In the fight against cancer, early diagnosis is critical for effective treatment. Traditional cancer diagnostic technologies, on the other hand, have limitations that make early detection difficult. Therefore, multi-functionalized nanoparticles (NPs) and nano-biosensors have revolutionized the era of cancer diagnosis and treatment for targeted action via attaching specified and biocompatible ligands to target the tissues, which are highly over-expressed in certain types of cancers. Advancements in multi-functionalized NPs can be achieved via modifying molecular genetics to develop personalized and targeted treatments based on RNA interference. Modification in RNA therapies utilized small RNA subunits in the form of small interfering RNAs (siRNA) for overexpressing the specific genes of, most commonly, breast, colon, gastric, cervical, and hepatocellular cancer.
1. Introduction
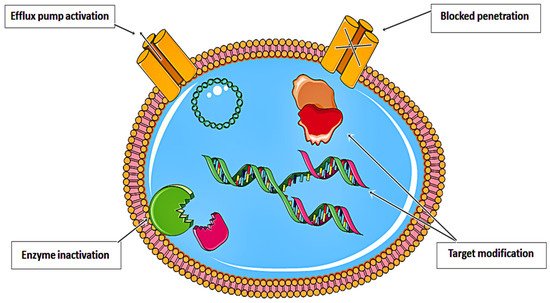
Despite decades of basic and clinical investigation, as well as trials of new therapeutic modalities, cancer remains a substantial cause of mortality worldwide
[1]. Various resistance strategies have led to the near inefficiency of anticancer drugs. As illustrated in
Figure 1, these drugs have been rendered almost ineffective. Recent approaches in nanomedicine have prompted the development of effective theranostic platforms for a myriad of biological and biomedical applications
[2]. Nanomaterials (i.e., niosomes
[3], polymer-based nanocapsules
[4], nanoparticles (NPs)
[5,6[5][6][7][8],
7,8], metal nanocages
[9], nanocomposites
[10], nanoliposomes
[11], and engineered nanohydrogels
[12]), with highly controlled geometry and physic-chemical properties, have been introduced as promising tools for recognizing cancer tissues and also serve as novel drug delivery systems (DDSs) to achieve active targeting
[2,13,14,15,16,17,18,19,20,21][2][13][14][15][16][17][18][19][20][21]. It is now believed that nanotechnology can purposefully improve the clinical outcome of cancer therapies through improving the tolerability of the efficacy of novel drugs
[22] or delivering proteins, DNA, RNA, and various types of molecules to cancerous cells
[23,24][23][24]. Several nanomaterial-based biosensors have also been developed for the accurate sensing of tumor markers
[24,25][24][25].
Figure 1. Resistance strategies developed by anticancer drugs that limit their therapeutic efficiency.
Similarly, biodegradable and biocompatible natural and engineered biomolecules (including proteins, peptides, polysaccharides, and nucleic acids) have been broadly examined to synthesize nanostructures
[26,27][26][27]. Biomolecule-based building blocks provide particular features that make it not feasible to be reproduced in these synthetic materials. However, multifunctional approaches can be developed by exploiting biomolecule-derived elements concerning cancer targeting and therapy
[27].
It is scientifically established that nucleic acids can be manipulated and designed to create many nanostructures
[28]. In the past, researchers have studied the preparation and characterization of DNA nanoparticles (DNA NPs), utilizing complex processes, such as coacervation
[29,30][29][30]. In this respect, charge-neural biodegradable DNA NPs were synthesized by compacting a small-sized single DNA molecule and loading it on magnetic NPs
[31]. This strategy is now considered a beneficial translatable gene therapy platform for overcoming challenging biological barriers by enhancing nuclear uptake across tiny nuclear pores of dividing cells and is being widely investigated to treat various conditions (i.e., malignancies and respiratory diseases)
[31,32,33][31][32][33].
Due to its increased thermodynamic stability, the RNA structure can be more flexible while folding into different structures (i.e., rigid structural motifs), and it produces diverse building blocks for numerous therapeutic applications
[34], including the fabrication of nanosensors and nanodevices
[28]. Furthermore, the thermal stability of RNA also allows it to produce multivalent nanostructures possessing specific stoichiometry
[28,35][28][35]. In this view, introducing novel methods for the re-assembling of RNA molecules has recently spurred interest in investigating the biomedical application of RNA nanostructures.
Nanomedicine has led to the development of a variety of nanoscale therapeutics and diagnostics to treat a variety of diseases, specifically cancer
[6,14,15,17,18,36,37,38,39,40,41,42,43,44,45,46][6][14][15][17][18][36][37][38][39][40][41][42][43][44][45][46]. This fact is broadly exploited in the field of DNA nanotechnology
[47]. Generally, methodologies for DNA nanotechnology can be applied to RNA nanotechnology
[48]. Despite their similarities, DNA and RNA nanotechnology differ in a few key aspects. Inter-and intra-molecular interactions, as well as stem-loops, are abundant in the RNA molecules. These allow the formation of complex secondary and tertiary structures (i.e., bulges, stems, junctions, loops, etc.) and thus can be utilized to create ‘dovetail’ joints among the building blocks
[35]. RNA can serve as a potential new therapeutic modality for cancer due to its lack of accumulation in vital organs
[49].
In the past, numerous RNA-NPs have been prepared by an automated self-assembly process and studied in the context of cancer research
[50,51][50][51]. A key challenge in programming RNA strands to assemble into nanostructures is to create a folding pathway to avoid kinetic traps
[52]. In addition, by introducing chemical modification into nucleotides without substantial changes in RNA content, RNA-NPs will escape host RNA decay pathways. In addition, RNA nanostructures might have immunologic properties, making them valuable tools for in-vivo applications
[49].
The conjugation of polymeric NPs with RNA molecules, such as RNA aptamers, cause these bio-conjugates to be easily absorbed by specific tumor cells, and therefore, can be considered as a beneficial strategy towards the controlled release of polymeric drug delivery vehicles
[53]. In the growing field of RNA nanotechnology to fight cancer, RNA aptamers have attracted great attention as tools for delivering other RNA therapeutics, such as short interfering RNAs (SiRNAs), to specific organs
[54]. Moreover, polyvalent RNA nanostructures have been effectively fabricated as carriers of siRNA, ribozyme, and anticancer agents to tumor sites
[55].
Using in-vitro and in-vivo experimental models, Yin et al. exploited RNA-based technology to efficiently deliver anti-microRNA to cancer cells derived from breast tissue
[56]. Ghimire and colleagues showed that RNA-NPs could be utilized as rubber for constraining vessel extravasation to improve the targeting of cancer cells and increase their renal excretion, thus reducing their toxic effects
[57]. Recently, radiolabeled RNA NPs were developed for specific targeting and efficient tumor accumulation with desirable in-vivo biodistribution
[58]. According to Kim et al., dual-targeting polymeric siRNA NPs were synthesized by multiple processes, including electrostatic deposition and poly-L-lysine condensation. Researchers found that these nanostructures are capable of efficiently delivering siRNA to tumor cells
[59]. Xu et al. successfully delivered delta-5-desaturase via dihomo-γ-linolenic acid-loaded RNA-NPs for suppressing the growth of cancerous colon cells via the induction of apoptotic cell death
[60]. Haque and colleagues systemically injected synergistic tetravalent RNA-NPs into the tail-vein of mice. They observed that these RNA nanostructures preserve their biological function within cancer cells without entering other tissues or organs
[61].
