Multiple sclerosis (MS) is a complex disease of the central nervous system (CNS) that involves an intricate and aberrant interaction of immune cells leading to inflammation, demyelination, and neurodegeneration. Due to the heterogeneity of clinical subtypes, their diagnosis becomes challenging and the best treatment cannot be easily provided to patients. Biomarkers have been used to simplify the diagnosis and prognosis of MS, as well as to evaluate the results of clinical treatments.
- biomarkers
- predictive
- diagnostic
- prognosis
- treatment response monitoring
- multiple sclerosis
- Introduction
1. Introduction
Multiple sclerosis (MS) is a disabling disease of the central nervous system (CNS) that probably results from the autoimmune attack of immune cells particularly T cells. Inflammation, demyelination and neurodegeneration are pathological hallmarks of MS during different stages of the disease. More than two million people across the globe suffer from MS and nearly 5–20 per 100,000 population suffer according to the current estimates in India [1,2][1][2]. Young adults between 20 and 40 years of age are mainly affected, although MS also occurs in children, teenagers, and the elderly [3]. According to the classification given by Lublin et al., MS manifests as relapsing remitting and progressive forms, with disease activity and/or progression [4]. Relapsing remitting clinical form of MS (RRMS) is seen in 90% of MS patients. A greater number of these patients eventually are transformed into secondary progressive MS (SPMS), which causes further deterioration and neurological disability [5]. The progressive form of MS is comprised of primary progressive MS (PPMS) and SPMS. Approximately 15% of patients are affected with PPMS, which is considered the subtype with the worst prognosis [6]. Due to heterogeneous clinical presentation of MS, there is no specific ‘diagnostic test’ available in the laboratory, leading to delays in diagnosis and determining a prognosis. Therefore, accurate diagnostic tools are required to understand the development and progression of disease, and the use of blood and cerebrospinal fluid (CSF) biomarkers would aid in the diagnosis, prognosis, and monitoring responses to current treatments [7]. The later objective would be of particular interest in SPMS, since most of the patients that have this MS-subtype do not respond well to new disease-modifying therapies (DMT) used to treat MS [8,9][8][9].
Therefore, the aforementioned data clearly justify a detailed investigation of biomarkers to better understand the factors that contribute to the development of MS, its exacerbation into the various subtypes, the effects of treatment, and the disease prognosis. Biomarkers would also be useful in differential diagnoses between MS and other CNS demyelinating diseases with similar signs or symptoms.
In recent years, several remarkable reviews have been published that highlight the importance of biomarkers in the prediction, diagnosis, and outcome of treatments in MS [10,11,12,13][10][11][12][13]. In this physically and mentally disabling disease, being able to make relevant information on the potential best biomarkers easily accessible to MS-clinicians is essential for daily practice.
- The Criterion to Be Considered as a Standard Biomarker
2. The Criterion to Be Considered as a Standard Biomarker
The characteristics used to classify a biomarker ideal are as follows:
(1) It must have the ability to differentiate between a patient and a healthy individual;
(2) It must be expressed at an early stage before the disease progresses;
(3) It must be easy to evaluate, safe for patients, and informative for subsequent management of the disease;
(4) It must offer reproducible results. Furthermore, the biomarker must be sensitive, specific, and must have a clear predictive value.
Fulfilling these characteristics, the identification of specific biomarkers can help to diagnose the disease, understand the progression of the disease, and what the response to therapeutic regimens would be. In MS, body fluids such as blood and CSF have different merits and demerits for use in identifying the specific biomarkers they contain. The detection of biomarkers in a blood sample is a safe, fast, and simple technique. In contrast, CSF biomarkers can be measured at different time points without undue inconvenience to patients. However, there are certain demerits to the blood sample method. For instance:
(1) The measurement of biomarkers in a blood sample may not necessarily reflect changes in the CNS, unlike CSF;
(2) In blood samples, markers are affected by many clinical and biological processes;
(3) The concentration of biomarkers in blood may be lower than the corresponding amount in the CSF.
Although measuring a biomarker in the CSF directly has the advantage of reflecting changes in the CNS, these samples must be obtained by lumbar puncture, which is a difficult and traumatic process that cannot be repeated with the frequency as the simple blood sample can be.
- Classification of Biomarkers
3. Classification of Biomarkers
Biomarkers are classified according to:
(A) Personal susceptibility or predictiveness;
(B) Diagnosis;
(C) Prognosis;
(D) Disease-associated activity;
(E) Response to treatment in different disease courses of MS (Table 1).
Table 1.
Classification and clinical uses of biomarkers.
|
Biomarkers |
Description |
|
Predictive |
Risk to Develop MS |
|
Diagnostic |
Fast Interpretation of Pathological State of MS |
|
Prognosis |
Outcome or Course of MS |
|
Disease-Associated Activity |
Demonstration of Current MS Condition as Inflammation, Demyelination, Cognitive Dysfunction, etc. |
|
Response to Treatment |
Predict Response to Therapy in MS Patients |
3.1. Predictive Biomarkers
The predictive biomarkers are useful for identifying particular individuals who are at risk for developing MS. People who are at an increased risk of developing MS and would benefit from targeted screening include:
(A) Children and siblings of MS patients;
(B) Healthy individuals diagnosed with clinically isolated syndrome (CIS) and other disorders related to the nervous system.
These risk groups were determined by identifying risk factors that correlate with MS [14]. The immune reaction to the Epstein–Barr virus (EBV) or human herpes virus (HHV)-6, or the presence of anti-myelin oligodendrocyte glycoprotein (Anti-MOG) and anti-myelin basic protein (Anti-MBP) antibodies are good examples of predictive biomarkers (Table 2).
Table 2. Radiological biomarkers and biomarkers identified in CSF and serum samples and their standard clinical use in MS.
Radiological biomarkers and biomarkers identified in CSF and serum samples and their standard clinical use in MS.
|
Predictive Biomarkers |
Diagnostic |
Prognostic |
Biomarkers of Dysfunction and Pathology |
|||
|
Epstein–Barr Virus (EBV) [15][16][17] |
Radiological biomarkers |
Oligoclonal bands (IgM) [67-72] |
Immunological Dysfunction |
|||
|
Human Herpesvirus Type-6 (HHV-6) [18][19][20][21][22][23] |
Oligoclonal Bands (IgG) and (IgM) |
Neurofilaments (NF) [73][74][75][76][77][78][79][80][81][82][83] |
Demyelination |
|||
|
Anti-MOG and |
Immunoglobulin |
Axonal Damage |
||||
|
|
|
|||||
|
|
Anti-Aquaporin-4(AQP4) Antibodies |
|
|
|||
- Epstein–Barr virus (EBV)
|
Predictive Biomarkers |
Diagnostic |
Prognostic |
Biomarkers of Dysfunction and Pathology |
|||
|
Epstein–Barr Virus (EBV) [15,16,17] |
Radiological biomarkers |
Oligoclonal bands (IgM) [67-72] |
Immunological Dysfunction |
|||
|
Human Herpesvirus Type-6 (HHV-6) [18,19,20,21,22,23] |
Oligoclonal Bands (IgG) and (IgM) |
Neurofilaments (NF) [73-83] |
Demyelination |
|||
|
Anti-MOG and |
Immunoglobulin |
Axonal Damage |
||||
|
|
|
|||||
|
|
Anti-Aquaporin-4(AQP4) Antibodies |
|
|
|||
- Epstein–Barr virus (EBV)
, suggesting that there is a definitive causal relationship between HHV-6 infection and the development of MS [19]. Evidence of HHV-6 neurotropism was also found in MS from viral DNA in the brain and CSF of MS patients. Furthermore, in clinical case-control studies, increased expression of HHV-6 genes, with higher levels of viral mRNA and proteins, were also found in the oligodendrocytes of MS patients [20–22][20][21][22]. In a more recent work, Dominguez-Mozo et al. described that higher titer of IgG HHV-6 antibodies are related to the occurrence of disease relapses in patients with MS and that treatment with natalizumab drastically reduces both the relapse rate and IgG HHV-6 antibody titers. Based on these data, the authors highlight the potential role of these antibodies not only as predictive factors, but also as early biomarkers of drug response in patients with MS [23].
- Anti-MOG and anti-MBP antibodies
There is considerable experimental evidence suggesting an association between EBV and MS due to the similarity in the geographical distribution of the appearance of both diseases, the existence of a greater number of reported cases of infectious mononucleosis in MS patients, and higher titters of specific EBV-associated antibodies in patients with an increased risk of MS [15]. Abrahamyan et al. studied the prevalence of Epstein–Barr nuclear antigen (EBNA)-1 and viral capsid antigen (VCA) antibodies in serum from a cohort of 901 patients with CIS or early RRMS [16]. The results showed that 100% of the patients with CIS/RRMS were EBV-seropositive, thus suggesting that a negative EBV serology in patients with suspected inflammatory disease of the CNS should alert clinicians to consider diagnoses other than MS. In addition, in the largest population of EBV-CIS evaluated to date (a cohort of 1047 cases of CIS), only one was seronegative for EBNA-1, demonstrating that, while it is possible to be truly seronegative for EBV and develop MS, it is extremely rare [17].
