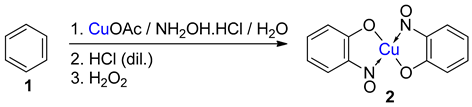
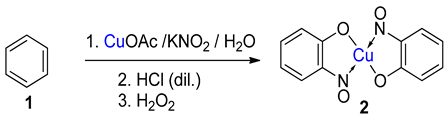
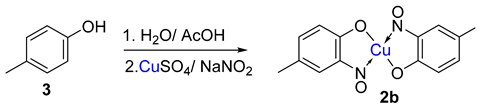
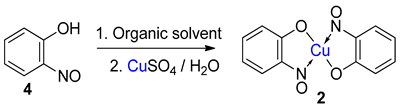
TMetal-nitrosophe syntheses of the title compoundsnolato complexes consist of a metal ion (most commonly copper(II)) flanked by typically two or more 2-nitrosophenolate ligands. The syntheses of them demonstrate a privileged introduction of a nitroso (and a hydroxyl via the Baudisch reaction) group to an aromatic ring. These complexes first appeared in the literature as early as 1939, and a range of applications has subsequently been published. However, optimisations of the preparative sequences were not considered, and as such, the reactions have seldom been utilised in recent years; indeed, there remains confusion in the literature as to how such complexes form.
- C-nitrosation
- copper complexes
- Baudisch reaction
- nitrosophenols
- ortho-nitrosophenols
- regioselective aromatic functionalisation
1. Introduction to Metal-Nitrosophenolato Complexes
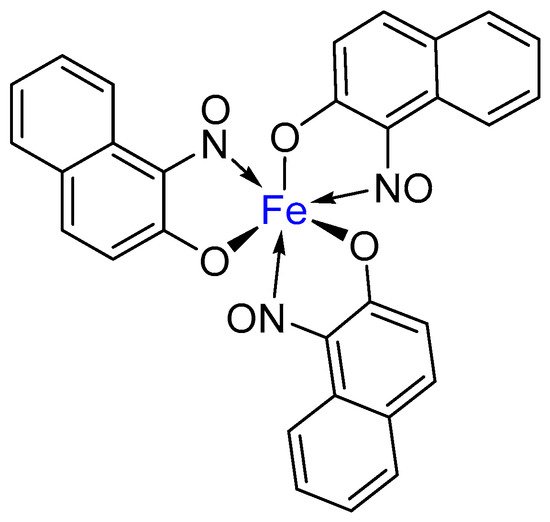
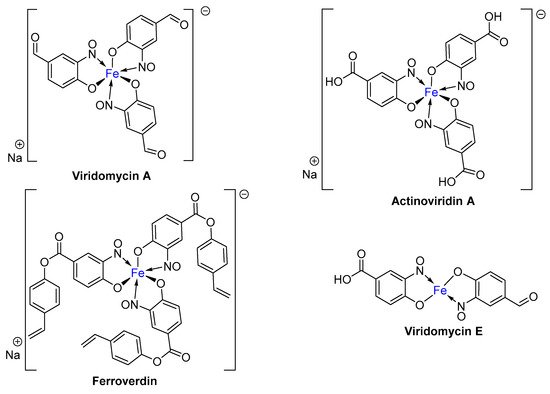
2. Metal-Nitrosophenolato Complexes in Nature
3. A Brief History of Metal-Nitrosophenolato Synthesis
4. Introduction to the Baudisch Reaction
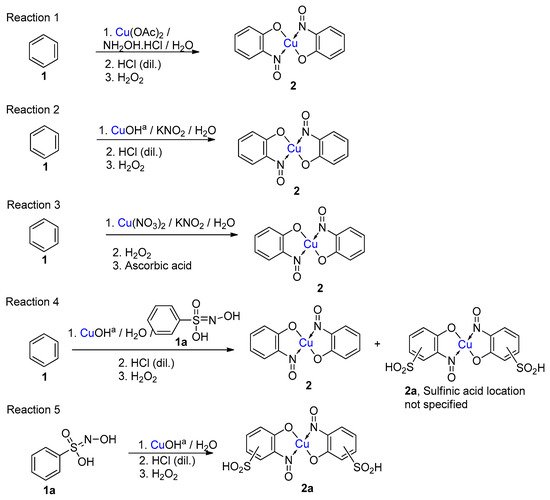