2. RNA Nanotechnology for Diagnosis of Cancers
Biosensors are diagnostic systems that convert a natural response into a programmable signal
[62]. The calculable signal can be electrochemical, optical, thermal, or piezoelectric. Low detection limits, accuracy, and high sensitivity make electrochemical biosensors the most reliable of all. Electrochemical biosensors have a great prospective in the real-sample analysis
[63]. The combination of nanotechnology with biosensors is a hallmark for disease assessment and the planning of its cure. Nanoscience is the science of the molecular and atomic manipulation of materials. It entails creating and managing chemical, physical, and systems in sizes of 1–200 nm. Nanotechnology has many applications in the biomedical field, especially in medical imaging for disease diagnosis
[64]. The exclusive physicochemical properties of NPs are used to develop biosensors of point-of-care accuracy, known as nanosensors. The performance of other electrochemical and enzymatic biosensors increases due to their small size as the distance between enzyme and electrode decreases. Some optical biosensors use noble metal NPs to improve optical properties and increase localized surface plasmon resonance (SPR); e.g., inter-particle plasmon coupling changes the color of NPs, which is used for improving the properties of biosensors grounded on the aggregation of NPs
[65]. DNA oligonucleotides were the first nucleic acid-based NPs that served as the foundation of DNA origami technology, but nowadays, RNA oligonucleotide nanotechnology is an important alternative to DNA technology
[49]. DNA and RNA have operational differences because of their different structural properties. RNA nanotechnology uses single-stranded oligonucleotides for designing diverse and functional RNA nanostructures. The specific structure and organization of functional groups in NPs make them an excellent tool for diagnosing and treating different diseases
[66]. The main edge of RNA NPs includes therapeutic elements, regulatory moieties, and targeting ligands. The field of RNA nanotechnology is different from traditional RNA research, which targets 2D/3D structure-function relationships and intra-RNA connections, as it emphasizes mainly quaternary exchanges and inter-RNA interactions
[51].
2.1. Benefits of RNA Nanotechnology in Targeting Cancer Treatment
RNA NPs are discrete entities, quite different from classical therapeutic RNA, including siRNA, anti-miRNA, miRNA, mRNA, ribosomal-RNA, and viral immune-stimulatory RNA. These traditional RNAs have a broad fundamental history of research in the RNA field. These small RNAs are caught by cells, and they stimulate RNA-sensing pattern recognition receptors (PRRs). Some of these are reported to be immunogenic.
There are a lot of benefits of RNA NPs as compared to traditional RNA, for example: (a) enhanced permeability and retention (EPR) effect, (b) the small size offers to promise pharmacokinetic and pharmacodynamic properties, (c) decreased liver accumulation, (d) Small non-coding RNAs or microRNAs serve as scaffolding and active elements in the bottom-up self-assembly of more complex nanomaterials, and (d) untraceable toxicity in-vivo
[67]. Multinational characteristics of RNA nanostructures, such as targeting ligands and multi-drug loading, are useful for combination therapy
[68]. RNA NPs show chemical, metabolic, and thermal stability in biological systems. The standardized volume of distribution V
d, i.e., 1.2 L/kg of pRNA NPs, indicates the presence of a valuable amount in peripheral tissues, especially in a tumor. The comparatively small amount of clearance (Cl) value indicates the insufficient filtration of the NPs from the kidneys. A specific targeted delivery can be achieved by incorporating traditional RNA into NPs by taking advantage of these properties. These NPs show specific targeted delivery, higher therapeutic efficacy, and an increased in-vivo half-life
[69].
Cancer nanotechnology faces a serious challenge of non-specific accumulation of delivered NPs in healthy organs, such as lungs, liver, spleen, and kidneys
[70]. The non-specific accumulation leads to poor transport of NPs to the tumor site, unwanted side effects, and toxicity. The Guo laboratory combined targeting ligands and a sequence of pRNA-3WJ-based NPs for advanced targeted delivery. After systemic delivery in tumor-bearing mice, these RNA NPs are precisely transported to tumors within almost 4 h. These NPs remain at the tumor site for more than 24 h. After more than a few hours of injection, no or minimal organ accumulation was detected. Several common cancer models were used to get consistent results, such as breast, colorectal, prostate, glioblastoma, gastric, and head and neck cancers. The targeting ligands were altered based on the overexpression of specific targets in tumor tissues
[71]. RNA with negative charge limits non-specific interactions with negatively charged cell membranes, which is important for high selectivity. The ratchet-like shape and strong elasticity of pRNA-3WJ-based NPs show improved EPR effects and higher tumor penetration. Overall, these findings showed that pRNA-3WJ-based NPs could be synthesized simply, with high selectivity and low side effects for healthy tissues. This promising bio-distribution is a significant signal of pharmacological profiles of RNA NPs
[51].
2.2. Nano-Biosensors as Developing Trend in Cancer Diagnostics
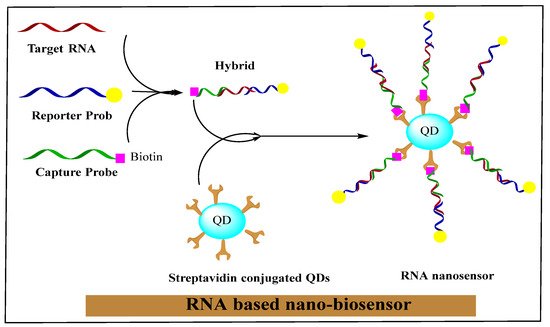
The nano-biosensor is an innovative unit that creates nano-conjugated biological systems, which function as signaling mediators to detect the specific contents of medical, biochemical, or physical agents. The related data is transferred in the form of signals using thermometric, piezoelectric, optical, magnetic, electrochemical, and micromechanical methods. The signals produced by these methods depend on the bio-recognition of a cancer cells-related surface or intracellular biomarkers through bio-ligands or antibodies. In a new era of research, nano-biosensors will increasingly be classified based on bio-recognition and signal transduction elements. RNA-based nano-biosensors are displayed in
Figure 2.
In the basic structure of a sensor, there are two main components: (i) the target analyte that can be a nucleic acid, antibody, drug, protein, or cell-surface molecule; and (ii) the transducer used for altering a signal into a form of energy. It can be detected electrochemically by detecting the energy it produces (voltage and current), (absorption and luminescence), and mechanically (resonance). The exclusive and tunable physicochemical properties of nanostructures, such as enhanced electric conductivity, greater area to volume ratio, high reactivity, unique magnetic properties, and powerful scattering and absorption, make them a fascinating tool for bio-sensing. The nanomaterials’ ability to interact effectively with biologically important analytes and convert those interactions into considerably enhanced signals has enabled a new class of early diagnostic procedures. Gold NPs (AuNPs) and QDs are excellent signal transducers because the existence of a specific analyte regulates the signal generated by the material’s optical properties. Nano and microsensors reduce the size of the receptor to improve their responsiveness. Increased signal-to-noise (S/N) ratio is responsible for this enhanced sensitivity. However, a running device’s success depends on its ability to detect a tremendously low concentration in a reasonable amount of time.