- Human herpesvirus type-6 (HHV-6)
The experimental results demonstrate high levels of HHV-6 virus expression in oligodendrocytes close to the MS plaques [18]
Oligodendrocytes are the myelin-forming cells of the CNS, and therefore any cellular damage to oligodendrocytes, for instance by autoantibodies, can lead to the loss of myelin sheath. The presence of antibodies against myelin proteins in the serum reflects an autoimmune attack against CNS myelin. Thus, anti-myelin autoantibodies, such as MOG and MBP, in the serum of patients with CIS can be considered as predictive biomarkers of disease. Berger et al. found antibodies against MBP in the serum of CIS patients who developed MS [24] and pointed out that the presence of anti-MBP antibodies in childhood increases the risk of demyelinating encephalomyelitis [25]. Subsequent studies showed that MS patients with anti-MOG antibodies are at an increased risk of developing MS and have a higher relapse rate [26]. Despite these results, the studies are not entirely conclusive since, for instance, Kuhle et al. observed that a clear connection between anti-MOG and anti-MBP presence and CIS to MS conversion is not apparent. For instance, Kuhle et al. observed that a clear connection between anti-MOG and anti-MBP presence and CIS to MS conversion is not apparent [27]. Furthermore, Kuerten et al. used microarrays to analyse antibodies against 205 myelin antigens in a cohort of 13 MS patients. Microarray significance analysis identified a subset of 64 myelin antigens, including MOG and MBP, for which widely elevated levels of anti-myelin autoantibodies could be detected in the plasma of MS patients. Despite this, the authors noted that the levels of significance were not high enough to serve these antibodies as a good predictive clinical biomarker for MS [27]. Based on these results, and those obtained by other authors (reviewed in [28]), anti-MOG antibodies would not be adequate biomarkers for the diagnosis or prognosis of MS, but rather for its differential diagnosis with MOG+-CNS demyelinating disease representing a new distinct disease entity.
3.2. Diagnostic Biomarkers
The purpose of diagnostic biomarkers is to confirm that a patient has a certain pathology, such as MS, and thus allow clinicians to discriminate between them and healthy people or those suffering from another potentially related disorder (Table 2 and Table 3). For MS, the diagnosis of the first clinical events of relapsing-remitting MS with disseminated inflammation in space can be confirmed by magnetic resonance imaging and by the existence of oligoclonal immunoglobulin bands detected in CSF. This modified McDonald MS diagnostic criteria include oligoclonal bands replacing the classic criteria of diffusion over time. Thus, when published MS data were compared by applying classic 2010 MS diagnostic criteria or 2017 modified McDonald MS diagnostic criteria, it was found that around 37% of the patients were correctly diagnosed with the 2010 criteria, and surprisingly, the number increased to 68% when the 2017 criteria were applied [29]. Therefore, the recent criteria, which include the existence of detected oligoclonal bands in CSF, provides a faster and more cost-effective approach to the diagnosis of MS than the classical criteria. However, the McDonald’s 2017 criteria have limitations when they are applied to patients with an atypical clinical syndrome or other inflammatory disorders of the CNS. These patients require expert MS clinicians for an accurate diagnosis of the disease.
3.2.1 MRI as a Diagnostic Biomarker
3.2.1 MRI as a Diagnostic Biomarker
Magneagnetic resonance imaging (MRI) is probably the single most important tool for diagnosing MS. This technique makes it possible to determine localized lesions in SNC. In MS, if lesions are found in the white matter of the brain, this indicates that MS is developing from CIS. The MRI study for MS includes:
- (A) T1-weighted or longitudinal magnetization relaxation time;
- (B) T2-weighted or transverse magnetization relaxation time;
- (C) A post-contrast scan. T1-weighted lesions are used primarily to detect any abnormalities in the integrity blood-brain barrier (BBB). Hypointense T1 lesions (also referred to as black holes) are used as a marker representing the loss of axons that occur during the development of MS.
MR imaging is classified into conventional and unconventional techniques (Table 3) and Figure 1 shows clinical examples of these techniques used in MS patients.
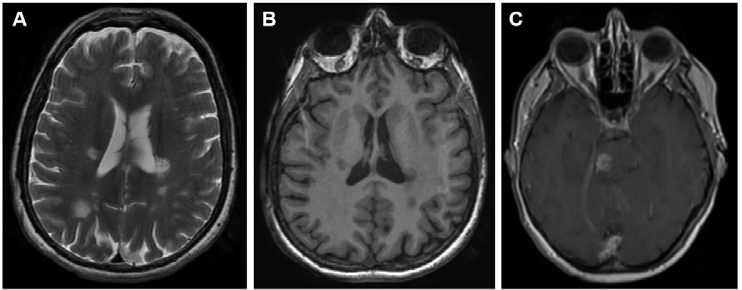
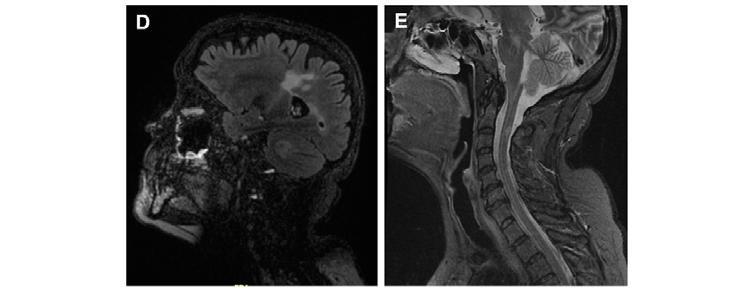
Figure 1. Representative images of MS lesions in the CNS obtained by the magnetic resonance techniques (MRI) most used for diagnosis in daily clinical practice. (A) Brain MRI T2-weighted. (B) Brain MRI T1-weighted. (C) Brain MRI T1-weighted after gadolinium administration. (D) Brain MRI FLAIR-weighted. (E) Spinal cord MRI STIR sequence.
Table 3. Currently imaging biomarkers used in MS, lesions that are mainly detected, and utility and limitations of each technique.
|
Imaging |
Lesion |
References |
Utility in MS |
Limitations |
|
T2-Weighted |
Periventricular Juxtacortical |
[30] |
Indicate Demyelination and |
Variations in the MR Images which is Due to Variable |
|
T1-Weighted |
Gd-Enhancing Lesions and Spinal Cord Lesions |
Shows About Axonal Loss and BBB |
Need of Reproducible and Reliable Method to Quantify Brain Atrophy |
|
|
MTR |
Gd-Enhancing Demyelinated |
Indicate Changes in Myelin Composition and Axonal Loss |
Measurements are Not Absolute and Results Vary |
|
|
DWI and DTI |
Demyelinating and Periventricular Lesions |
Demonstrates about Demyelination and Axonal Loss |
Lack of Pathology Specificity. Required Variable Samples According to Different Type of Lesions and Different |
|
|
MRS |
Periventricular and Cortical |
Assess Measure Biochemicals as NAA, GABA, etc. which Reflect Axonal Damage and Shows Abnormalities in Pathology of MS |
||
|
OCT |
Optic Nerve Lesions |
Used to Measure RNFL Thickness and Macular Volume also Gives Data about |
Movement of Patient Can Diminish the Quality of Image |
|
|
PET |
Periventricular Necrotic |
Assess Inflammation in Cortex, NAWM and Gives Information about |
Invasive and Expensive Tool Not Available all the Time. During Acquisition, if Patient Moves the Activity Will Blur Over Brain Structures. Resolution Will be Degraded and Interpretation of Results Will be Impossible |
|
Imaging |
Lesion |
References |
Utility in MS |
Limitations |
|
T2-Weighted |
Periventricular Juxtacortical |
[30] |
Indicate Demyelination and |
Variations in the MR Images which is Due to Variable |
|
T1-Weighted |
Gd-Enhancing Lesions and Spinal Cord Lesions |
Shows About Axonal Loss and BBB |
Need of Reproducible and Reliable Method to Quantify Brain Atrophy |
|
|
MTR |
Gd-Enhancing Demyelinated |
Indicate Changes in Myelin Composition and Axonal Loss |
Measurements are Not Absolute and Results Vary |
|
|
DWI and DTI |
Demyelinating and Periventricular Lesions |
[35,36,37] |
Demonstrates about Demyelination and Axonal Loss |
Lack of Pathology Specificity. Required Variable Samples According to Different Type of Lesions and Different |
|
MRS |
Periventricular and Cortical |
[38,39] |
Assess Measure Biochemicals as NAA, GABA, etc. which Reflect Axonal Damage and Shows Abnormalities in Pathology of MS |
|
|
OCT |
Optic Nerve Lesions |
[40,41] |
Used to Measure RNFL Thickness and Macular Volume also Gives Data about |
MTR: Magnetization Transfer Ratio, DWI: Diffusion-Weighted Imaging, DTI: Diffusion Tensor Imaging, PET: Positron Emission Tomography, OCT: Optical Coherence Tomography, MRS: Magnetic Resonance Spectroscopy; RNFL: retinal nerve fiber layer.
- Oligoclonal bands (IgG) and (IgM)
Movement of Patient Can Diminish the Quality of Image | ||||
PET | ||||
|
Periventricular Necrotic |
[42,43] |
Assess Inflammation in Cortex, NAWM and Gives Information about |
Invasive and Expensive Tool Not Available all the Time. During Acquisition, if Patient Moves the Activity Will Blur Over Brain Structures. Resolution Will be Degraded and Interpretation of Results Will be Impossible |
- Oligoclonal bands (IgG) and (IgM)
Oligoclonal bands (OCB) are immunoglobulins (Ig) produced intrathecally and, al- though they are considered an immunological characteristic of MS, they are not exclusive to MS, as they are also found in other CNS pathologies [44–46][44][45][46]. OCBs are present in the CSF of more than 95% of MS patients, are absent in serum, and serve as an important criterion for the diagnosis of MS (Table 3). Among the oligoclonal bands, those of the IgG type (OCGB), produced by B cells, are possibly the most important biomarker attributed to CNS demyelinating spectrum disorders. The OCGB serves as a diagnostic element in patients with Clinically Isolated Syndrome (CIS), and is also associated with the development of Clinically Defined MS (CDMS), due to the presence of a higher level of IgG in the CSF of these patients. The presence of OCGB is also essential to predict the progression of Radiologically Isolated Syndrome (RIS) patients to CIS and from CIS to MS [47[47][48],48], whose sensitivity and specificity is 88% and 86%, respectively [49]. The presence of high levels of OCGB in the CSF of patients with CIS favors the transition to CDMS [50,51][50][51] and the subsequent progression of patients to definitive MS [52][52].
