation (range).
| Character | Merlinius brevidens | M. brevidens | M. brevidens | M. brevidens | M. brevidens | M. brevidens | M. brevidens | M. microdorus | M. nanus |
|---|---|---|---|---|---|---|---|---|---|
| Reference | Present study (China) | Canada: Munawar et al. (2021) | India: Bharti et al. (2020) | Greece: Tzortzakakis et al. (2018) | Iran: Alvani et al. (2017) | UK: Bridge (1971) | USA: Allen (1955) | Belgium: Geraert (1966) | USA: Allen (1955) |
| N | 14 | 15 | 10 | 3 | 8 | 10 | 11 | 14 | 8 |
| L | 655.3 ± 25.5 (631.3–707.9) | (591–811) | (572–644) | (490–698) | (600–718.5) | (526–676) | (540–690) | (580–700) | (520–640) |
| a | 34.0 ± 2.0 (31.7–39.9) |
(25.5–38.5) | (26.1–31.6) | (22.9–30.3) | (23.9–29.0) | (21.5–27) | (23–27) | (24.0–28.5) | (27–31) |
| b | 5.6 ± 0.4 (5.1–6.6) |
(4.6–5.9) | (4.2–5.3) | (4.0–5.1) | (4.5–5.2) | (4.5–5.1) | (4.2–5.2) | ||
| - | |||||||||
| V | 59.0 ± 2.0 (54.7–62.4) |
(53.8–61.0) | (54–58) | (52.0–58.0) | (54.5–57.0) | (53–58.5) | (52–58) | (55–59) | (52–57) |
| Lip height | 5.3 ± 0.4 (4.6–6.0) |
(3.1–4.0) | (3–4) | - | - | - | - | - | - |
| Lip width | 9.9 ± 0.6 (9.0–11.0) |
(6.1–7.7) | (6–8) | - | - | - | - | - | - |
| Stylet length | 18.8 ± 0.6 (17.4–19.5) |
(15–17.5) | (13–14) | (13.0–16.0) | (16–16.5) | (14–15.5) | (14–16) | (12–14) | (12–15) |
| Stylet base diameter | 15.2 ± 0.5 (14.1–15.9) |
- | (12–13) | - | - | - | - | - | - |
| Anterior to median bulb valve |
61.8 ± 3.2 (54.5–65.9) |
- | (55–64) | - | - | - | - | - | - |
| Pharynx length | 116.8 ± 6.8 (107.2–124.6) |
(120.3–144) | (107–123) | (123–136) | (118.5–141) | - | - | - | - |
| Anterior to end of pharyngeal glands |
134.5 ± 7.0 (123.1–142.0) |
- | - | - | - | - | - | - | - |
| Anterior to excretory pore | 112.1 ± 2.6 (106.6–114.8) |
- | (84–102) | - | (90.5–110) | - | - | - | - |
| Maximum body width | 19.3 ± 0.7 (17.7–20.5) |
(15.5–21.2) | (19–24) | (19–23) | (21.5–30) | - | - | - | - |
| Anterior end to vulva | 386.8 ± 27.3 (345.1–439.1) |
- | (313–361) | - | (327–392) | - | - | - | - |
| Body width at vulva | 22.3 ± 1.3 (20.1–24.6) |
(16.4–22.3) | (17–24) | - | (21.5–30) | - | - | - | - |
| Tail length | 51 ± 7 (41–63) |
(46–59.8) | (41–54) | (38–41) | (42–53) | (46) | - | (34–63) | - |
| Anal body diameter | 14.4 ± 1.4 (11.5–16.3) |
(10.3–14.2) | (10–16) | (12–14) | (15.5–19) | (19) | - | (17) | - |
The studied population also differs from female Merlinius microdorus [23][30] Siddiqi, [24][31] by the tail shape (blunt and sub-cylindrical vs. rounded and sub-cylindrical); it is further distinguished by the presence of 18–22 tail annuli with smooth broadly rounded or truncated tail terminus vs. 53–54 tail annuli with a bluntly pointed terminus in M. microdorus [18][20][18,27]. The studied population can also be distinguished from Merlinius nanus [14] Siddiqi [24][31], by having a robust stylet vs. delicate; blunt and sub-cylindrical tail vs. pointed and conoid tail; tail terminus hemispherical and smooth vs. bluntly pointed and annulated [13][18][13,18]. Lateral fields with 6 longitudinal incisures were present vs. 6–10 incisures [13]. Additionally, female tail with 18–22 annuli in present isolates vs. 55–60 in M. nanus [13][18][13,18]. The differences in morphometric data of females of the present isolate and other described Merlinius species are given in Table 34.