The overall sensitivities of a sensor are often affected by mass transport limitations, such as when the substance is transported to the receptor by diffusion mechanism. The limiting factor in saturating the receptor for a specific geometry of the sensor is the time required by the analytes to reach the sensor area. As the receptor area becomes smaller, the time grows higher. The decreased number of analytes obstructs the detection of extremely dilute solutions. Many practical solutions have been tested to concentrate the dilute solution straight onto the sensor surface. For example, Melli et al. formulated a unique solution with a series of micropillars with superhydrophobic surfaces to simplify the management of samples with a low concentration of analytes. A diluted solution can be placed on these micropillars, the target molecule will become concentrated by the evaporation of liquid, and the detection time is significantly decreased
[72].
Halo et al. used the same theory to detect colorectal cells by mRNA quantification. They developed a NanoFlare platform comprising a single layer of single-stranded DNA (ssDNA) coated on spherical AuNP. A fluorescent reporter was added to a short DNA counterpart that was hybridized to the ssDNA recognition sequence. The reporter fluorophore was slaked while it was close to the AuNPs, but it broadened when the target mRNA displaced the DNA, allowing the fluorescence reading. The detection limit of the nanosensor was about 100 cancer cells/mL blood
[72].
A particular type of RNA aptamer that causes small-molecule fluorophores to emit fluorescence is called a light-up aptamer. Several new light-up aptamers have been developed that can bind to different biocompatible fluorogenic ligands and make their way to the design of modern RNA-based molecular strategies for sensing applications. The Systemic Evolution of Ligands by EXponential enrichment (SELEX) is a combinatorial procedure for identifying such aptamers. RNA aptamer libraries were exposed to recurrent cycles of collection and amplification in this procedure, resulting in RNA aptamers having the strongest selectivity for the target ligand. Various RNA NPs can be coupled with light-up aptamers having programmable sensing capabilities to create dynamic reporting entities
[73].
2.3. RNA Nano-Biosensors
RNA/DNA nano-biosensors can measure the responses produced by aptamer hybridization or nucleic acid conversion processes as promising diagnostic tools
[74]. As aptamers, RNA or DNA are single-stranded nucleic acid oligomers whose structure is extremely organized and complex, forming a strong connection with the target molecules (
Figure 1)
[75]. The RNA with a functional group usually reacts with molecular labels with an orthogonal reactive group in a suitable reaction environment. Fluorescent compounds activated by NHS are attached directly to an NH
2 group of the RNA fragment, and similarly, thiol to maleimide and alkyne to azide, etc. RNA was directly manufactured, and classical pairing reactive groups, e.g., amino –NH
2-COOH, azide, maleimide, alkyne, and thiol, were incorporated by similar methods for the production of polyvalent RNA NPs
[76]. Single-stranded RNA NPs that are used for many purposes, such as biodistribution and diagnostic studies, are the subsequent derivatives of fluorescent dyes (FITC, Cy5, Cy3, and AF-647) attached to RNA
[77].
RNA polymer is conjugated with NPs (such as iron oxide NPs, quantum dots, and gold NPs), and a sequence of research studies used siRNA, pRNA, and phi29 for this purpose. The siRNA was coupled with many nano-based imaging agents to form multifunctional NPs exhibiting diagnostic and therapeutic moieties.
Bhatia et al. established tumor-highlighted peptides (F3) and siRNA coupled to the PEGylated QDs core as a framework. The F3 peptide was conjugated to amine group-modified QD via sulfo-LC-SPDP (sulfosuccinimidyl 6-(3′-(2-pyridyldithio)-propionamido) hexanoate as a heterofunctional cross-linker, and thiol modified siRNA used sulfo-SMCC (sulfosuccinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate) for conjugation. The QD-siRNA/F3 conjugate NPs were proficiently transported to HeLa cells and unconfined from their endosomal setup, which provided the demolished EGFP signal. These siRNA-NPs conjugates exhibited both imaging and therapeutic properties. Furthermore, the siRNA was coupled to iron oxide NPs, which showed magnetic characteristics for biomedical applications. The linkage of siRNA to iron NPs exhibited a double response, such as the in-vivo delivery of siRNA and gathering of siRNA in the tumor by MRI and the near-infrared fluorescent (NIRF) in-vivo optical imaging. The amine groups of iron oxide NPs were treated with m-maleimidobenzoyl N-hydroxysuccinimde ester (MBS) for linkage between siRNA and magnetic NPs. After that, the reduced thiol group of RNA was treated, and magnetic NPs were coupled with and near-infrared Cy5.5 dye and membrane translocation peptides. The MRI and NIRF were used simultaneously to see the siRNA-magnetic NP uptake. The coupling of AuNPs with RNA is studied to enhance the accessibility of tethered RNA splicing enhancers. Guo et al., in 2007, produced a pRNA of the phi29 and DNA-packaging motor linkage to AuNPs to study the phage assembly. In this case, the SH-labeled DNA oligonucleotide was merged with 3′ terminal of pRNA to incorporate the thiol (-SH) group into pRNA. Then, the thiol-treated pRNA was conjugated to gold NPs. The pRNA/AuNPs conjugate was attached to procapsid by an in-vitro phage assembly. Guo’s group proved that the RNA polymer was coupled with the AuNPs effortlessly, and this procedure can be used for imaging purposes. Currently, pRNA-3WJ was coupled straight to the quantum dot for resistive biomemory applications. They introduced a sephadex G-100 resin-recognizing RNA aptamer in the biotin-labeled pRNA/3WJ (SEP
apt/3WJ/Bio) theme for the conjugation of pRNA-3WJ to the quantum dot (QD). Firstly, the SEPapt/3WJ/Bio was attached to G-100 resin, and then the streptavidine-labeled quantum dot was linked to SEP
apt/3WJ/Bio by streptavidine-biotin coupling on Sephadex G-100 and STV/QD-SEP
apt/3WJ/Bio conjugates were subjected to dissociation in the elution buffer. Later, the STV/QD-Bio/3WJ
b, 3WJ
a, and SEP
apt/3WJ
c were split by elution buffer. After that, the STV/QD-Bio/3WJ
b fragment was filtered and reconvened with pRNA 3WJ
a and thiol-labeled pRNA 3WJ
c for resistive biomemory application. Hence, the RNA polymer can be easily incorporated with other NPs and used for various diagnostic, therapeutic, and bio-electronic fields
[77].
Table 1 summarizes the role of RNA-based nanostructures in diagnosing cancers.