The presence of OCGB is also useful in predicting Optic Neuritis (ON) in MS [53[53][54],54], although they are not able to report the intensity of a second relapse. Several lines of evidence indicate that patients with OCGB present in their CSF exhibit a higher level of inflammatory activity, which leads to significant tissue damage [55–57][55][56][57], a greater degree of lesions, and greater brain atrophy [58–61][58][59][60][61].
- Immunoglobulin G index (IgG index)
The levels of IgG and IgM OCBs released intrathecally in MS patients indicate the clonal extension of B cells and plasma cells in the CNS. The relative amount of IgG in the CSF, compared to that present in serum, is evaluated by the IgG index. The IgG index is estimated as the ratio of IgG to albumin in CSF compared to the ratio of IgG to albumin in serum [62]. On the other hand, the albumin quotient (albumin in CSF/albumin in serum) indicates the alteration to the integrity of the blood–brain barrier in MS [63]. It has been estimated that a patient may be diagnosed as having MS when the IgG index exceeds the value of 0.7, which means an increased synthesis of intrathecal IgG antibodies in the CNS which in turns triggers the symptoms of MS. Thus, the IgG index serves as an important biomarker for the diagnosis of MS and is routinely determined during the MS diagnostic.
- Anti-Aquaporin-4 (AQP4) antibodies
Aquaporin-4 (AQP4) is expressed in CNS astrocytes and its function is to transport water through the cell membrane and maintain the homeostatic balance within the CNS. MS patients lack the expression of this protein, while 38–75% of patients with Neuromyelitis Optica (NMO) have AQP4 antibodies [64]. Since NMO is a rare disease in which the immune system attacks myelin surrounding the optic nerve and spinal cord, the differenti- ation between NMO and MS is challenging due to their similar clinical features. Patients with NMO have specific IgG antibodies in their serum directed against AQP4, which is expressed in astrocytes. Therefore, the specific immunoreactivity of this biomarker helps to differentiate between patients with NMO and MS, and also improves the determination of other disorders related to immunity also affecting the CNS [65,66][65][66].
3.3. Biomarkers of Prognosis
Prognostic biomarkers are used to predict the potential response of a patient to treatment in terms of efficacy and/or safety, which allows clinicians to make the most appropriate clinical and therapeutic decisions.
- Oligoclonal bands (IgM)
The IgM-type immunoglobulins (OCMBs) were shown to correlate with MS activity [67] and predict an aggressive course in patients with RRMS during the early stages of the disease [68,69,70,71][68][69][70][71]. The presence of OCMB is associated with an increase in retinal axonal loss in MS [70], the thinning of the retinal nerve fibre layer [71], and its deposition influences inflammatory processes in the brain, generating greater lesions in the CNS [72].
- Neurofilaments (NF)
Neurofilaments (NFs) belong to type IV intermediate filaments and shape neurons. In the CNS they are abundant in the cytoplasm of neurons and are composed of four subfilaments of different molecular weights:
(a) NF-L (polypeptide light chain) of 68 kDa;
(b) NF-M (neurofilament of medium size) of 150 kDa;
(c) NF-H (heavy chain) from 190 to 210 kDa and α-internexin [73].
If axonal damage occurs in the CNS, the filaments are secreted into CSF or blood serum [74]. The NF-L light chain can be considered as a prognostic biomarker that provides information on the transformation of CIS into RRMS [75,76][75][76]. Likewise, a high level of NF-L in CSF is considered a predictive marker of disease severity and progression to SPMS [77], and of disability and cognitive impairment during the conversion of patients to CDMS [78,79][78][79]. On the other hand, Gunnaarsson et al. observed that NF-L is associated with CNS damage, so it can also be used as a neurodegeneration biomarker in MS [80]. Kuhle et al. further demonstrated that NF-L is a marker of tissue damage and disease activity in patients with RRMS, suggesting that it is also a prognostic biomarker [81]. Patients with RRMS and SPMS have a high concentration of NF-H, and this NF is used as a prognostic marker in the development of MS and the future disability of patients [82,83][82][83].
3.4. Biomarkers of Dysfunction and Pathology
MS-type neurological disease activity is defined as the appearance of new neurological symptoms, the recurrence of a previous condition, identifiable radiological activity, or the progression of disability. Clinicians assign a score that predicts whether symptoms have resumed or ceased and whether it is necessary to continue or modify medication to control the disease.
3.4.1. Biomarkers of Immunological Dysfunction
- Cytokines
Neuroinflammation occurs during the relapsing phase of MS, releasing numerous cytokines and chemokines into the CSF, which in turn produces CNS lesions characteristic of MS. Many articles have been published in the literature that describes the changes in certain cytokines during the course of MS, and that are detectable in the CSF of patients [84–88][84][85][86][87][88], or in their serum [88-91][88][89][90][91]. Various authors have proposed cytokines as potential biomarkers of immunological dysfunction in MS.
The chemokine CXCL13, interacts with the CXCR5 receptor, and results in the activation of B and T helper cells in demyelination lesions. Furthermore, a higher level of CXCL13 is found to be associated with the conversion of CIS to MS [84]. Mouzaki et al. found that, through cytokines, it is easy to differentiate MS patients from other inflammatory CNS disorders [85]. Kim et al. also showed that any imbalance in the IL-1 signalling leads to CNS demyelination [86]. Huang et al. investigated protein biomarkers in CSF and plasma using a highly sensitive proteomic immunoassay. The cases from two independent cohorts were compared with healthy controls and patients with other neurological diseases. Ten up-regulated proteins were identified in CSF, including CCL11, IL-12B, CD5, MIP-1a, and CXCL9 in MS patients [87]. Moreover, CCL11 was associated with disease duration, particularly in patients with SPMS subtype [87]. Bai et al., in a meta-analysis of 226 studies with 13,526 MS patients and 8428 healthy controls, showed that 13 CSF cytokines are significantly associated with MS, and among them, CCL21, IL-15, CCL19, CCL11, CCL3, and CXCL13 showed larger standardized mean differences (measured as ES parameter or effective sizes differences between groups with statistical significance) in cytokine concentrations between MS patients and healthy controls [88].
Cytokines have also been proposed as potential biomarkers in blood serum, a clearing fluid that is easier and less traumatic to obtain, which is why it would be highly useful in daily clinical practice. For instance, the relapse rate and prevalence of MS patients was correlated with the levels of interleukin IL-6 in their blood serum [89] and any imbalance in IL-1 signalling leads to increased CNS demyelination [90]. Mouzaki et al. pointed out that measuring some cytokines in serum may allow clinicians to differentiate MS patients from other CNS inflammatory disorders at an early stage [85]. In the same meta-analysis by Bai et al., of the 37 serum cytokines analysed in the meta-analysis, 21 cytokines were significantly associated with MS. Among these, CCL20, IL-23, IL-21, IL-12p40, IL17F, IL22, and IL2R had large ES values which differentiated between MS patients and healthy controls [88] [88].
3.4.2. Biomarkers of Demyelination
- Myelin basic protein (MBP)
Although the levels of the MBP protein increase in the CSF of patients with MS during acute demyelination, they are not considered a good prognostic biomarker [92,93][92][93]. This is because MBPs in CSF tend to remyelinate demyelinating lesions, but the reverse pathway has not been yet demonstrated [94,95][94][95]. The literature reveals that a higher level of MBP has been found in the CSF of MS patients [96] and that MBP levels increase dramatically during the relapse of MS patients [97]. Despite this, it is not considered a good pathology biomarker, given the inconsistency of the results obtained.
3.4.3. Biomarkers of Axonal Damage
- Neurofilaments (NF)
MS patients with high levels of axonal degeneration and neuronal death have a higher level of neurofilament type NF-H in the progressive clinical course of MS [98,99][98][99]. Likewise, higher levels of NF-H have been found in patients with CIS and RRMS [100], and correlate with the relapse activity of patients with CIS and RRMS [101]. For its part, the NF-L type, after dissociation, disperses from the parenchyma into the CSF due to its low molecular mass and hypophosphorylation [101], and a high level is also found in patients with MS or CIS [102,103][102]. It has even been shown that NF-L levels increase in the CSF during acute MS relapse and in MS patients with higher relapse rates. Finally, it was reported that in cases of transition from RRMS to SPMS, a higher level of NF-L is also found [75]. Therefore, neurofilaments are good candidates to be used as a prognostic biomarker to determine axonal damage and estimate the efficacy of MS treatment.
4. Conclusions
The dearth of knowledge about the pathophysiology of MS and the clinical variation in MS subtypes makes it implausible to establish a single biomarker which guarantees the full evaluation of the disease. Although progress has been made in the search for this ‘magical unique biomarker’, MS continues to be extremely unpredictable, with more unanswered questions than absolute certainties, the finding of it seems still a long way off. There remains a need for a panel of validated biomarkers that are capable of predicting and monitoring the efficiency of the growing number of treatment strategies available, with the aim of reducing the recurrence of relapses, and stopping the progression and disability of patients with MS.
Funding: This work has been supported by a grant from the Health Institute Carlos III (PI20/01446) and FEDER funding.
Acknowledgments: The authors do not have any conflict of interest.
References:
- Dilokthornsakul, P.; Valuck, R.J.; Nair, K.V.; Corboy, J.R.; Allen, R.R.; Campbell, J.D. Multiple Sclerosis prevalence in the United States Commercially insured population. Neurology 2016, 86, 1014–1021. [CrossRef]
- Bhatia, R.; Bali, P.; Chowdhary, R. Epidemiology and genetic aspects of multiple sclerosis in India. Ann. Indian Acad. Neurol. 2015, 18, 6. [CrossRef]
- Huang, W.J.; Chen, W.W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [CrossRef]
- Weinshenker, B.G.; Bass, B.; Rice, G.P.; Noseworthy, J.; Carriere, W.; Baskerville, J.; Ebers, G.C. The natural history of multiple sclerosis: A geographically based study. I. Clinical course and disability. Brain 1989, 112, 133–146. [CrossRef] [PubMed]
- Confavreux, C.; Vukusic, S. Natural history of multiple sclerosis: A unifying concept. Brain 2006, 129, 606–616. [CrossRef] [PubMed]
- Wallin, M.T.; Wilken, J.A.; Turner, A.P.; Williams, R.M.; Kane, R. Depression and multiple sclerosis: Review of a lethal combination. J. Rehabil. Res. Dev. 2006, 43, 45–46. [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. The role of oxidative stress in the pathogenesis of multiple sclerosis: The need for effective antioxidant therapy. J. Neurol. 2004, 251, 261–268. [PubMed]
- Kleinschnitz, C.; Meuth, S.G.; Wiendl, H. The trials and errors in MS therapy. Int. MS J. 2008, 15, 79–80.
