Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Hui Sun and Version 2 by Dean Liu.
Polymeric nanomaterials have been widely studied as carriers for constructing antimicrobial agent delivery systems and have shown advantages including high biocompatibility, sustained release, targeting and improved bioavailability.
- antimicrobial agent
- polymeric nanomaterial
- self-assembly
- antimicrobial peptide
1. Combating MDR Bacteria
The generation of drug resistance of pathogens is typically caused by the accumulation of drug resistant genes through mutation with the long-term use, especially overuse and improper use of antibiotics [1][25]. Therefore, the exploration of highly efficient delivery system to reduce dosage and improve bioavailability of antibiotics, as well as the delivery of non-antibiotic antimicrobial agents including AMPs, AgNPs, metal oxides, gases and so forth, is a promising strategy for reducing drug resistance [2][3][134,135]. Polymeric nanomaterial-based antimicrobial agent delivery systems have widely been used in combating MDR bacteria [4][5][136,137]. For instance, Liu et al. [6][138] conjugated quercetin and acetylcholine on the surface of selenium nanoparticles to combat MDR bacteria, which could effectively eliminate MRSA by destroying the membrane due to the synergy between quercetin, acetylcholine and selenium nanoparticles. Cationic polymeric star-shaped nanoparticles or dendrimers have also shown excellent antimicrobial activity against MDR bacteria even without loading antimicrobial agents [7][8][9][139,140,141], demonstrating the great potentials of polymeric nanomaterials in combating MDR bacteria.
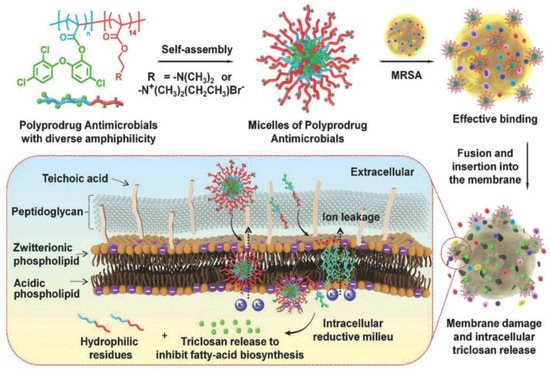
Hu et al. [10][142] prepared polyprodrug antimicrobials to combat MRSA by membrane damage and concurrent drug release, as shown in Figure 19. Triclosan was covalently linked with acrylic acid to produce a triclosan prodrug monomer (TMA). Then, TMA was copolymerized with quaternized N,N-dimethylaminoethyl methacrylate (QDMA), affording PQDMA-b-PTMA, which could self-assemble into prodrug micelles with positively charged surfaces. The hydrophilic–hydrophobic balance of the prodrug micelles was optimized to enhance interaction with bacterial cell membranes, resulting in improved antimicrobial activity. They proposed that the antimicrobial mechanism was as follows: (1) the prodrug micelles attached onto the surface of MRSA due to strong electrostatic interaction; (2) the prodrug micelles fused with and inserted into the cell membrane of MRSA; (3) the cell membrane of MRSA was damaged due to charge disorder, and prodrug micelles were encapsulated into the cell; (4) prodrug micelles were disassembled, and the linkage between triclosan and acrylic acid was broken due to the reductive milieu environment, resulting in the in situ release of triclosan and death of MRSA. It was noteworthy that no detectable resistance was observed due to the synergistic antibacterial mechanism, and prodrug micelles exhibited remarkable bacterial inhibition and low hemolysis toward red blood cells compared with commercial triclosan and vancomycin.
The combination of different classes of antimicrobial agents such as antimicrobials and AgNPs could afford synergistic effects, resulting in the efficient inhibition of MDR bacteria that is far better than its individual components [11][12][143,144]. Webster and coworkers [13][145] prepared polymer vesicles to co-deliver ampicillin and AgNPs simultaneously in the hydrophilic cavity and hydrophobic membrane, respectively. The AgNPs-embedded polymersomes exhibited potent antibacterial activity against E. coli transformed with a gene for ampicillin resistance in a dose-dependent fashion, while the free ampicillin, AgNPs decorated polymersomes without ampicillin and ampicillin loaded polymersomes without AgNPs had no effect on bacterial growth. TEM images in Figure 210 revealed that the interactions between vesicles, AgNPs and bacterial cells might result in the deformation and disruption of bacterial envelopes and consequently result in the death of bacteria. Later, the same group [14][146] functionalized proline-rich AMP PR-39 on the corona of polymer vesicles with AgNPs embedded in the membrane to combat MRSA with a AMP/AgNPs ratio-dependent behavior. A ratio of AgNPs-to-AMP of 1:5.8 corresponding to 11.6 μg mL−1 of AgNPs and 14.3 × 10−6 M of AMP exhibited the best MRSA inhibition activity, demonstrating the potentials of binary or ternary antimicrobial agent co-delivery systems in combating MDR bacteria.