Table 1. Summary of RNA-based nanostructures in diagnosis of cancers.
| RNA-Based Nanoparticles |
Key Feature |
| Immune-Magnetic Exosome RNA (iMER) |
Exosomal analysis of glioblastoma multiforme (GBM). |
| Anti-RNA aptamer |
Initial detection and analysis of residual GBM. |
| RNA tetrahedrons |
Target triple-negative breast cancer cells. |
| Oligonucleotide-treated Au-NPs |
Analyzing circulating tumor cells (CTCs) of the prostate. |
| miR-122 mimicked using cationic lipid NPs |
Theranostic agent against hepatocellular carcinoma. |
| Superparamagnetic iron oxide NPs (PEG-g-PEI-SPION) |
Initial detection and analysis of gastric cancer. |
Figure 2. RNA-based nano-biosensor. Reprinted with permission from
[75]. Copyright 2021 Elsevier.
Graphene consists of a single-atom dense two-dimensional honeycomb framework made of sp
2-bonded carbon atoms. Many graphite structures, such as nanotubes, graphite, and fullerenes are produced using graphene. British scientists Andre Geim and Konstantin Novoselov were awarded Nobel Prize in Physics in 2010 at the University of Manchester for their revolutionary research on graphene.
Presently, novel functional materials can be manufactured, and siRNA can be delivered to cancer cells by immobilizing RNA on graphenes. Hu et al. produced polydisperse and stable RNA-graphene oxide nanosheets by covalently immobilizing an RNA aptamer on grapheme oxide. Sharifi’s group exfoliated graphene flakes from nano-crystalline graphite to yield conducting and transparent RNA-graphene-labeled thin films by using RNA as a surfactant. These thin films are used in a lot of electronic applications
[77]. Proteins and peptides are used to form nucleic acid carriers because the nucleotides are shortened by electrostatic linkages with positively charged amino acids of proteins, which are used to transport nucleic acids and small non-coding RNA molecules. On the other hand, the amino acids impart bioreversible polyplex stabilization of the system, endosomal escape, and targeted delivery
[78].
2.4. RNA Nanotechnology in Diagnosis of Different Cancers
2.4.1. GBM
Shao et al. developed a microfluidic platform entitled immune-Magnetic Exosome RNA (iMER) for the exosomal analysis of GBM. Three functional sections were combined by iMER, i.e., real-time RNA analysis, targeted up-gradation of extracellular vesicles, and on-chip RNA isolation. The up-gradation or enrichment process detached the cancer exosomes immunomagnetically from host-derived exosomes, and the later analysis was executed on enriched populations. Later, a glass-bead filter was used to pass the lysate collected after the lysation of exosomes. RNA was immobilized onto glass beads by forming electrostatic bonds during this method and then extracted and quantified by qPCR. Magnetic microbeads with anti-epidermal growth factor receptor (EGFR) antibodies were used to identify and enrich GBM-derived exosomes. These GBM-derived exosomes were incubated with beads, and the whole surface seemed thickly covered. A large quantity of mRNA was found in these vesicles, having mRNA of nuclear proteins as well
[72].
Spherical nucleic acids SNA are a type of NPs that are made up of a NP core treated with oligonucleotide structures and comprised of RNA interference RNAi reporter molecules and therapeutics. The exclusive 3D SNA structures are resilient to nuclease degradation and co-opt into the cells despite the lack of transfection agents. SNAs act as diagnostic agents and have the potential to detect two unique mRNA sites at a time inside of a cell
[77]. These SNAs can effectively cross blood-brain and blood–tumor barriers and are broadly experimented against the most violent and widespread type of malignant brain cancer, i.e., GBM models
[79]. Currently, much attention has been given to EGFRvIII, which is an EGFR receptor variant and is associated with GBM progression. DNA and RNA aptamers have been fabricated and used for GBM detection by the involvement of EGFR.
Iqbal et al. sequestered an anti-RNA aptamer from purified human protein by creative selection. The authors confirmed the ‘aptamer’s ability to detect and seize murine and human GBM cells after its immobilization on a glass substrate. Aptamer binds with wild-type and mutant EGFR with excellent specificity and affinity (Kd = 2.4 nM). This technique is used for the initial detection and analysis of residual disease. Similarly, Iqbal’s group fabricated a flow-through lab-on-chip tool that used surface-attached aptamer’s affinity for GBM’s overexpressed biomarker, i.e., EGFR, to prove that a microfluidic-based technique can be used to detect and capture GBM cells. Later, the same group took radical steps in diagnostics related to anti-EGFR aptamers and came up with two succeeding articles on tracing the differential dynamics of GBM cell structure on substrates grafted with aptamers. They analyzed the dynamic morphology of GBM in computational single-cell metrics to identify and capture tumor cells
[80,81][80][81].
Choulier et al. linked cell-SELEX and protein to separate RNA aptamers. These aptamers have the potential to bind specifically to integrin α5β1, which is an αβ heterodimeric receptor related to cancer angiogenesis and GBM ferociousness. The authors used in histo-fluorescence analysis on patient-based xenografts and a fluorescence-related analysis on cell lines to verify the diagnostic capability of tagged aptamers
[82]. Recently, some specific aptamers were considered as markers for metastasis and recurrence of GBM stem cells (GSCs) and brain tumor-initiating cells (TICs).
Rich et al. designed a pool of DNA aptamers distinguishing TICs with an extremely low dissociation constant (Kd between 0.12 and 3.75), and an aptamer-Cy3-related fluorescence proved the binding. In 2019, Affinito et al. used an RNA library on basic human GSCs to design a 20-F-RNA aptamer A40s, which was explicitly related to the GBM cells. The authors established the detection of A40s-based analysis in GBM cells and GSCSs of human tissue sections
[83]. Here, the aptamer showed a great affinity (Kd = 41.92 nM) for target cells in a less nanomolar range. As stem cells play an important role in chemo-resistance and metastasis, these aptamers can be used in the clinical field to detect violent areas and analyze GBM treatments
[81].
2.4.2. Breast Cancer
Currently, three-dimensional RNA NPs with tetrahedral structures have been designed. RNA tetrahedrons are used in various applications in nanomedicine and nanomaterials because of their structural permanence and mechanical rigidity. The EGFR aptamer was bonded with the RNA tetrahedron structure to target triple-negative breast cancer cells. After IV administration, the drug-loaded NPs face a sequence of biological blockades. NPs face quick opsonization and succeeding sequestration by local macrophages under physiological conditions. As a result, healthy organs, such as the spleen and liver, accumulate very high levels of NPs
[84]. To address these issues, RNA NPs were used to specifically target tumor cells and avoid renal clearance and organ accumulation. For example, Prna-3WJ NPs were fixed with an RNA aptamer specific for EGFR. These NPs specifically targeted triple-negative breast cancer (TNBC). Fluorescence confocal microscopy was used for the histological analysis of tumors to detect the precise directing and retention of RNA NPs in a cancer-frozen cross-section. Both treated and control groups were compared, and it became evident that pRNA-3WJ-EGFR showed extraordinary accumulation at tumor cells without disturbing the healthy organs
[69].
In another study, QD-mi RNA let-7a-gold NPs (QD-RNA-Au NP) were conjugated with Chitosan-based nano-formulation, including negatively charged poly (g-glutamic acid) (PGA) for transfer to breast cancer cells. In the cells, the QDs were separated by dicer-mediated release and showed fluorescence for theranostic applications
[85].