3.2. Molecular Characterization and Phylogenetic Analysis of Merlinius brevidens
The amplification of the D2–D3 fragments of 28S rRNA and ITS-rRNA genes yielded single fragments of approximately 776 and 980–981 bp, respectively. The BLASTN search of the generated D2–D3 sequences of the present strain showed 100% similarities with M. brevidens Iranian populations (KP313844, KJ585416); 99% similarities with M. brevidens populations from China (MT856989) and South Africa (MN262457), and 99% similarities with Merlinius spp. accessioned KX789700 (Iran) and KY750832 (Mexico); differing from M. brevidens populations (KP313844, KJ585416, MT856989, and MN262457) by 20–75 bp; and from unidentified Merlinius spp. (KX789700, KY750832) by a high genetic distance of between 191 and 476 bp (20–38%). The present strain shows no interspecific differences from the next closest related species (Pratylenchoides leiocauda populations; MN539649, MN539650, and MN510993) deposited into GenBank from China, all with 96% identities. The six newly generated ITS sequences showed an intraspecific variation of 1 bp (0.1%), with a relative level of homology to Indian Merlinius spp. (MK981336); differing by 106 bp (11%). In addition, genetic distances of between 39 and 43 bp (4%) were also observed when compared with sequences of the closest related population (Helicotylenchus digonicus; GQ906352, GQ906353, and GQ906351) from China and Germany.
The phylogenetic relationships between merliniidae and other tylenchids were based on the D2-D3 region of partial sequences from the 28S-rRNA and ITS-rRNA genes, as inferred from Bayesian interference (BI) analysis with general time-reversible (GTR + I + G) substitution model (Figure 5 and Figure 6). The current population formed a well-supported clade with some previously deposited sequences of M. brevidens (KP313842, KP313844, KP313845, and KJ585416) in NCBI from Iran, with 95–100% bootstrap support values according to the phylogenetic tree inferred from the study of the D2–D3 region of the 28S rRNA gene sequence alignment. They specifically belong to the same clade as other M. brevidens isolates from Mexico, Greece, Canada, the United States of America, and South Africa, suggesting that they are conspecific. However, a sub-clade of M. brevidens was formed by a few additional populations from Iran (KP313842, KP313845, KP313847, Alvani et al. [25][32]; MN947623, unpublished). The authors did not provide morphological or morphometric data for these last populations, implying that they should be re-evaluated using detailed integrative taxonomy. Additionally, M. brevidens isolates cluster together in the same sister clade as M. nanus from the United States and appeared as a different, well-supported clade with 100% bootstrap support.
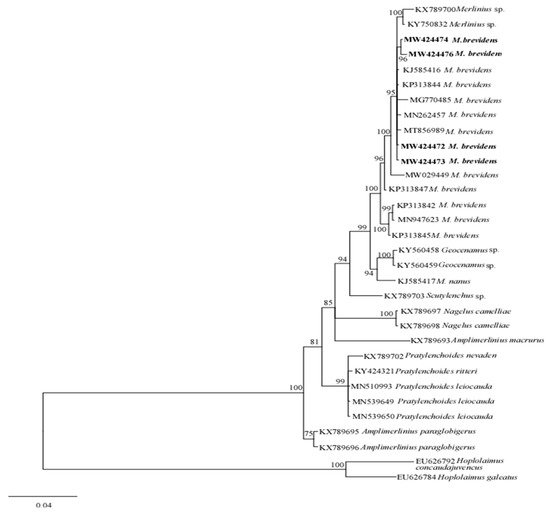
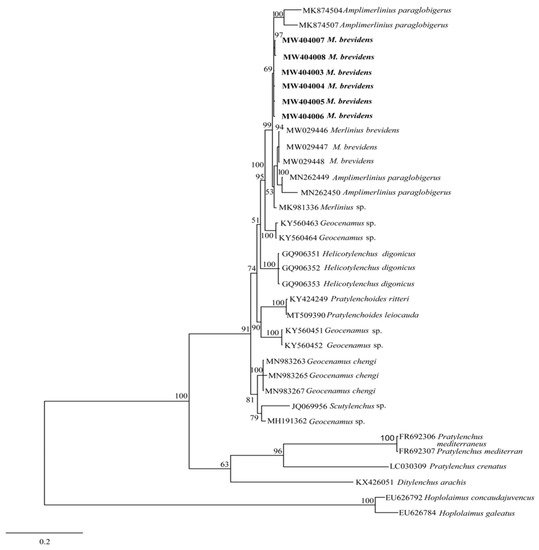

Figure 5. Bayesian tree inferred under the GTR + G + I model from sequences of 28S-rRNA region of the newly sequenced species of Merliniidae indicated in bold and other tylenchids sequences. Posterior probability and bootstrap values exceeding 50% are given on appropriate clades.

Figure 6. Bayesian tree inferred under the GTR + G + I model from sequences of ITS rRNA region of the newly sequenced species of Merliniidae indicated in bold and other tylenchids sequences. Posterior probability and bootstrap values exceeding 50% are given on appropriate clades.
These populations also form a clade with more closely related species of Amplimerlinius and A. paraglobigerus (MK874504 and MK874507) sequences from South Africa, with 69% bootstrap support, and together they formed a sister clade to M. brevidens from Canada (MW029446-MW029448) and A. paraglobigerus from S/Africa (MN262449) based on ITS-rRNA sequences. Furthermore, only a few Merlinius species have been molecularly identified; the studied M. brevidens population was also found in the same major clade as G. chengi and unidentified Geocenamus species.
M. brevidens is a cosmopolitan species that has been reported from numerous countries, according to a literature review [13][20][13,27]. However, few or no integrative taxonomic descriptions were included with any of the M. brevidens sequences that were submitted to GenBank. As a result, determining their genuine identity is challenging.