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkers in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029058. [CrossRef]
- Gul, M.; Jafari, A.A.; Shah, M.; Mirmoeeni, S.M.S.; Haider, S.U.; Moinuddin, S.; Chaudhry, A. Molecular Biomarkers in Multiple Sclerosis and Its Related Disorders: A Critical Review. Int. J. Mol. Sci. 2020, 21, 6020. [CrossRef]
- Porter, L.; Shoushtarizadeh, A.; Jelinek, G.A.; Brown, C.R.; Lim, C.K.; de Livera, A.M.; Jacobs, K.R.; Weiland, T.J. Metabolomic Biomarkers of Multiple Sclerosis: A Systematic Review. Front. Mol. Biosci. 2020, 7, 574133. [CrossRef]
- Toscano, S.; Patti, F. CSF biomarkers in multiple sclerosis: Beyond neuroinflammation. Neuroimmunol. Neuroinflamm. 2021, 8, 14–41.
- Corvol, J.C.; Pelletier, D.; Henry, R.G.; Caillier, S.J.; Wang, J.; Pappas, D.;Casazza, S.; Okuda, D.T.; Hauser, S.L.; Oksenberg, J.R.; et al. Abrogation of T cell quiescence characterizes patients at high risk for multiple sclerosis after the initial neurological event. Proc. Natl. Acad. Sci. USA 2008, 105, 11839–11844. [CrossRef]
- Hedström, A.K.; Huang, J.; Michel, A.; Butt, J.; Brenner, N.; Hillert, J.; Waterboer, T.; Kockum, I.; Olsson, T.; Alfredsson, L. High Levels of Epstein–Barr virus nuclear antigen1-specific antibodies and infectious mononucleosis act both independently and synergistically to Increase multiple sclerosis risk. Front. Neurol. 2020, 10, 1368. [CrossRef] [PubMed]
- Abrahamyan, S.; Eberspächer, B.; Muna, H.M.M.; Hoshi, M.; Aly, L.; Luessi, F.; Groppa, S.; Klotzl, L.; Meuth, S.G.; Schroeder, C.; et al. Complete Epstein-Barr virus seropositivity in a large cohort of patients with early multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 681–686. [CrossRef]
- Dobson, R.; Kuhle, J.; Middeldorp, J.; Giovannoni, G. Epstein-Barr-negative MS: A true phenomenon? Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e318. [CrossRef]
- Ascherio, A.; Munger, K.L. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann. Neurol. 2007, 61, 288–299. [CrossRef] [PubMed]
- Leibovitch, E.C.; Jacobson, S. Evidence linking HHV-6 with multiple sclerosis: An update. Curr. Opin. Virol. 2014, 9, 127–133. [CrossRef] [PubMed]
- Merelli, E.; Bedin, R.; Sola, P.; Barozzi, P.; Mancardi, G.L.; Ficarra, G.; Franchini, G. Human herpes virus 6 and human herpes virus 8 DNA sequences in brains of multiple sclerosis patients, normal adults and children. J. Neurol. 1997, 244, 450–454. [CrossRef]
- Barres, B.A.; Schmid, R.; Sendnter, M.; Raff, M.C. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development 1993, 118, 283–295. [CrossRef]
- Opsahl, M.L.; Kennedy, P.G. Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain 2005, 128, 516–527. [CrossRef]
- Dominguez-Mozo, M.I.; Perez-Perez, S.; Villar, L.M.; Oliver-Martos, B.; Villarrubia, N.; Matesanz, F.; Costa-Frossard, L.; PintoMedel, M.J.; García-Sánchez, M.I.; Ortega-Madueño, I.; et al. Predictive factors and early biomarkers of response in multiple sclerosis patients treated with natalizumab. Sci. Rep. 2020, 10, 14244. [CrossRef]
- Berger, T.; Rubner, P.; Schautzer, F.; Egg, R.; Ulmer, H.; Mayringer, I.; Dilitz, E.; Deisenhammer, F.; Reindl, M. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N. Engl. J. Med. 2003, 349, 139–145. [CrossRef] [PubMed]
- O’Conner, K.C.; Lopez-Amaya, C.; Gagne, D.; Lovato, L.; Moore-Odom, N.H.; Kennedy, J.; Krupp, L.; Tenembaum, S.; Ness, J.; Belman, A.; et al. Antimyelin antibodies modulate clinical expression of childhood Multiple Sclerosis. J. Immunol. 2010, 223, 92–99.
- Spadaro, M.; Gerdes, L.A.; Krumbholz, M.; Ertl-Wagner, B.; Thaler, F.S.; Schuh, E.; Metz, I.; Blaschek, A.; Dick, A.; Bruck, W.; et al. Autoantibodies to MOG in a distinct subgroup of adult Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e257. [CrossRef]
- Kuerten, S.; Lanz, T.V.; Lingampalli, N.; Lahey, L.J.; Kleinschnitz, C.; Mäurer, M.; Schroeter, M.; Braune, S.; Ziemssen, T.; Ho, P.P.; et al. Autoantibodies against central nervous system antigens in a subset of B cell-dominant multiple sclerosis patients. Proc. Natl. Acad. Sci. USA 2020, 117, 21512–21518. [CrossRef] [PubMed]
- Ziemssen, T.; Akgün, K.; Brück, W. Molecular biomarkers in multiple sclerosis. J. Neuroinflammation 2019, 16, 272. [CrossRef]
- Schwenkenbecher, P.; Wurster, U.; Konen, F.F.; Gingele, S.; Sühs, K.W.; Wattjes, M.P.; Stangel, M.; Skripuletz, T. Impact of the McDonald Criteria 2017 on Early Diagnosis of Relapsing-Remitting Multiple Sclerosis. Front. Neurol. 2019, 10, 188. [CrossRef] [PubMed]
- Zivadinov, R.; Bergsland, N.; Dwyer, M.G. Atrophied brain lesion volume, a magnetic resonance imaging biomarker for monitoring neurodegenerative changes in multiple sclerosis. Quant. Imaging Med. Surg. 2018, 8, 979–983. [CrossRef]
- Sahraian, M.A.; Radue, E.W.; Haller, S.; Kappos, L. Black holes in multiple sclerosis: Definition, evolution, and clinical correlations. Acta Neurol. Scand. 2010, 122, 1–8. [CrossRef] [PubMed]
- Grossman, R.I.; Gonzalez-Scarano, F.; Atlas, S.W.; Galetta, S.; Silberberg, D.H. Multiple sclerosis: Gadolinium enhancement in MR imaging. Radiology 1986, 161, 721–725. [CrossRef]
- Dousset, V.; Grossman, R.I.; Ramer, K.N.; Schnall, M.D.; Young, L.H.; Gonzalez-Scarano, F.; Lavi, E.; Cohen, J.A. Experimental allergic encephalomyelitis and multiple sclerosis: Lesion characterization with magnetization transfer imaging. Radiology 1992, 182, 483–491. [CrossRef]
- Deloire-Grassin, M.S.; Brochet, B.; Quesson, B.; Delalande, C.; Dousset, V.; Canioni, P.; Petry, K.G. In vivo evaluation of remyelination in rat brain by magnetization transfer imaging. J. Neurol. Sci. 2000, 178, 10–16. [CrossRef]
- Avila, M.G.S.; Claudio, A.O.; Zabala, E.L.; Teledo, J.D. Diffusion weighted imaging changes in multiple sclerosis patients, frequency and co-relation to disease activity. Austin Neurol. 2018, 3, 1012.
- Abolhasani Foroughi, A.; Salahi, R.; Nikseresht, A.; Heidari, H.; Nazeri, M.; Khorsand, A. Comparison of diffusion-weighted imaging and enhanced T1-weighted sequencing in patients with multiple sclerosis. Neuroradiol. J. 2017, 30, 347–351. [CrossRef] [PubMed]
- Aung, W.Y.; Mar, S.; Benzinger, T.L. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med. 2013, 5, 427–440. [CrossRef] [PubMed]
- Akbar, N.; Rudko, D.A.; Parmar, K. Magnetic resonance imaging of multiple sclerosis. Sci. J. Mult. Scler. 2017, 1, 008–020.
- Narayana, P.A. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J. Neuroimaging. 2005, 15, 46S–57S. [CrossRef]
- Grazioli, E.; Zivadinov, R.; Weinstock-Guttman, B.; Lincoff, N.; Baier, M.; Wong, J.R.; Hussein, S.; Cox, J.L.; Hojnacki, D.; Ramanathan, M. Retinal nerve fiber layer thickness is associated with brain MRI outcomes in multiple sclerosis. J. Neurol. Sci. 2008, 268, 12–17. [CrossRef]
- Britze, J.; Frederiksen, J.L. Optical coherence tomography in multiple sclerosis. Eye 2018, 32, 884–888. [CrossRef]
- Oh, U.; Fujita, M.; Ikonomidou, V.N.; Evangelou, I.E.; Matsuura, E.; Harberts, E.; Fujimura, Y.; Richert, N.D.; Ohayon, J.; Pike, V.W.; et al. Translocator protein PET imaging for glial activation in multiple sclerosis. J. Neuroimm. Pharmacol. 2011, 6, 354–361. [CrossRef]
- Stankoff, B.; Poirion, E.; Tonietto, M.; Bodini, B. Exploring the heterogeneity of MS lesions using positron emission tomography: A reappraisal of their contribution to disability. Brain Path. 2018, 28, 723–734. [CrossRef]
- Pryce, G.; Baker, D. Oligoclonal bands in multiple sclerosis; Functional significance and therapeutic implications. Does the specificity matter? Mult. Scler. Relat. Disord. 2018, 25, 131–137. [CrossRef]
- Trbojevic-Cepe, M. Detection of oligoclonal Ig bands: Clinical significance and trends in methodological improvement. EJIFCC 2004, 15, 86–94.