Mediley et al. described the use of an AuNP aggregation-related colorimetric sensor for straight cancer cell detection. However, the signal produced by aptamers after binding with cancerous cells was too low to detect CCRF-CM cells due to their weak binding affinity. To solve this problem, Lu et al. used S6 RNA aptamer-linked multifunctional oval-shaped AuNPs and the monoclonal anti-HER2/c-erb-2 antibody for multivalent attachment of AuNPs with target cells for extremely sensitive analysis of SK-BR-3 breast cancer cells. AuNP-linked colorimetric techniques are used for initial and sensitive in-vitro recognition of cancerous cells
[86].
2.4.3. Prostate Cancer
Sioss et al. fabricated a nanowire-resonator array sensor for signal detection using oligonucleotide-treated AuNPs to analyze RNA in circulating tumor cells (CTCs). In this method, RNAs were attached to AuNPs previously immobilized on the sensor by hybridization. The resonance frequency of the sensor was changed by AuNPs after adding mass to it. The authors calculated this change in resonance frequency and detected PCA3 RNA, a nucleic acid prostate cancer marker. After measurement of RNA and volume used, they calculated that the level of development was 1 CTC/10 mL blood. The sensors used were extremely sensitive and specific, showing a single-nucleotide inconsistency calculation. AuNPs have a large surface area, and they are used as a support to increase signal formation by enhancing molecular binding events
[87].
The prostate-specific antigen (PSA) was spotted in prostate cancer cells and the neovasculature of many types of malicious neoplasms, such as breast cancer
[88], lung
[89], and some other tumor cells
[90]. One study reported ligand-receptor relation of RNA NPs with PSA aptamers. The 3WJ-RNA NPs were attached to PSA aptamer (PSA-RNA), indicating extraordinary tumor accumulation in the bio-distribution analysis
[77].
Mohamadi et al. fabricated a microelectrode biosensor based on the PSA mRNA and magnetic NPs-based circulating tumor cells. Another study demonstrated aptamer-based in-vivo targeting by pRNA-3WJ NPs containing the anti-prostate-specific membrane antigen (PSA) RNA aptamer. On the other hand, folate can be coupled with pRNA-3WJ NPs as an innovative strategy for improving nanocarrier distribution.
The pRNA-3WJ-folate conjugates have been used in many cancer cells in which FR is overexpressed, such as colorectal, gastric, head and neck cancers, and glioblastoma. The in-vivo distribution of RNA NPs was studied using pRNA-X NPs containing fluorophore or folate. These NPs were injected into athymic mice with KB cells xenografts. Whole-body images were taken at different time intervals and indicated the high accumulation of RNA NPs in cancer cells within 4 h. Specific localization of pRNA-X NPs in the tumor was calculated at the 8th hour of organ imaging, without accumulating in healthy organs
[69].
2.4.4. Liver Cancer
Cationic lipids (CLs) are used for liposome-oriented DNA/miRNA transport. These lipids are made up of a linker to which a hydrophobic part is linked to a cationic head part. The positively charged head group is attached to the negatively charged phosphate group of nucleic acids
[91]. Liposomes have advantages of less risk of immunological reaction, less toxicity, and are easy to handle, making them a useful tool for non-viral drug delivery and diagnostics. Lipid NPs containing lactosylated gramicidin were exploited to transfer anti-mir-155 to hepatocellular carcinoma. Hsu et al. transferred miR-122 mimic using cationic lipid NPs to suppress miR-122 in hepatocellular carcinoma
[92].
2.4.5. Gastric Cancer
Chen et al. developed an MRI-visible system based on superparamagnetic iron oxide NPs (PEG-g-PEI-SPION) and polyethylenimine conjugated to polyethylene glycol (PEG) for the delivery of siRNA to gastric cancer. Despite its use in cancer gene down-regulation, this PEG-g-PEI-SPION has verified itself as an extremely effective contrast agent for in-vivo MRI scanning as well. In the same way, Sun et al. designed Micro-RNA-16-loaded magnetic NPs to solve drug resistance challenges in the mouse gastric cancer model. Polyethylene glycol (PEG)-coated iron oxide (Fe
3O
4) NPs were used in this study. These magnetic NPs showed highly efficient in-vivo imaging along with increased (human gastric cancer cell line 7901) SGC7901 sensitivity to the drug Adriamycin
[93].
Rychahou et al. developed the precise delivery of folate-linked pRNA NPs into colorectal cancer. After IV injection, the RNA NPs showed accumulation in metastatic cells of colorectal cancer, lung, liver, and lymph node cancer, but no accumulation was found inside healthy organs
[69].
3. RNA-Nanomaterials for Targeted Therapy of Different Cancers
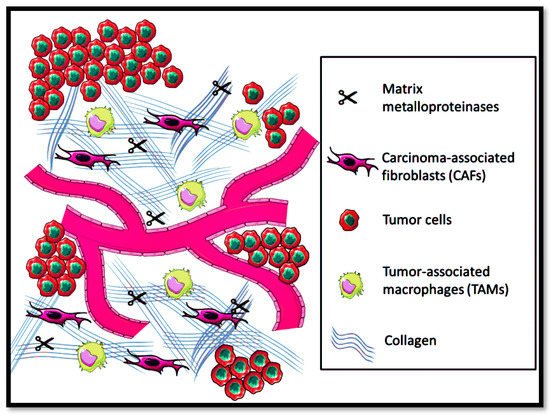
Extracellular matrix (ECM) is composed of glycoproteins, elastin, collagen, and hyaluronic for providing solid structural support for cellular processes, i.e., proliferation, cell migration, and growth
[94]. Moreover, ECM also systematizes the intracellular communication of cytokines and growth factors and acts as a source of physical barrier against the tumor microenvironment
[95]. However, in solid metastatic tumors, the ECM equilibrium in maintaining homeostasis gets affected, leading to disorganization of the physicochemical and biochemical features, as shown in
Figure 3.
Figure 3. Tumor microenvironment prevalence in the extracellular matrix.
Conventional cancer treatment has failed to mitigate the impact of malignancies
[96]. Therefore, multi-functionalized NPs were preferred to be synthesized for targeted action via attachment of specified ligands to target the tissues that are highly over-expressed in certain diseases. The development of targeted and personalized therapeutics based on RNA interference has also been made possible due to advances in molecular genetics
[97]. The modification in RNA therapies utilized small RNA subunits in the form of small interfering RNAs (siRNA) for overexpressing the specific genes of the related cancers
[98]. The RNAi technology can be exploited to change the oncogenic characteristics of breast cancer cells, making them highly conducive to apoptosis cell death. Combination therapies are useful approaches to generating apoptotic effects mediated via carcinogenic pathways and help to overcome drug resistance
[99].