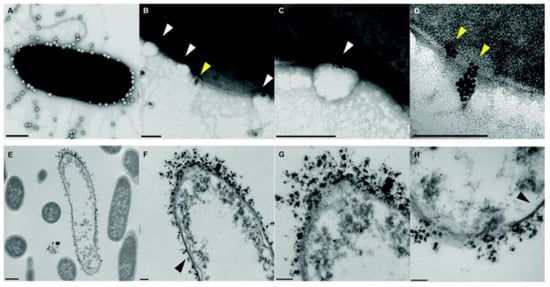
Figure 210. TEM images of bacteria-polymer vesicles interactions. Scale bars are 100 nm for (A–D,F–H) and 500 nm for (E), respectively. White arrows pointed out the indentation of bacterial cell membrane in regions of AgNPs loaded polymer vesicles; yellow arrows implied the polarization indicative of hydrophobic interactions of AgNPs inside the vesicles; black arrows revealed that the regions of the outer membrane with little AgNPs loaded polymer vesicles contact appeared morphologically normal (reproduced with permission from Geilich et al. [13][145], Nanoscale; published by Royal Society of Chemistry, 2015).
2. Anti-Biofilm
Biofilms are matrix-enclosed communities of bacteria that show increased drug resistance and capability to evade the immune system [15][47]. It has been widely recognized that bacteria exist in the form of biofilms in many instances, which is hard to eliminate due to the protection of extracellular polymeric substances (EPS), a complex matrix composed of proteins, nucleic acids, phospholipids, polysaccharides, blood components and humic substances produced by bacteria [16][147]. Therefore, it is difficult for antimicrobials to penetrate the EPS to kill bacteria, resulting in the occurrence of drug resistance. The efficient delivery of antimicrobial agents by polymeric nanomaterials is considered a promising strategy for penetrating the biofilm and delivering antimicrobial agents to the deep end of the matrix to kill pathogens [17][18][19][148,149,150]. For example, Deoxyribonuclease I functionalized ciprofloxacin-loaded PLGA nanoparticles were prepared to target and disassemble the P. aeruginosa biofilm by degrading extracellular DNA that stabilizes the biofilm matrix and released ciprofloxacin inside the biofilm to effectively eliminate P. aeruginosa, as reported by Torrents and coworkers [20][151].
Webster et al. [21][152] prepared bifunctional polymersomes with methicillin encapsulated in the hydrophilic cavity and superparamagnetic iron oxide nanoparticles (SPIONs) embedded in the membrane, as illustrated in Figure 311. The iron oxide-encapsulated polymersomes (IOPs) penetrated into the S. epidermidis biofilm with high efficiency, promoted by external magnetic field. Comparing with individual SPIONs, methicillin and SPION co-encapsulated polymersomes showed enhanced penetration capability up to 20 μm due to the improved relaxivity and magneticity (Figure 311c). Thus, methicillin could be released into the deep end of the biofilm, resulting in the effective eradication of pathogens. The confocal microscopy images and the 3D reconstructions of z-stacks of the bacterial biofilm revealed the capability of IOPs to eradicate biofilms with and without methicillin, as shown in Figure 311d. When there was no methicillin, only bacteria in the bottom layer of the biofilm were killed. On the contrary, all bacteria throughout the biofilm were eliminated by the methicillin loaded IOPs. These organic/inorganic hybrid nanocarriers showed great promise as new weapons for eradicating persistent biofilm or drug-resistant bacteria.