- Ziemssen, T.; Ziemssen, F. The role of the humoral immune system in multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE). Autoimmun. Rev. 2005, 4, 460–467. [CrossRef]
- Kuhle, J.; Disanto, G.; Dobson, R.; Adiutori, R.; Bianchi, L.; Topping, J.; Bestwick, J.P.; Meier, U.-C.; Marta, M.; Dalla Costa, G.; et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult. Scler. 2015, 21, 1013–1024. [CrossRef]
- Matute-Blanch, C.; Villar, L.M.; Álvarez-Cermeño, J.C.; Rejdak, K.; Evdoshenko, E.; Makshakov, G.; Nazarov, V.; Lapin, S.; Midaglia, L.; Vidal-Jordana, A.; et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain 2018, 141, 1085–1093. [CrossRef] [PubMed]
- Dobson, R.; Ramagopalan, S.; Davis, A.; Giovannoni, G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: A meta-analysis of prevalence, prognosis and effect of latitude. J. Neurol. Neurosurg. Psychiatry 2013, 84, 909–914. [CrossRef] [PubMed]
- Katsavos, S.; Anagnostouli, M. Biomarkers in Multiple Sclerosis: An up-to-date overview. Mult. Scler. Int. 2013, 2013, 340508. [CrossRef]
- Nilsson, P.; Larsson, E.M.; Maly-Sundgren, P.; Perfekt, R.; Sandberg-Wollheim, M. Predicting the outcome of optic neuritis: Evaluation of risk factors after 30 years of follow-up. J. Neurol. 2005, 252, 396–402. [CrossRef] [PubMed]
- Tintore, M.; Rovira, À.; Río, J.; Otero-Romero, S.; Arrambide, G.; Tur, C.; Comabella, M.; Nos, C.; Arévalo, M.J.; Negrotto, L.; et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 2015, 138, 1863–1874. [CrossRef]
- Skov, A.G.; Skov, T.; Frederiksen, J.L. Oligoclonal bands predict multiple sclerosis after optic neuritis: A literature survey. Mult. Scler. 2011, 17, 404–410. [CrossRef]
- Söderström, M.; Ya-Ping, J.; Hillert, J.; Link, H. Optic neuritis: Prognosis for multiple sclerosis from MRI, CSF, and HLA findings. Neurology 1998, 50, 708–714. [CrossRef]
- Freedman, M.S.; Thompson, E.J.; Deisenhammer, F.; Giovannoni, G.; Grimsley, G.; Keir, G.; Ohman, S.; Racke, M.K.; Sharief, M.; Sindic, C.J.; et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch. Neurol. 2005, 62, 865–870. [CrossRef]
- Farina, G.; Magliozzi, R.; Pitteri, M.; Reynolds, R.; Rossi, S.; Gajofatto, A.; Benedetti, M.D.; Facchiano, F.; Monaco, S.; Calabrese, M. Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: A combined CSF and MRI study. J. Neuroinflammation 2017, 14, 40. [CrossRef] [PubMed]
- Graner, M.; Pointon, T.; Manton, S.; Green, M.; Dennison, K.; Davis, M.; Braiotta, G.; Craft, J.; Edwards, T.; Polonsky, B.; et al. Oligoclonal IgG antibodies in multiple sclerosis target patient-specific peptides. PLoS ONE 2020, 15, e0228883.
- Rojas, J.I.; Patrucco, L.; Tizio, S.; Cristiano, E. Oligoclonal bands in the cerebrospinal fluid and increased brain atrophy in early stages of relapsing-remitting multiple sclerosis. Arq. Neuropsiquiatr. 2012, 70, 574–577. [CrossRef] [PubMed]
- Ferreira, D.; Voevodskaya, O.; Imrell, K.; Stawiarz, L.; Spulber, G.; Wahlund, L.O.; Hillert, J.; Westman, E.; Karrenbauer, V.D. Multiple sclerosis patients lacking oligoclonal bands in the cerebrospinal fluid have less global and regional brain atrophy. J. Neuroinmun. 2014, 274, 149–154. [CrossRef] [PubMed]
- Villar, L.M.; Sádaba, M.C.; Roldán, E.; Masjuan, J.; González-Porqué, P.; Villarrubia, N.; Espiño, M.; García-Trujillo, J.A.; Bootello, A.; Alvarez-Cermeño, J.C. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J. Clin. Investig. 2005, 115, 187–194. [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [CrossRef]
- Villar, L.; García-Barragán, N.; Espiño, M.; Roldán, E.; Sádaba, M.; Gómez-Rial, J.; González-Porqué, P.; Alvarez-Cermeño, J. Influence of oligoclonal IgM specificity in multiple sclerosis disease course. Mult. Scler. 2008, 14, 183–187. [CrossRef]
- Monreal, E.; Sainz de la Maza, S.; Costa-Frossard, L.; Walo-Delgado, P.; Zamora, J.; Fernández-Velasco, J.I.; Villarrubia, N.; Espiño, M.; Lourido, D.; Lapuente, P.; et al. Predicting aggressive multiple sclerosis with intrathecal IgM synthesis among patients with a clinically isolated syndrome. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1047. [CrossRef]
- LeVine, S.M. Albumin and multiple sclerosis. BMC Neurol. 2016, 16, 47. [CrossRef]
- Brebner, J.A.; Stockley, R.A. Polyclonal free light chains: A biomarker of inflammatory disease or treatment target? F1000 Med. Rep. 2013, 5, 4. [CrossRef] [PubMed]
- Presslauer, S.; Milosavljevic, D.; Brücke, T.; Bayer, P.; Hübl, W. Elevated levels of kappa free light chains in CSF support the diagnosis of multiple sclerosis. J. Neurol. 2008, 255, 1508–1514. [CrossRef] [PubMed]
- Rinker, J.R., 2nd; Trinkaus, K.; Cross, A.H. Elevated CSF free kappa light chains correlate with disability prognosis in multiple sclerosis. Neurology 2006, 67, 1288–1290. [CrossRef]
- Villar, L.M.; Espino, M.; Costa-Frossard, L.; Muriel, A.; Jimenez, J.; Alvarez-Cermeño, J.C. High levels of cerebrospinal fluid free kappa chains predict conversion to multiple sclerosis. Clin. Chim. Acta 2012, 413, 1813–1816. [CrossRef]
- Arneth, B.; Birklein, F. High sensitivity of free lambda and free kappa light chains for detection of intrathecal immunoglobulin synthesis in cerebrospinal fluid. Acta Neurol. Scand. 2009, 119, 39–44. [CrossRef]
- Flanagan, E.P.; Cabre, P.; Weinshenker, B.G.; Sauver, J.S.; Jacobson, D.J.; Majed, M.; Lennon, V.A.; Lucchinetti, C.F.; McKeon, A.; Matiello, M.; et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann. Neurol. 2016, 79, 775–783. [CrossRef]
- McCreary, M.; Mealy, M.A.; Wingerchuk, D.M.; Levy, M.; DeSena, A.; Greenberg, B.M. Updated diagnostic criteria for neuromyelitis optica spectrum disorder: Similar outcomes of previously separate cohorts. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318815925. [CrossRef] [PubMed]
- Paul, F.; Jarius, S.; Aktas, O.; Bluthner, M.; Bauer, O.; Appelhans, H.; Franciotta, D.; Bergamaschi, R.; Littleton, E.; Palace, J.; et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med. 2007, 4, e133. [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments at a glance. J. Cell Sci. 2012, 125, 3257–3263. [CrossRef] [PubMed]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Pieh, L.F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nature Rev. Neurol. 2018, 14, 577–589. [CrossRef] [PubMed]
- Salzer, J.; Svenningsson, A.; Sundstrom, P. Neurofilament light as a prognostic marker in multiple sclerosis. Mult. Scler. 2010, 16, 287–292. [CrossRef] [PubMed]
- Arrambide, G.; Espejo, C.; Eixarch, H. Neurofilament light chain level is a weak risk factor for the development of MS. Neurology 2016, 87, 1076–1084. [CrossRef] [PubMed]
- Modvig, S.; Degn, M.; Roed, H.; Sørensen, T.L.; Larsson, H.B.; Langkilde, A.R.; Frederiksen, J.L.; Sellebjerg, F. Cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain predict multiple sclerosis development and disability after optic neuritis. Mult. Scler. 2015, 21, 1761–1770. [CrossRef] [PubMed]
- Barro, C.; Leocani, L.; Leppert, D.; Comi, G.; Kappos, L.; Kuhle, J. Fluid biomarker and electrophysiological outcome measures for progressive MS trials. Mult. Scler. 2017, 23, 1600–1613. [CrossRef]
- Novakova, L.; Zetterberg, H.; Sundström, P.; Axelsson, M.; Khademi, M.; Gunnarsson, M.; Malmeström, C.; Svenningsson, A.; Olsson, T.; Piehl, F.; et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017, 89, 2230–2237. [CrossRef]
- Gunnarsson, M.; Malmeström, C.; Axelsson, M.; Sundström, P.; Dahle, C.; Vrethem, M.; Olsson, T.; Piehl, F.; Norgren, N.; Rosengren, L.; et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol. 2011, 69, 83–89. [CrossRef]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92, e1007–e1015. [CrossRef]
- Petzold, A. Neurofilament phosphoforms: Surrogate markers for axonal injury, degeneration and loss. J. Neurol. Sci. 2005, 233, 183–198. [CrossRef] [PubMed]
- Teunissen, C.E.; Iacobaeus, E.; Khademi, M.; Brundin, L.; Norgren, N.; Koel-Simmelink, M.J.; Schepens, M.; Bouwman, F.; Twaalfhoven, H.A.; Blom, H.J.; et al. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology 2009, 72, 1322–1329. [CrossRef] [PubMed]
- Khademi, M.; Kockum, I.; Andersso, M.L.; Iacobaeus, E.; Brundin, L.; Sellebjerg, F.; Hillert, J.; Piehl, F.; Olsson, T. Cerebrospinal Fluid CXCL13 in multiple sclerosis: A suggestive prognostic marker for the disease course. Mult. Scler. J. 2010, 17, 335–343. [CrossRef] [PubMed]
- Mouzaki, A.; Rodi, M.; Dimisianos, N.; Emmanuil, A.; Kalavrizioti, D.; Lagoudaki, R.; Grigoriadis, N.C.; Papathanasopoulos, P. Immune Parameters That Distinguish Multiple Sclerosis Patients from Patients with Other Neurological Disorders at Presentation. PLoS ONE 2015, 10, e0135434.