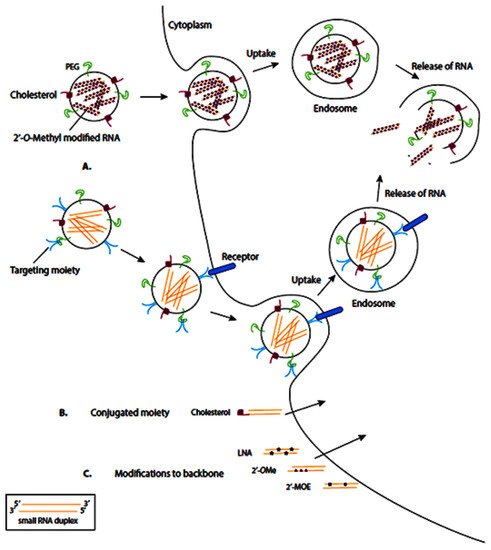
RNA-based drugs are often entrapped or attached to the surface of different nanovehicles to deliver the cargo to the cells. These nanovehicles can be modified with various moieties, including polyethylene glycol (PEG) and cholesterol, which enhance them by the membrane of target cells, where the nanovehicle can enter the target cells via endocytosis (
Figure 4A). Meanwhile, some RNA-based therapeutics are conjugated to moieties directly, which facilitates their transmembrane transport (
Figure 4B). In another therapeutic approach, the synthesized RNA-therapeutics are chemically modified to enhance their binding affinity, stability, and biocompatibility (
Figure 4C).
Figure 4. Schematic representation of common delivery methods for RNA-based therapies. LNA: locked nucleic acid (2′4′-methylene; 2′OMe: 2′-O-methyl; 2′MOE: 2′-O-methoxyethyl. Nanocarriers can enter the target cells via endocytosis (
A), direct conjugation to moieties (
B), and chemical modification (
C). Reprinted from
[100].
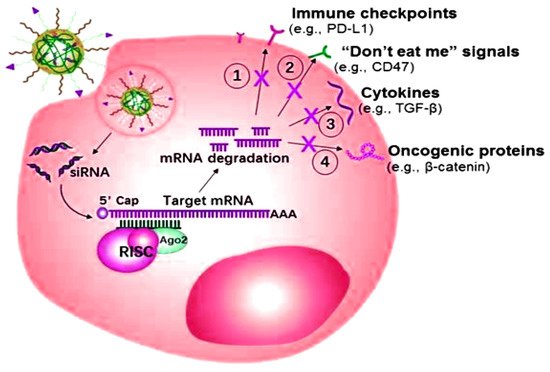
The miRNA or siRNA possesses the capability to bind with the enzyme-containing molecule RNA-induced silencing complex (RISC), resulting in the enzymatic cleavage of the target mRNA
[101]. Targeted mRNA has the capability of silencing over-expressed genes as well as inducing programmed death-ligand 1 (PD-L1) efficacy towards programmed apoptosis against the deadliest cancer cells, as shown in
Figure 5. The targeted role of mRNA as a ligand in various cancers is shown in
Table 2.
Figure 5. Small interfering RNAs (siRNA) mechanism of action for overexpressing the specific genes of the related cancers.
Table 2. Summary of RNA-based nanostructures in treatment of cancers.
| Nanostructure |
Key Feature |
Ref |
| Ultra-thermostable RNA NPs |
RNA ligand proved to dramatically inhibit the growth of breast cancer with non-detectable toxicity and immune responses in mice. |
[102] |
| Selenium-siRNA NPs |
Small interfering RNA (siRNA) showed great potential in advanced therapeutics because of its highly sequential ability for silencing HeLa genes for cervical cancer |
[103] |
| MSN-anti-miR-155 NPs |
miR-155 was highly over-expressed in colorectal tissues and cell lines as compared to the control groups and showed enhanced therapeutic efficacy. |
[104] |
| Survivin-siRNA NPs |
The novel nanocarrier system was able to initiate a specified and safe cellular uptake with increased transfection efficacy, promoting the downregulation of HCC cells. |
[105] |
| Enveloped siRNA NPs |
siRNA multi-functionalized nano-enveloped carriers can strongly silence target genes expressions as well as strongly pre-dominant genes, such as prohibitin 1 (PHB1), resulting in significantly culminating prostate tumor growth |
[106] |
| FA-PEI-Fe | 3 | O | 4 | -siRNA NPs |
Effective targeted PD-L1-knockdown therapy as well as a diagnosis in gastric cancers, thus favoring towards the best theranostic approach |
[107] |
| PLL-siRNA-MSN NPs |
MSNPs-PLL proved to be an accomplished candidate for non-invasive transdermal drug delivery in alleviating skin cancer cells division |
[108] |
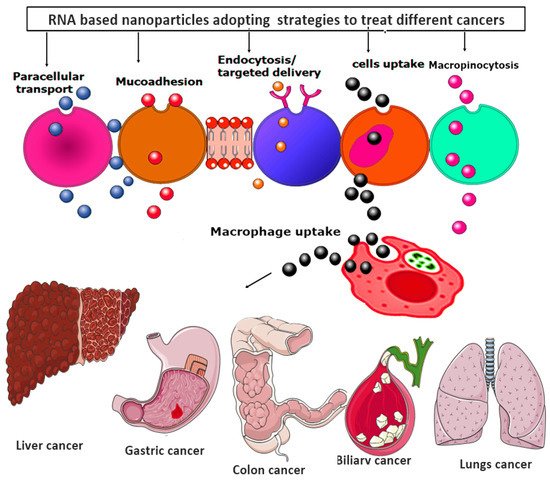
Moreover, all the RNA-based nanoparticles tend to adopt various advanced strategies to provide the targeted and efficacious treatment against various metastatic cancer, as shown in
Figure 6.
Figure 6. RNA-based nanoparticles various advanced strategies for providing the targeted and efficacious treatment against various metastatic cancers.
3.1. RNA NPs
Cancer of the breast is the deadliest disease in the world
[109]. Breast cancer therapy includes radiation therapy, chemotherapy, adjunct therapy (radiation therapy and chemotherapy), endocrine, and ligand-mediated therapy
[110]. Guo et al. (2020)
[102] conjugated PTX with RNA via the synthesizing prodrug for PTX as PTX-N3. PTX-N3 prodrug was synthesized by admixing the PTX, and other substituents in a 20 mL DCM solvent. The reaction mixture was then stirred at room temperature for 36 h following filtration and rotary evaporation to attain the yield’s crude product. The crude product was then purified by silica gel chromatography using n-Hexane: ethyl acetate as an eluent. RNA-6 alkynes oligomers were synthesized via a stranded solid-phase RNA synthesis and purified through desalting. RNA sequences of nine types were isolated and conjugated to PTX using copper (I)-catalyzed alkyne-azide cycloaddition by click addition. The reaction mixture was then diluted with ethanol followed by overnight incubation for RNA precipitation in DEPC-H
2O. The precipitated were re-dissolved and purified by the ion-pair reverse phase HPLC in a PTX-labeled RNA for assembling NPs. Finally, an RNA four-way junction NP (4WJ-X nanostructure) with ultra-thermodynamic stability to solubilize and load PTX for targeted cancer therapy was developed by a 3D computational model generated using Swiss PDB Viewer and PyMOL Molecular Graphics System. Assembly of NPs was confirmed using a specified buffer solution. EGFR NPs were also conjugated with one of the aptameric RNA oligomers and 4WJ-X-24 PTXs. It was observed that each RNA NPs was found to be successfully attached via covalent interaction to twenty-four molecules of PTX as a prodrug. The developed RNA-PTX complex was found to be structurally stable and rigid. It was concluded that RNA NPs proved to help increase the water solubility of the BCS class II drug PTX by 32,000-fold, which possesses the issue of low water solubility. This treatment strategy of RNA functionalization in the form of intravenous injections resulted in the specified cancer targeting. RNA ligand proved to dramatically inhibit the growth of breast cancer with non-detectable toxicity and immune responses in mice. Moreover, no mortalities were observed at the LD
50 dose of PTX
[102].