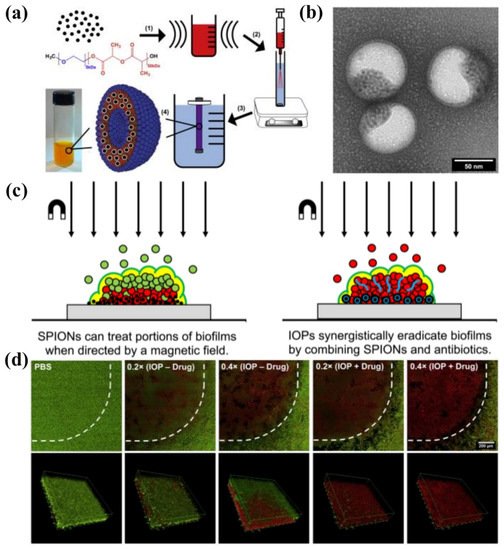
Figure 311. (a) Synthesis of IOPs loaded with SPION and methicillin. (1) 5 nm monodisperse hydrophobic SPIONs are combined with mPEG-PDLLA co-polymer in organic solvent and ultrasonicated to create a uniform suspension. (2) This organic phase is injected through an atomizer into an actively stirring aqueous phase containing PBS and methicillin. (3) The mixture is dialyzed against pure PBS to remove the organic solvent and unencapsulated drug to yield (4) highly stable polymersome solution. (b) TEM image of SPIONs loaded polymersomes. (c) Magnetic field induced treatment of biofilm using SPIONs and/or antimicrobials. (d) Confocal microscopy images of LIVE/DEAD staining of S. epidermidis biofilms treated with IOPs with an external magnetic field (reproduced with permission from Geilich et al. [21][152], Biomaterials; published by Elsevier, 2017).
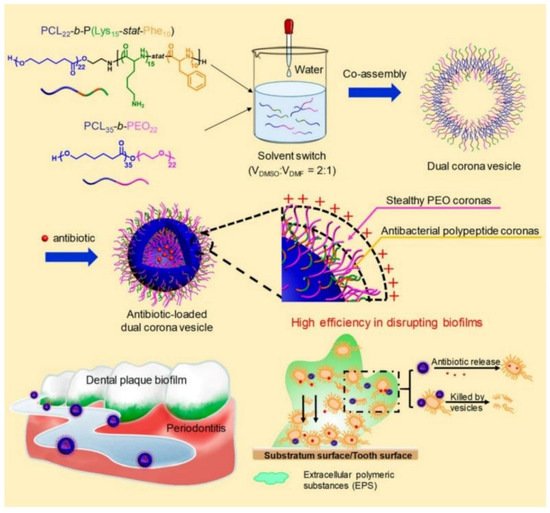
Recently, Du and coworkers [22][153] reported the treatment of periodontitis by efficiently disrupting biofilms using a dual corona antimicrobials-loaded polymer vesicle with stealthy poly(ethylene oxide) (PEO) corona to penetrate the biofilm and antibacterial polypeptide corona to provide intrinsic antimicrobial activity, as shown in Figure 412. The dual corona polymer vesicles were prepared by the co-assembly of two polymers PCL-b-poly(lysine-stat-phenylalanine) [PCL-b-P(Lys-stat-Phe)] and PEO-b-PCL with the same hydrophobic biodegradable PCL segment and different hydrophilic chains. Ciprofloxacin could be efficiently encapsulated in the cavity of the vesicles. Due to the protein-repelling ability of PEO, dual corona polymer vesicles penetrated the EPS of the biofilms with high efficiency, while the positive charged P(Lys-stat-Phe) allowed the vesicle to target and kill bacteria via electrostatic interaction. In addition, the encapsulated ciprofloxacin could be released as the polymer vesicle reached the deep end of the biofilm, resulting in a reduced dosage of the antimicrobials up to 50% to eradicate E. coli or S. aureus biofilms. In vivo experiment results demonstrated excellent performance of the dual corona vesicles in reducing dental plaque and alleviating inflammation using a rat periodontitis model.
Despite the strategy of delivering antibiotics to the deep end of biofilms by polymeric nanocarriers in order to reduce dosage and enhance antimicrobial activity, the efficient delivery of non-antibiotic antimicrobial agents including AMPs, AgNPs, photosensitizers and so forth for eliminating biofilms was also widely studied [23][24][25][154,155,156]. For instance, Haldar et al. [26][157] fabricated biodegradable polymer-coated AgNPs nanocomposite to eradicate biofilms, which reduced MRSA burden both on the catheter (>99.99% reduction) and in tissues surrounding the catheter (>99.999% reduction) in a mice model. Ji and coworkers [27][158] developed targeted photodynamic therapy strategies by using a supramolecular delivery system for the treatment of biofilms. The photosensitizer Chlorin e6 was grafted onto α-cyclodextrin, and the targeting group AMP Magainin I was covalently bound with PEG. Taking advantage of supramolecular recognition between α-cyclodextrin and PEG, targeting supramolecular micelles loaded with Chlorin e6 were formed, which exhibited excellent bacterial targeting effects and enhanced biofilm eradication ability against P. aeruginosa biofilm and MRSA biofilm. These results proved the versatility and great potential of polymeric nanomaterial-based antimicrobial agent delivery systems for eradicating biofilms.