- Kim, B.S.; Jin, Y.H. IL-1 signal affects both protection and pathogenesis of virus-induced chronic CNS demyelinating disease. J. Neuroinflammation 2012, 9, 217.
- Huang, J.; Khademi, M.; Fugger, L.; Lindhe, Ö.; Novakova, L.; Axelsson, M.; Malmeström, C.; Constantinescu, C.; Lycke, J.; Piehl, F.; et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 12952–12960. [CrossRef]
- Bai, Z.; Chen, D. Cerebrospinal fluid and blood cytokines as biomarkers for multiple sclerosis: A systematic review and meta-analysis of 226 studies with 13,526 patients. Front. Neurosci. 2019, 13, 1026. [CrossRef]
- Chen, Y.C.; Yang, X.; Miao, L. Serum level of interleukin-6 in Chinese patients with multiple sclerosis. J. Immunol. 2012, 249, 109–111. [CrossRef] [PubMed]
- Ozenci, V.; Kouwenhoven, M.; Huang, Y.M.; Kivisakk, P.; Link, H. Multiple sclerosis is associated with an imbalance between tumor necrosis factor -alpha and IL- 10 secreting blood cells that is corrected by interferon-beta treatment. Clin. Exp. Immunol. 2000, 120, 147–153. [CrossRef]
- Ascherio, A.; Munger, K.L. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann. Neurol. 2007, 61, 288–299. [CrossRef] [PubMed]
- Cohen, S.R.; Herndon, R.M.; McKhann, G.M. Radioimmunoassay of myelin basic protein in spinal fluid: An index of active demyelination. N. Engl. J. Med. 1976, 295, 1455–1457. [CrossRef] [PubMed]
- Whitaker, J.N. Myelin encephalitogenic protein fragments in cerebrospinal fluid of persons with multiple sclerosis. Neurology 1977, 27, 911–920. [CrossRef]
- Romme Christensen, J.; Börnsen, L.; Khademi, M.; Olsson, T.; Jensen, P.E.; Sørensen, P.S. Sellebjerg F CSF inflammation and axonal damage are increased and correlate in progressive multiple sclerosis. Mult. Scler. 2013, 19, 877–884. [CrossRef]
- Harris, V.K.; Sadiq, S.A. Disease biomarkers in multiple sclerosis: Potential for use in therapeutic decision making. Mol. Diagn. Ther. 2009, 13, 225–244. [CrossRef]
- Wekerle, H.; Lassmann, H. The immunology of inflammatory demyelinating disease. In McAlpine’s Multiple Sclerosis, 4th ed.; Compston, A., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2005.
- Sellebjerg, F.; Christiansen, M.; Garred, P. MBP, anti-MBP and anti-PLP antibodies, and intrathecal complement activation in multiple sclerosis. Mult. Scler. 1998, 4, 127–131. [CrossRef]
- Gresle, M.M.; Shaw, G.; Jarrott, B.; Alexandrou, E.N.; Friedhuber, A.; Kilpatrick, T.J.; Butzkueven, H. Validation of a novel biomarker for acute axonal injury in experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2008, 86, 3548–3555. [CrossRef] [PubMed]
- Petzold, A.; Eikelenboom, M.J.; Keir, G.; Grant, D.; Lazeron, R.H.; Polman, C.H.; Uitdehaag, B.M.; Thompson, E.J.; Giovannoni, G. Axonal damage accumulates in the progressive phase of multiple sclerosis: Three years follow up study. J. Neurol. Neurosurg. Psychiatry 2005, 76, 206–211. [CrossRef] [PubMed]
- Brettschneider, J.; Petzold, A.; Junker, A.; Tumani, H. Axonal damage markers in the cerebrospinal fluid of patients with clinically isolated syndrome improve predicting conversion to definite multiple sclerosis. Mult. Scler. 2006, 12, 143–148. [CrossRef] [PubMed]
- Rejdak, K.; Petzold, A.; Stelmasiak, Z.; Giovannoni, G. Cerebrospinal fluid brain specific proteins in relation to nitric oxide metabolites during relapse of multiple sclerosis. Mult. Scler. 2008, 14, 59–66. [CrossRef]
- Lycke, J.N.; Karlsson, J.E.; Andersen, O.; Rosengren, L.E. Neurofilament protein in cerebrospinal fluid: A potential marker of activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1998, 64, 402–404. [CrossRef]
- Malmeström, C.; Haghighi, S.; Rosengren, L.; Andersen, O.; Lycke, J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 2003, 61, 1720–1725. [CrossRef] [PubMed]
References
- Dilokthornsakul, P.; Valuck, R.J.; Nair, K.V.; Corboy, J.R.; Allen, R.R.; Campbell, J.D. Multiple Sclerosis prevalence in the United States Commercially insured population. Neurology 2016, 86, 1014–1021.
- Bhatia, R.; Bali, P.; Chowdhary, R. Epidemiology and genetic aspects of multiple sclerosis in India. Ann. Indian Acad. Neurol. 2015, 18, 6.
- Huang, W.J.; Chen, W.W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166.
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286.
- Weinshenker, B.G.; Bass, B.; Rice, G.P.; Noseworthy, J.; Carriere, W.; Baskerville, J.; Ebers, G.C. The natural history of multiple sclerosis: A geographically based study. I. Clinical course and disability. Brain 1989, 112, 133–146.
- Confavreux, C.; Vukusic, S. Natural history of multiple sclerosis: A unifying concept. Brain 2006, 129, 606–616.
- Wallin, M.T.; Wilken, J.A.; Turner, A.P.; Williams, R.M.; Kane, R. Depression and multiple sclerosis: Review of a lethal combination. J. Rehabil. Res. Dev. 2006, 43, 45–46.
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. The role of oxidative stress in the pathogenesis of multiple sclerosis: The need for effective antioxidant therapy. J. Neurol. 2004, 251, 261–268.
- Kleinschnitz, C.; Meuth, S.G.; Wiendl, H. The trials and errors in MS therapy. Int. MS J. 2008, 15, 79–80.
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkers in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029058.
- Gul, M.; Jafari, A.A.; Shah, M.; Mirmoeeni, S.M.S.; Haider, S.U.; Moinuddin, S.; Chaudhry, A. Molecular Biomarkers in Multiple Sclerosis and Its Related Disorders: A Critical Review. Int. J. Mol. Sci. 2020, 21, 6020.
- Porter, L.; Shoushtarizadeh, A.; Jelinek, G.A.; Brown, C.R.; Lim, C.K.; de Livera, A.M.; Jacobs, K.R.; Weiland, T.J. Metabolomic Biomarkers of Multiple Sclerosis: A Systematic Review. Front. Mol. Biosci. 2020, 7, 574133.
- Toscano, S.; Patti, F. CSF biomarkers in multiple sclerosis: Beyond neuroinflammation. Neuroimmunol. Neuroinflamm. 2021, 8, 14–41.
- Corvol, J.C.; Pelletier, D.; Henry, R.G.; Caillier, S.J.; Wang, J.; Pappas, D.;Casazza, S.; Okuda, D.T.; Hauser, S.L.; Oksenberg, J.R.; et al. Abrogation of T cell quiescence characterizes patients at high risk for multiple sclerosis after the initial neurological event. Proc. Natl. Acad. Sci. USA 2008, 105, 11839–11844.
- Hedström, A.K.; Huang, J.; Michel, A.; Butt, J.; Brenner, N.; Hillert, J.; Waterboer, T.; Kockum, I.; Olsson, T.; Alfredsson, L. High Levels of Epstein–Barr virus nuclear antigen1-specific antibodies and infectious mononucleosis act both independently and synergistically to Increase multiple sclerosis risk. Front. Neurol. 2020, 10, 1368.
- Abrahamyan, S.; Eberspächer, B.; Muna, H.M.M.; Hoshi, M.; Aly, L.; Luessi, F.; Groppa, S.; Klotzl, L.; Meuth, S.G.; Schroeder, C.; et al. Complete Epstein-Barr virus seropositivity in a large cohort of patients with early multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 681–686.
- Dobson, R.; Kuhle, J.; Middeldorp, J.; Giovannoni, G. Epstein-Barr-negative MS: A true phenomenon? Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e318.
- Ascherio, A.; Munger, K.L. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann. Neurol. 2007, 61, 288–299.
- Leibovitch, E.C.; Jacobson, S. Evidence linking HHV-6 with multiple sclerosis: An update. Curr. Opin. Virol. 2014, 9, 127–133.
- Merelli, E.; Bedin, R.; Sola, P.; Barozzi, P.; Mancardi, G.L.; Ficarra, G.; Franchini, G. Human herpes virus 6 and human herpes virus 8 DNA sequences in brains of multiple sclerosis patients, normal adults and children. J. Neurol. 1997, 244, 450–454.
- Barres, B.A.; Schmid, R.; Sendnter, M.; Raff, M.C. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development 1993, 118, 283–295.