3.2. Nanotechnology for Transfer of Therapeutic RNAs
By using nanotechnology for RNA, we are able to overcome many shortcomings of naked RNA molecules, such as poor chemical stability, easy degradation by nucleases, and extremely short half-lives for in-vivo applications. NPs act as a multipurpose and targeted system for the safe transfer of naked RNA molecules. The NP-based protect RNA from enzymatic cleavage and immune system threats. Because of their EPR properties, these nanostructures facilitate excellent RNA accumulation at the tumor site. Nowadays, the nanocarriers used for RNA delivery are lipid-based nanosystems
[111], polymeric nanomaterials, bio-inspired nanovesicles, and inorganic NPs
[112].
RNA NPs can easily include targeting ligands, counting RNA aptamers, and chemical ligands to impart specific targeting against proteins, fluorescent chemicals, and cell surface receptors. Chemical ligands, e.g., folate, are attached to the end of the RNA strand while the RNA is synthesized by modified RNA phosphoamidites. These ligands can also be attached to the RNA strand after RNA synthesis by the chemical conjugation method. The RNA aptamer strand, such as EGFR aptamer, can be manufactured by elongation of the scaffold strand.
The binding efficiency in-vitro and in-vivo of RNA NPs with ligands is tested by labeling them with radioisotopes and fluorescent dyes
[113]. The labeled RNA NPs are incubated with cells for in-vitro binding and internalization studies. After incubation, these NPs are quantified by flow cytometry or fluorescence confocal microscopy
[77]. Folic acid, FA, has attracted much attention for targeted siRNA delivery due to its small size, outstanding in-vivo stability, low immunogenicity, high binding affinity for folate receptors FRs, and great specificity to cancer cells. Folate receptors play an important role in diagnosing and therapeutics of inflammatory diseases and carcinoma. Folate receptors are overexpressed in cancer cells, and folic acid-decorated siRNA transporters bind precisely to FRs. The site-specific distribution of FAsiRNA conjugates and enhances siRNA concentration at the target site
[112,114][112][114].
Folate receptors are overexpressed in epithelial cancer cells surfaces, permitting the folic acid conjugated NPs to target these cancer cells at a frequency higher than the normal cells.
RNA NPs can efficiently target cancer metastasis that is difficult to target because of the spread of cancerous cells to far-off cells and lymph nodes. Folic acid was used as a targeting agent by RNA NPs to simultaneously target colon cancer cells in the chief sites of metastasis, such as lungs, liver, and lymph nodes
[49]. The 3WJ-based design procedure was used to form highly branched RNA dendrimers. RNA dendrimers used pRNA nanosquare as a symmetrical core for their formation. The formation of higher-ordered structures may face steric hindrance that is reduced by the square shape. Targeted delivery of NPs highly lessens the off-target toxicity and accumulation of NPs in healthy organs. RNA aptamers are an emergent field of therapeutics in which single-stranded RNA sequences form 3D structures by folding up and binding to extracellular domains of cell surface receptors with high selectivity and affinity. Nowadays, cell surface receptors are targeted by tens of RNA aptamers, such as prostate cancer (e.g., PSA), colon cancer (e.g., EpCAM), ovarian cancer (e.g., E-selection), glioblastoma (e.g., EGFRvIII), lymphoma (e.g., CD19) and breast cancer (e.g., EGFR, HER2, HER3)
[49].
3.3. Small Interfering RNA-Selenium NPs
Small interfering RNA (siRNA) showed great potential in advanced therapeutics because of its highly sequential ability for silencing genes
[115]. One of the most common causes of death in women is cervical cancer
[116]. As a result, the right medication is necessary to minimize the severity of this cancer. For this purpose, Yu Xia synthesized biocompatible selenium NPs (SeNPs) and loaded them with the arginyl glycyl aspartic acid (RGD) Fc peptide for the sake of active targeting
[103]. The RGDfC peptide bore a rich cationic charge and functionalized SeNPs for enhanced gene delivery. RGDfC-SeNPs have the capability of binding with HeLa cervical cancer lines.
Furthermore, Derlin 1-siRNA can be adjunct to the formulated conjugate of RGDfC-SeNPs via electrostatic interaction. The RGDfC-Se@siRNA successful conjugation and synthesis was confirmed via size determination by a zeta sizer, transmission electron microscopy (TEM), and Fourier transform infrared spectroscopy (FTIR). Elemental compositions of RGDfC-SeNPs were studied by energy dispersive spectroscopy (EDS). RGDfC-Se@siRNA characterization results proved that it followed Clathrin-mediated endocytosis for specifically reaching HeLa cancer cell lines and exhibited triggered siRNA release in a tumor microenvironment as compared to the biological microenvironment. However, in qPCR and Western blotting assays, both techniques showed eminent chances of gene silencing in HeLa cells. RGDfC-Se@siRNA was found to suppress the tumor invasion and division in HeLa cells via triggering the apoptosis pathway. Moreover, another crucial mechanistic approach of mitochondrial membrane disruption and reactive oxygen species generation (ROS) in HeLa cells was quite convincing in understanding that mitochondrial dysfunction mediated by ROS might play a significant role in RGDfC-Se@siRNA-induced apoptosis. Interestingly, advanced nanotherapeutics also presented substantial antitumor activity in a HeLa tumor-bearing mouse model
[103].
3.4. siRNA-Polymeric NPs
Prostate cancer malignancy is one of the significant reasons for mortality worldwide. Prostate cancer is a non-skin malignancy causing the second biggest number of deaths in men when differentiated with different tumors. Conventional therapies are expected to cause erectile dysfunction, libido, obesity, and bone mass loss. Nanotechnology has modernized the field of medication to sidestep conventional therapies against metastatic cancers and different intracellular infections
[117]. Xiao ding Xu et al. (2017)
[106] synthesized siRNA multi-functionalized enveloped NPs for prostate cancer advanced therapy. SiRNA was self-assembled in the NPs via utilizing the library of oligo arginine and sharp pH-responsive polymers. Moreover, siRNA-functionalized and self-assembled NPs resulted in prolonged blood circulation, and the pH triggered a drug release through the activation of the endosomal membrane penetration. Furthermore, modification of the synthesized nanocarrier was done via attaching a specified molecular ligand that can recognize the PSA receptor. Synthesized nano-enveloped particles were characterized for size and zeta potential via DLS. The morphology of NPs was determined by TEM, fluorescence intensity, encapsulation efficiency, in-vitro siRNA release, luciferase silencing, endosomal escape, flow cytometry, in-vitro PHB1 silencing, digestion assay, Western blot, immunofluorescence staining, in-vitro inhibition of cell proliferation, a xenograft tumor model, and pharmacokinetic studies. siRNA multi-functionalized nano-enveloped carriers have the capability to strongly silence target genes expressions as well as strongly pre-dominant genes, such as prohibitin 1 (PHB1), resulting in significantly culminating tumor growth. Moreover, these advanced NPs also possess great potential for a robust siRNA delivery vehicle for prostate cancer-targeted therapy
[106].