3. Wound Healing
Wound infections induced by pathogens have become one of the main problems in wound care management systems, which impede the healing process and may result in life threatening complications. One of the approaches for treating wound infection is the use of wound dressings with antibacterial agents possessing broad-spectrum antimicrobial activity [28][159]. Typically, the moisture environment provided by the dressing has been shown to promote ulcer healing and to reduce pain experienced by patients [29][160]. Moreover, there are other requirements for wound dressings such as separating the wound with external environments and providing good breathability to promote wound healing. Polymeric nanomaterial-based delivery systems have shown considerable potentials in wound healing, especially polymer nanofibers and hydrogels [30][31][99,161]. For example, Lakshminarayanan et al. [32][162] prepared polydopamine crosslinked polyhydroxy antimicrobials loaded gelatin nanofiber mats for advanced wound dressings with long-term antimicrobial activity up to 20 days. The morphology of the nanofiber mats was retained for 1 month in an aqueous environment and showed comparable wound closure compared to commercially available silver-based dressings. Cai and coworkers [33][163] prepared composite hydrogels embedded with copper nanoparticles that could effectively convert NIR laser irradiation energy into localized heat for photothermal therapy. The synergistic effect of photothermal performance and rapid release of copper ions upon laser irradiation were responsible for excellent antimicrobial activity, reduced inflammatory response and promoted angiogenesis ability.
Antimicrobial agents including AMPs [34][49], antibiotics [35][164], AgNPs [36][165], metal oxide such as ZnO [37][166], photothermal sensitizers including porphyrin [38][167] and heavy metal ions [33][163] are usually used to improve the antimicrobial activity of polymeric wound dressings by covalent linkage, physical interaction or encapsulation. For example, Liu et al. [39][168] decorated chloramine on the surface of chitosan films by electrostatic interaction to heal MRSA infected wounds. Zhou and coworkers [38][167] prepared porphyrin containing alternating copolymer vesicles for the disinfection of drug-resistant bacteria infected wounds via photothermal effect. Fahimirad et al. [40][169] loaded recombinant LL37 AMP into chitosan nanoparticles for the elimination of MRSA infection during wound healing process with ultrahigh encapsulation efficiency of 78.52% and improved the activity and stability of LL37 AMP under thermal, salts and acidic pH treatments. Guo et al. [41][170] prepared injectable antimicrobial conductive quaternized chitosan hydrogels by loading graphene oxide via covalent bond for drug resistant bacterial disinfection and infectious wound healing, and the hybrid hydrogels showed excellent performance in the treatment of MRSA infected full-thickness defect mouse model.
Very recently, polymer vesicles loaded with antimicrobials have been explored as dressings in promoting wound healing by spraying onto wounds [38][42][43][44][167,171,172,173]. Du and coworkers [44][173] reported bifunctional polymer vesicles loaded with antimicrobials and antioxidant for healing infected diabetic wounds, as presented in Figure 513. As one of the chronically infected wounds, the diabetic wounds are difficult to heal due to high ROS concentration and recurrent infections, resulting in the occurrence of diabetic ulcers and chronic diabetic complications with very high mortality rate. Therefore, scavenging ROS is very important in the treatment of diabetic wounds. In this study, well-dispersed ceria nanoparticles were deposited on the membrane of ciprofloxacin-loaded polymer vesicles (CIP-Ceria-PVs). The CIP-Ceria-PVs could inhibit peroxide free radicals up to 50% at extremely low cerium concentrations of 1.25 μg mL−1, protecting normal L02 cells from the damage of peroxide free radicals. Moreover, CIP-Ceria-PVs exhibited enhanced antimicrobial activity compared with free ciprofloxacin due to scavenging ROS. In vivo studies in Figure 513b demonstrated the excellent wound healing capability of CIP-Ceria-PVs, and the diabetic wound was completely healed within 14 days. At the same time, they developed a H2S delivery polymer vesicle, which was capable of long-term H2S generation to promote the proliferation, migration of epidermal and endothelial cells and angiogenesis, accelerating the complete healing of diabetic wounds [43][172].