- Opsahl, M.L.; Kennedy, P.G. Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain 2005, 128, 516–527.
- Dominguez-Mozo, M.I.; Perez-Perez, S.; Villar, L.M.; Oliver-Martos, B.; Villarrubia, N.; Matesanz, F.; Costa-Frossard, L.; PintoMedel, M.J.; García-Sánchez, M.I.; Ortega-Madueño, I.; et al. Predictive factors and early biomarkers of response in multiple sclerosis patients treated with natalizumab. Sci. Rep. 2020, 10, 14244.
- Berger, T.; Rubner, P.; Schautzer, F.; Egg, R.; Ulmer, H.; Mayringer, I.; Dilitz, E.; Deisenhammer, F.; Reindl, M. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N. Engl. J. Med. 2003, 349, 139–145.
- O’Conner, K.C.; Lopez-Amaya, C.; Gagne, D.; Lovato, L.; Moore-Odom, N.H.; Kennedy, J.; Krupp, L.; Tenembaum, S.; Ness, J.; Belman, A.; et al. Antimyelin antibodies modulate clinical expression of childhood Multiple Sclerosis. J. Immunol. 2010, 223, 92–99.
- Spadaro, M.; Gerdes, L.A.; Krumbholz, M.; Ertl-Wagner, B.; Thaler, F.S.; Schuh, E.; Metz, I.; Blaschek, A.; Dick, A.; Bruck, W.; et al. Autoantibodies to MOG in a distinct subgroup of adult Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e257.
- Kuerten, S.; Lanz, T.V.; Lingampalli, N.; Lahey, L.J.; Kleinschnitz, C.; Mäurer, M.; Schroeter, M.; Braune, S.; Ziemssen, T.; Ho, P.P.; et al. Autoantibodies against central nervous system antigens in a subset of B cell-dominant multiple sclerosis patients. Proc. Natl. Acad. Sci. USA 2020, 117, 21512–21518.
- Ziemssen, T.; Akgün, K.; Brück, W. Molecular biomarkers in multiple sclerosis. J. Neuroinflammation 2019, 16, 272.
- Schwenkenbecher, P.; Wurster, U.; Konen, F.F.; Gingele, S.; Sühs, K.W.; Wattjes, M.P.; Stangel, M.; Skripuletz, T. Impact of the McDonald Criteria 2017 on Early Diagnosis of Relapsing-Remitting Multiple Sclerosis. Front. Neurol. 2019, 10, 188.
- Zivadinov, R.; Bergsland, N.; Dwyer, M.G. Atrophied brain lesion volume, a magnetic resonance imaging biomarker for monitoring neurodegenerative changes in multiple sclerosis. Quant. Imaging Med. Surg. 2018, 8, 979–983.
- Sahraian, M.A.; Radue, E.W.; Haller, S.; Kappos, L. Black holes in multiple sclerosis: Definition, evolution, and clinical correlations. Acta Neurol. Scand. 2010, 122, 1–8.
- Grossman, R.I.; Gonzalez-Scarano, F.; Atlas, S.W.; Galetta, S.; Silberberg, D.H. Multiple sclerosis: Gadolinium enhancement in MR imaging. Radiology 1986, 161, 721–725.
- Dousset, V.; Grossman, R.I.; Ramer, K.N.; Schnall, M.D.; Young, L.H.; Gonzalez-Scarano, F.; Lavi, E.; Cohen, J.A. Experimental allergic encephalomyelitis and multiple sclerosis: Lesion characterization with magnetization transfer imaging. Radiology 1992, 182, 483–491.
- Deloire-Grassin, M.S.; Brochet, B.; Quesson, B.; Delalande, C.; Dousset, V.; Canioni, P.; Petry, K.G. In vivo evaluation of remyelination in rat brain by magnetization transfer imaging. J. Neurol. Sci. 2000, 178, 10–16.
- Avila, M.G.S.; Claudio, A.O.; Zabala, E.L.; Teledo, J.D. Diffusion weighted imaging changes in multiple sclerosis patients, frequency and co-relation to disease activity. Austin Neurol. 2018, 3, 1012.
- Abolhasani Foroughi, A.; Salahi, R.; Nikseresht, A.; Heidari, H.; Nazeri, M.; Khorsand, A. Comparison of diffusion-weighted imaging and enhanced T1-weighted sequencing in patients with multiple sclerosis. Neuroradiol. J. 2017, 30, 347–351.
- Aung, W.Y.; Mar, S.; Benzinger, T.L. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med. 2013, 5, 427–440.
- Akbar, N.; Rudko, D.A.; Parmar, K. Magnetic resonance imaging of multiple sclerosis. Sci. J. Mult. Scler. 2017, 1, 008–020.
- Narayana, P.A. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J. Neuroimaging. 2005, 15, 46S–57S.
- Grazioli, E.; Zivadinov, R.; Weinstock-Guttman, B.; Lincoff, N.; Baier, M.; Wong, J.R.; Hussein, S.; Cox, J.L.; Hojnacki, D.; Ramanathan, M. Retinal nerve fiber layer thickness is associated with brain MRI outcomes in multiple sclerosis. J. Neurol. Sci. 2008, 268, 12–17.
- Britze, J.; Frederiksen, J.L. Optical coherence tomography in multiple sclerosis. Eye 2018, 32, 884–888.
- Oh, U.; Fujita, M.; Ikonomidou, V.N.; Evangelou, I.E.; Matsuura, E.; Harberts, E.; Fujimura, Y.; Richert, N.D.; Ohayon, J.; Pike, V.W.; et al. Translocator protein PET imaging for glial activation in multiple sclerosis. J. Neuroimm. Pharmacol. 2011, 6, 354–361.
- Stankoff, B.; Poirion, E.; Tonietto, M.; Bodini, B. Exploring the heterogeneity of MS lesions using positron emission tomography: A reappraisal of their contribution to disability. Brain Path. 2018, 28, 723–734.
- Pryce, G.; Baker, D. Oligoclonal bands in multiple sclerosis; Functional significance and therapeutic implications. Does the specificity matter? Mult. Scler. Relat. Disord. 2018, 25, 131–137.
- Trbojevic-Cepe, M. Detection of oligoclonal Ig bands: Clinical significance and trends in methodological improvement. EJIFCC 2004, 15, 86–94.
- Ziemssen, T.; Ziemssen, F. The role of the humoral immune system in multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE). Autoimmun. Rev. 2005, 4, 460–467.
- Kuhle, J.; Disanto, G.; Dobson, R.; Adiutori, R.; Bianchi, L.; Topping, J.; Bestwick, J.P.; Meier, U.-C.; Marta, M.; Dalla Costa, G.; et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult. Scler. 2015, 21, 1013–1024.
- Matute-Blanch, C.; Villar, L.M.; Álvarez-Cermeño, J.C.; Rejdak, K.; Evdoshenko, E.; Makshakov, G.; Nazarov, V.; Lapin, S.; Midaglia, L.; Vidal-Jordana, A.; et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain 2018, 141, 1085–1093.
- Dobson, R.; Ramagopalan, S.; Davis, A.; Giovannoni, G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: A meta-analysis of prevalence, prognosis and effect of latitude. J. Neurol. Neurosurg. Psychiatry 2013, 84, 909–914.
- Katsavos, S.; Anagnostouli, M. Biomarkers in Multiple Sclerosis: An up-to-date overview. Mult. Scler. Int. 2013, 2013, 340508.
- Nilsson, P.; Larsson, E.M.; Maly-Sundgren, P.; Perfekt, R.; Sandberg-Wollheim, M. Predicting the outcome of optic neuritis: Evaluation of risk factors after 30 years of follow-up. J. Neurol. 2005, 252, 396–402.
- Tintore, M.; Rovira, À.; Río, J.; Otero-Romero, S.; Arrambide, G.; Tur, C.; Comabella, M.; Nos, C.; Arévalo, M.J.; Negrotto, L.; et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 2015, 138, 1863–1874.
- Skov, A.G.; Skov, T.; Frederiksen, J.L. Oligoclonal bands predict multiple sclerosis after optic neuritis: A literature survey. Mult. Scler. 2011, 17, 404–410.
- Söderström, M.; Ya-Ping, J.; Hillert, J.; Link, H. Optic neuritis: Prognosis for multiple sclerosis from MRI, CSF, and HLA findings. Neurology 1998, 50, 708–714.
- Freedman, M.S.; Thompson, E.J.; Deisenhammer, F.; Giovannoni, G.; Grimsley, G.; Keir, G.; Ohman, S.; Racke, M.K.; Sharief, M.; Sindic, C.J.; et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch. Neurol. 2005, 62, 865–870.
- Farina, G.; Magliozzi, R.; Pitteri, M.; Reynolds, R.; Rossi, S.; Gajofatto, A.; Benedetti, M.D.; Facchiano, F.; Monaco, S.; Calabrese, M. Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: A combined CSF and MRI study. J. Neuroinflammation 2017, 14, 40.
- Graner, M.; Pointon, T.; Manton, S.; Green, M.; Dennison, K.; Davis, M.; Braiotta, G.; Craft, J.; Edwards, T.; Polonsky, B.; et al. Oligoclonal IgG antibodies in multiple sclerosis target patient-specific peptides. PLoS ONE 2020, 15, e0228883.
- Rojas, J.I.; Patrucco, L.; Tizio, S.; Cristiano, E. Oligoclonal bands in the cerebrospinal fluid and increased brain atrophy in early stages of relapsing-remitting multiple sclerosis. Arq. Neuropsiquiatr. 2012, 70, 574–577.
- Ferreira, D.; Voevodskaya, O.; Imrell, K.; Stawiarz, L.; Spulber, G.; Wahlund, L.O.; Hillert, J.; Westman, E.; Karrenbauer, V.D. Multiple sclerosis patients lacking oligoclonal bands in the cerebrospinal fluid have less global and regional brain atrophy. J. Neuroinmun. 2014, 274, 149–154.