3.5. siRNA-Superparamagnetic Iron Oxide NPs
Globally, death from gastric cancer accounts for the majority of deaths. Conventional treatments have not been able to alleviate the consequences of this deadly disease. The development of targeted therapies for gastric cancer requires new technologies
[118]. The most novel approach being considered is finding the primary targets, such as immune checkpoints such as PD-L1, in gastric cancer
[119]. PD-L1 presents an over-expression on the activated T cells, resulting in the endosomal escape by cancer cells. The mechanism of inhibiting cancer cell regulation via PD-L1 cells lies in promoting and maintaining the T-cell responses in a controlled manner
[120]. Xin Luo promoted siRNA delivery system for knocking down PD-L1 by developing folic acid (FA) and disulfide (SS)-polyethylene glycol (PEG)-conjugated polyethylenimine (PEI), complexed with superparamagnetic iron oxide Fe
3O
4 NPs (SPIONs)
[107]. SPIONs were encapsulated with FA-PEG-SS-PEI via a ligand-exchange method, and then this conjugate was combined with synthesized siRNA-complexed cationic micelles. Furthermore, synthesized NPs were characterized based on binding capability, cytotoxicity, cellular internalization, and transfection efficacy. Cell viability assays demonstrated negligible toxicity and maximum cellular uptake as well. Cellular magnetic resonance imaging (MRI) presented that the NPs depicted maximum contrast for the T2 weight for cancer MRI. Furthermore, PD-L1 siRNAs displayed nominal knockdown of PD-L1 in the PD-L1-overexpressing gene. However, the co-culture model of activated T cells and the over-expressed gene cells represented an increased level of secreted cytokines. Therefore, these findings highlight the potential of this class of multi-functionalized polyplexed NPs for effective targeted PD-L1-knockdown therapy and diagnosis in gastric cancers, thus favoring the best theranostic approach
[107].
Hepatocellular carcinoma (HCC) is the most common malignancy of the liver and the most common cause of morbidity and mortality
[121,122][121][122]. A few molecular targeting drugs, such as sorafenib (SO), have been approved for advanced HCC, which also show a peripheral survival chance compared to conventional therapeutics. Unfortunately, its efficacy for HCC patients stayed substandard. Thus, the development of new methods for diagnosing and managing HCC is of the utmost importance. Gene delivery is the most preferred therapy involving RNA interference for the purpose of post-transcriptional gene silencing. Zhuo Wu et al. (2017)
[105] developed a new class of amylose NPs, where the cationic amylose was used as the backbone functionalized with folate for targeting. Furthermore, SPIONs were utilized for the purpose of imaging and diagnosis for delivering specified surviving siRNA to hepatocellular carcinoma cells. The synthesized siRNA and multi-functionalized NPs were characterized based on zeta sizing, NMR, FTIR, cytotoxicity studies, cellular uptake, gene silencing, apoptosis signaling, and magnetic resonance imaging (MRI), and the results of these characterization techniques assured the successful conjugation of a new class of amylose NPs for the targeted delivery to HCC cells. Moreover, the novel nanocarrier system was able to initiate a specified and safe cellular uptake with increased transfection efficacy, promoting the downregulation of HCC cells. The resulting conjugate biocompatible complex based on cationic amylose could be used as a well-organized, prompt, and innocuous gene delivery vector. Furthermore, upon SPION addition, it holds great potential as a theranostic carrier for the gene therapy of HCC
[105].
3.6. RNA-Mesoporous Silica NPs
Timely diagnosis and therapy are the prime responsibility of the healthcare system
[123]. Several advancements in molecular genetics have allowed pathogenic mutations in diagnosing and classifying unique skin infections, such as psoriasis, atopic dermatitis, and skin cancer
[124]. Advancements in molecular genetics also allowed the development of targeted and personalized therapeutics based on RNA interference
[97]. Modification in RNA therapies utilized small RNA subunits in the form of small interfering RNAs (siRNA) for overexpressing the specific genes of the related disorders
[98]. Therefore, Daniel Chin Shiuan Lio et al. developed mesoporous silica NPs nucleotide complexes of 200 nm with a pore size of 4 nm and mixed it with oligonucleotides of RNA, followed by overnight stirring. After stirring, the mesoporous silica NPs-oligonucleotide conjugate were coated with poly-L-lysine (PLL) in 1:1 for 10 min
[108]. The excessive amount of PLL was removed via centrifugation at the speed of 10,000 rpm for 15 min. Excessive PLL-removed NPs were again re-suspended in the phosphate-buffered saline (PBS). Characterization of the PLL-coated nanocarriers was done via size analysis, labeling cells with MSNPs for the time-course study, and post-treatment in-vivo studies with a xenograft tumor model, primer sequence, real-time-polymerase chain reaction (RT-PCR), histological sectioning, Western blot, and flow cytometry. Results concluded that the loading of NPs-based oligonucleotides by poly-L-lysine resulted in improved transdermal drug delivery, increased zeta potential, and enhanced stability
[125]. The MSNPs-PLL evaluation on skin squamous cell carcinoma (SCC) cells in-vitro showed a safety profile and increased penetration. Therefore, we are bound to believe that MSNPs-PLL proved to be accomplished candidates for non-invasive transdermal drug delivery in alleviating the skin cancer cells division
[108].
Mesoporous silica NPs (MSNs) have been considered the most promising nanocarriers for attached targeted moieties owing to unique features of tunable pore structure, greater surface area, and pore volume
[126,127,128][126][127][128]. These flexible features of the MSNs are accountable for successful conjugation, thermal stability, and biocompatibility. Colorectal cancer is a heterogeneous and lethal disease, proceeding towards the development of malignant tumors in the inner walls of the colon and rectum in the form of polyps. MicroRNA-155 (miR-155) is an oncogenic microRNA, and is over-expressed in many cancers, including colorectal cancer (CRC). Therefore, targeting the approach in treating cancers by using miR-155 is a nominal strategy for treating cancer. Therefore, Yang Li (2018)
[104] developed anti-miR-155-loaded MSNs functionalized with polymeric dopamine (PDA) and AS1411 aptameric (MSNs-anti-miR-155@PDA-Apt) for the targeted therapy of CRC. The prepared NPs were characterized based on size, cell uptake studies, in-vitro cytotoxicity, xenograft tumor model, in-vivo imaging and biodistribution, in-vivo antitumor efficacy, and systemic toxicity. The results showed that miR-155 was highly over-expressed in CRC tissues, resulting in a significantly high targeted therapy and enhanced therapeutic efficacy
[104].