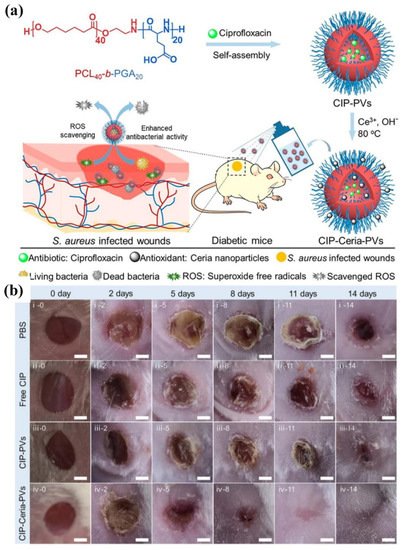
Figure 513. (a) Illustration of the preparation of CIP-Ceria-PVs and the combined antioxidant-antimicrobials treatment of infected diabetic wounds. (b) Digital images of infected diabetic wounds at different time intervals under treatment. Scale bar: 2 cm (reproduced with permission from Wang et al. [44][173], ACS Nano; published by American Chemical Society, 2021).
4. Tissue Engineering
The regeneration of adult tissue following an injury or degeneration is quite a limited process. Usually, the injury site is vulnerable to bacterial infections, which causes complications and delay of the regeneration of tissues [45][174]. Therefore, the prerequisite of tissue regeneration is to eliminate localized bacterial infections, followed by the delivery of bioactive molecules such as growth factor to the defected tissues. Antimicrobial polymer coatings on the surface of implants can provide appropriate biointerfaces to promote the regeneration of tissues. For instance, ZnO nanoparticles embedded PLA was dip coated on magnesium alloy, which helped to control the degradation and increase antibacterial activity [46][175]. Suteewong et al. [47][176] deposited polymethylmethacrylate (PMMA)/chitosan-silver hybrid nanoparticles on rubber substrate, which exhibited enhanced antibacterial activity toward E. coli and S. aureus and reduced cytotoxicity to L-929 fibroblast cells, demonstrating the potential of this hybrid nanoparticle coating at soft substrates. In addition, antimicrobial agents loaded with polymer nanomaterials can be used as bioadhesives to repair damaged soft tissues. Gu and coworkers [48][177] developed fast and high strength bioadhesives based on polysaccharides and peptide dendrimers with inherent hemostatic ability and antibacterial properties. Moreover, the bioadhesive showed a remarkable 5-fold increase in adhesion strength comparing with commercial bioadhesive Coseal.
Biocompatible polymeric nanoparticles have been investigated as delivery vehicles for various tissue engineering applications [49][178]. For example, Du and coworkers [50][179] prepared antibacterial peptide-mimetic alternating copolymers (PMACs) vesicles loaded with growth factor for bone regeneration. They designed a series of PMACs with different repeating units, and the PMAC with a repeating unit of 14 exhibited the best antibacterial activity against both E. coli and S. aureus with ultralow MICs of 8.0 μg mL−1. After self-assembling into vesicles in pure water, the antimicrobial activity of the vesicles was well-preserved. Growth factor could be encapsulated in antimicrobial vesicles and released during the long-term antibacterial process to promote the regeneration of bone with a 20 mm defect model in rabbits. Micro-CT, bone mineral content and BMD were used to evaluate the repair of bone defects with scaffolds at 4 weeks and 6 weeks after implantation. After 6 weeks, the defect in the rabbit bone was completely repaired, demonstrating the excellent bone repair capability of antimicrobial growth factor-loaded vesicles.