- Villar, L.M.; Sádaba, M.C.; Roldán, E.; Masjuan, J.; González-Porqué, P.; Villarrubia, N.; Espiño, M.; García-Trujillo, J.A.; Bootello, A.; Alvarez-Cermeño, J.C. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J. Clin. Investig. 2005, 115, 187–194.
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173.
- Villar, L.; García-Barragán, N.; Espiño, M.; Roldán, E.; Sádaba, M.; Gómez-Rial, J.; González-Porqué, P.; Alvarez-Cermeño, J. Influence of oligoclonal IgM specificity in multiple sclerosis disease course. Mult. Scler. 2008, 14, 183–187.
- Monreal, E.; Sainz de la Maza, S.; Costa-Frossard, L.; Walo-Delgado, P.; Zamora, J.; Fernández-Velasco, J.I.; Villarrubia, N.; Espiño, M.; Lourido, D.; Lapuente, P.; et al. Predicting aggressive multiple sclerosis with intrathecal IgM synthesis among patients with a clinically isolated syndrome. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1047.
- LeVine, S.M. Albumin and multiple sclerosis. BMC Neurol. 2016, 16, 47.
- Brebner, J.A.; Stockley, R.A. Polyclonal free light chains: A biomarker of inflammatory disease or treatment target? F1000 Med. Rep. 2013, 5, 4.
- Presslauer, S.; Milosavljevic, D.; Brücke, T.; Bayer, P.; Hübl, W. Elevated levels of kappa free light chains in CSF support the diagnosis of multiple sclerosis. J. Neurol. 2008, 255, 1508–1514.
- Rinker, J.R., 2nd; Trinkaus, K.; Cross, A.H. Elevated CSF free kappa light chains correlate with disability prognosis in multiple sclerosis. Neurology 2006, 67, 1288–1290.
- Villar, L.M.; Espino, M.; Costa-Frossard, L.; Muriel, A.; Jimenez, J.; Alvarez-Cermeño, J.C. High levels of cerebrospinal fluid free kappa chains predict conversion to multiple sclerosis. Clin. Chim. Acta 2012, 413, 1813–1816.
- Arneth, B.; Birklein, F. High sensitivity of free lambda and free kappa light chains for detection of intrathecal immunoglobulin synthesis in cerebrospinal fluid. Acta Neurol. Scand. 2009, 119, 39–44.
- Flanagan, E.P.; Cabre, P.; Weinshenker, B.G.; Sauver, J.S.; Jacobson, D.J.; Majed, M.; Lennon, V.A.; Lucchinetti, C.F.; McKeon, A.; Matiello, M.; et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann. Neurol. 2016, 79, 775–783.
- McCreary, M.; Mealy, M.A.; Wingerchuk, D.M.; Levy, M.; DeSena, A.; Greenberg, B.M. Updated diagnostic criteria for neuromyelitis optica spectrum disorder: Similar outcomes of previously separate cohorts. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318815925.
- Paul, F.; Jarius, S.; Aktas, O.; Bluthner, M.; Bauer, O.; Appelhans, H.; Franciotta, D.; Bergamaschi, R.; Littleton, E.; Palace, J.; et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med. 2007, 4, e133.
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments at a glance. J. Cell Sci. 2012, 125, 3257–3263.
- Khalil, M.; Teunissen, C.E.; Otto, M.; Pieh, L.F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nature Rev. Neurol. 2018, 14, 577–589.
- Salzer, J.; Svenningsson, A.; Sundstrom, P. Neurofilament light as a prognostic marker in multiple sclerosis. Mult. Scler. 2010, 16, 287–292.
- Arrambide, G.; Espejo, C.; Eixarch, H. Neurofilament light chain level is a weak risk factor for the development of MS. Neurology 2016, 87, 1076–1084.
- Modvig, S.; Degn, M.; Roed, H.; Sørensen, T.L.; Larsson, H.B.; Langkilde, A.R.; Frederiksen, J.L.; Sellebjerg, F. Cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain predict multiple sclerosis development and disability after optic neuritis. Mult. Scler. 2015, 21, 1761–1770.
- Barro, C.; Leocani, L.; Leppert, D.; Comi, G.; Kappos, L.; Kuhle, J. Fluid biomarker and electrophysiological outcome measures for progressive MS trials. Mult. Scler. 2017, 23, 1600–1613.
- Novakova, L.; Zetterberg, H.; Sundström, P.; Axelsson, M.; Khademi, M.; Gunnarsson, M.; Malmeström, C.; Svenningsson, A.; Olsson, T.; Piehl, F.; et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017, 89, 2230–2237.
- Gunnarsson, M.; Malmeström, C.; Axelsson, M.; Sundström, P.; Dahle, C.; Vrethem, M.; Olsson, T.; Piehl, F.; Norgren, N.; Rosengren, L.; et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol. 2011, 69, 83–89.
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92, e1007–e1015.
- Petzold, A. Neurofilament phosphoforms: Surrogate markers for axonal injury, degeneration and loss. J. Neurol. Sci. 2005, 233, 183–198.
- Teunissen, C.E.; Iacobaeus, E.; Khademi, M.; Brundin, L.; Norgren, N.; Koel-Simmelink, M.J.; Schepens, M.; Bouwman, F.; Twaalfhoven, H.A.; Blom, H.J.; et al. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology 2009, 72, 1322–1329.
- Khademi, M.; Kockum, I.; Andersso, M.L.; Iacobaeus, E.; Brundin, L.; Sellebjerg, F.; Hillert, J.; Piehl, F.; Olsson, T. Cerebrospinal Fluid CXCL13 in multiple sclerosis: A suggestive prognostic marker for the disease course. Mult. Scler. J. 2010, 17, 335–343.
- Mouzaki, A.; Rodi, M.; Dimisianos, N.; Emmanuil, A.; Kalavrizioti, D.; Lagoudaki, R.; Grigoriadis, N.C.; Papathanasopoulos, P. Immune Parameters That Distinguish Multiple Sclerosis Patients from Patients with Other Neurological Disorders at Presentation. PLoS ONE 2015, 10, e0135434.
- Kim, B.S.; Jin, Y.H. IL-1 signal affects both protection and pathogenesis of virus-induced chronic CNS demyelinating disease. J. Neuroinflammation 2012, 9, 217.
- Huang, J.; Khademi, M.; Fugger, L.; Lindhe, Ö.; Novakova, L.; Axelsson, M.; Malmeström, C.; Constantinescu, C.; Lycke, J.; Piehl, F.; et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 12952–12960.
- Bai, Z.; Chen, D. Cerebrospinal fluid and blood cytokines as biomarkers for multiple sclerosis: A systematic review and meta-analysis of 226 studies with 13,526 patients. Front. Neurosci. 2019, 13, 1026.
- Chen, Y.C.; Yang, X.; Miao, L. Serum level of interleukin-6 in Chinese patients with multiple sclerosis. J. Immunol. 2012, 249, 109–111.
- Ozenci, V.; Kouwenhoven, M.; Huang, Y.M.; Kivisakk, P.; Link, H. Multiple sclerosis is associated with an imbalance between tumor necrosis factor -alpha and IL- 10 secreting blood cells that is corrected by interferon-beta treatment. Clin. Exp. Immunol. 2000, 120, 147–153.
- Cohen, S.R.; Herndon, R.M.; McKhann, G.M. Radioimmunoassay of myelin basic protein in spinal fluid: An index of active demyelination. N. Engl. J. Med. 1976, 295, 1455–1457.
- Whitaker, J.N. Myelin encephalitogenic protein fragments in cerebrospinal fluid of persons with multiple sclerosis. Neurology 1977, 27, 911–920.
- Romme Christensen, J.; Börnsen, L.; Khademi, M.; Olsson, T.; Jensen, P.E.; Sørensen, P.S. Sellebjerg F CSF inflammation and axonal damage are increased and correlate in progressive multiple sclerosis. Mult. Scler. 2013, 19, 877–884.
- Harris, V.K.; Sadiq, S.A. Disease biomarkers in multiple sclerosis: Potential for use in therapeutic decision making. Mol. Diagn. Ther. 2009, 13, 225–244.
- Wekerle, H.; Lassmann, H. The immunology of inflammatory demyelinating disease. In McAlpine’s Multiple Sclerosis, 4th ed.; Compston, A., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2005.
- Sellebjerg, F.; Christiansen, M.; Garred, P. MBP, anti-MBP and anti-PLP antibodies, and intrathecal complement activation in multiple sclerosis. Mult. Scler. 1998, 4, 127–131.
- Gresle, M.M.; Shaw, G.; Jarrott, B.; Alexandrou, E.N.; Friedhuber, A.; Kilpatrick, T.J.; Butzkueven, H. Validation of a novel biomarker for acute axonal injury in experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2008, 86, 3548–3555.
- Petzold, A.; Eikelenboom, M.J.; Keir, G.; Grant, D.; Lazeron, R.H.; Polman, C.H.; Uitdehaag, B.M.; Thompson, E.J.; Giovannoni, G. Axonal damage accumulates in the progressive phase of multiple sclerosis: Three years follow up study. J. Neurol. Neurosurg. Psychiatry 2005, 76, 206–211.
- Brettschneider, J.; Petzold, A.; Junker, A.; Tumani, H. Axonal damage markers in the cerebrospinal fluid of patients with clinically isolated syndrome improve predicting conversion to definite multiple sclerosis. Mult. Scler. 2006, 12, 143–148.
- Rejdak, K.; Petzold, A.; Stelmasiak, Z.; Giovannoni, G. Cerebrospinal fluid brain specific proteins in relation to nitric oxide metabolites during relapse of multiple sclerosis. Mult. Scler. 2008, 14, 59–66.
- Lycke, J.N.; Karlsson, J.E.; Andersen, O.; Rosengren, L.E. Neurofilament protein in cerebrospinal fluid: A potential marker of activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1998, 64, 402–404.
- Malmeström, C.; Haghighi, S.; Rosengren, L.; Andersen, O.; Lycke, J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 2003, 61, 1720–1725.