5. Anticancer
The anticancer application of antimicrobial agents is an attracting field since cancers are often accompanied by inflammation, and the drug resistance of cancer cells is becoming increasingly concerning [51][180]. Theoretically, antimicrobial agents that kill bacteria via non-selective behaviors such as damage of the cell membrane [52][181], elevating temperature [53][182] and induced degeneration of proteins and genetic materials [54][183] can also kill cancer cells. For instance, Shim et al. [54][183] prepared AgNPs loaded chitosan-alginate composite, exhibiting broad-spectrum antimicrobial activity and high toxicity toward breast cancer cell line MDA-MB-231; Jothivenkatachalam and coworkers [55][184] fabricated chitosan-copper nanocomposite for the inhibition of various microorganisms and A549 cancer cells by photocatalytic effect. In addition, AMPs with specific sequences and proper positive charge densities have shown anticancer and antiviral activities, such as cecropin A and B, magainins, melittin, defensins, lactoferricin and so forth, as summarized by Hoskin’s and Franco’s group, respectively [52][56][181,185]. However, the AMPs are vulnerable to enzymes and can easily cause immune responses; thus, the delivery system is critical for in vivo applications of AMPs. Hazekawa et al. [57][186] conjugated antimicrobial human peptide, LL-37 peptide fragment analog, with a PLGA copolymer. The formed micellar system significantly improved the permeability of the peptide to cancer cells, and the proliferation, migration and invasion in various cancer cell lines were effectively exhibited. The intracellular delivery of peptides by polymer carriers in oncology applications has been summarized by Pun et al. very recently [58][187].
Another strategy for eliminating cancer cells using antimicrobial delivery systems is the co-delivery of antimicrobial and anticancer agents simultaneously or loading anticancer drugs with antimicrobial carriers [59][60][188,189]. For instance, Du and coworkers [61][190] proposed the concept of “armed” carrier to co-deliver anticancer and antiepileptic drugs with antibacterial polypeptide-grafted chitosan-based nanocapsules. Mahkam et al. [62][191] designed pH-responsive antibacterial clay/polymer nanocomposite as a carrier to deliver anticancer drug methotrexate and antibacterial agent ciprofloxacin with an ultrahigh efficiency of >90%, which showed enhanced antimicrobial and anticancer activity compared with free methotrexate and ciprofloxacin, demonstrating the potential of antibacterial nanocarriers in cancer therapy. Lei and coworkers [63][192] developed a class of multifunctional polymeric hybrid micelles (PHM) with high antibacterial activity for the efficient delivery of siRNA to cancer cells, as illustrated in Figure 614. The PHM was prepared by the co-assembly of EHP-FA and EHE, for which their structures were presented in Figure 614A. Due to the existence of positively charged poly(ethylene imine) (PEI) and poly-ε-l-lysine (EPL), the PHM showed high antibacterial activity against S. aureus in vitro and in vivo. On the contrary, PHM exhibited good hemocompatibility and lower cytotoxicity toward A549, HeLa, HepG2 and C2C12 cells benefiting from the shield effect of PEG. siRNA could be complexed onto PHM by electrostatic interaction, and PHM with folic acid decorated on the surface could effectively target FA receptor overexpressed HeLa cells and other low-expressed cancer cells, resulting in the targeted delivery of siRNA. In vitro experiments revealed that the PHM showed a high p65 gene silencing efficiency above 90% in various cancer cells, which is significantly higher than EHP-FA and EHE, demonstrating the potential of PHM as a safe and effective siRNA vector with high antibacterial activity for multifunctional gene therapy.
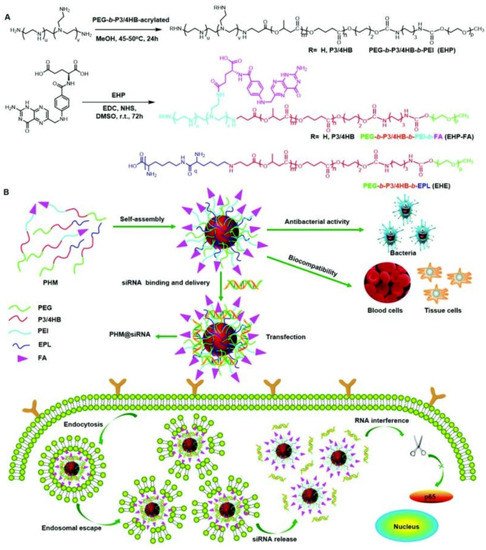
Figure 614. Scheme showing the synthesis and potential application of the PHM copolymer in siRNA delivery. (A) EHP and EHP-FA were synthesized by Michael addition and esterification reaction, respectively; PHM micelles were prepared by mixing EHP-FA and EHE copolymer; (B) schematic illustration of the application of PHM in siRNA delivery (reproduced with permission from Zhou et al. [63][192], Nanoscale; published by Royal Society of Chemistry, 2018).