5. Copper-Mediated Aromatic Nitrosation
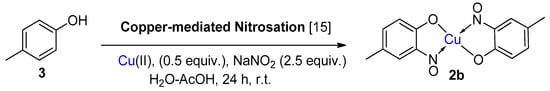
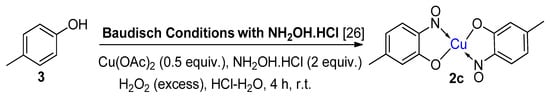
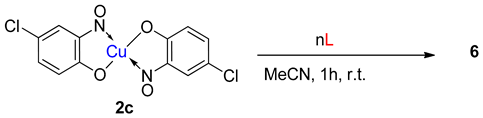
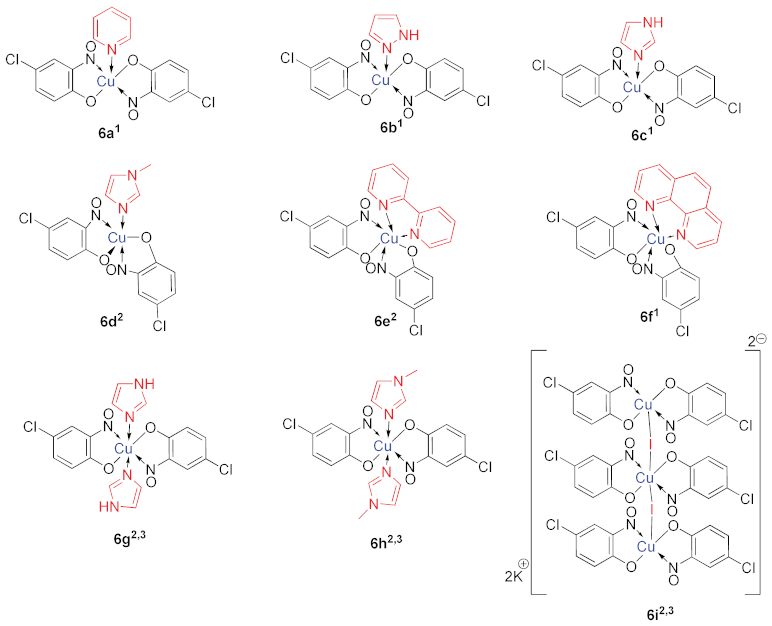
6. Additional Syntheses of Metal-Nitrosophenolato Complexes
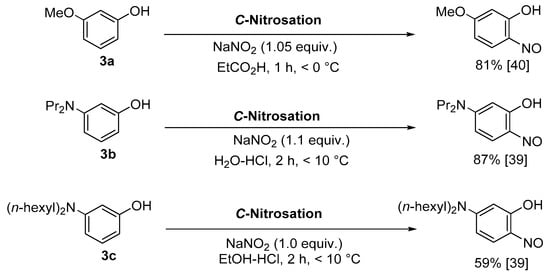
Description | Accepted Starting Materials | ||||
|---|---|---|---|---|---|
Baudisch conditions with hydroxylamine | A range of aromatics including benzene, phenols, catechols, naphthalenes and phenylsulfinic acids [20,42,43][20][42][43]. | ||||
| |||||
Baudisch conditions with nitrous acid | Shown to accept benzene. Similar aromatics expected to work, though scope not investigated [20]. | ||||
| |||||
Copper-mediated aromatic nitrosation | Phenols with sufficiently electron-rich aromatic ring and at least one non-functionalised carbon atom ortho to the phenol [15,41][15][41]. | ||||
| |||||
Association of free 2-nitrosophenols with copper | |||||
|
7. Scope of Metal-Nitrosophenolato Synthesis
8. Properties of Metal-2-Nitrosophenolato Complexes
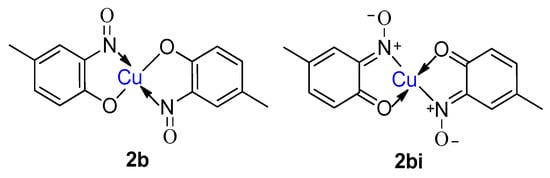
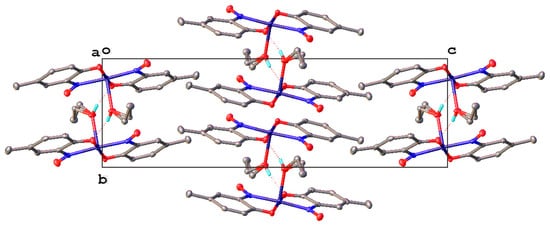
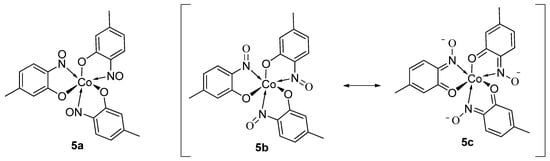
9. Derivatisation of Complexes
10. Conclusion
References
- Charalambous, J.; Frazer, M.J.; Taylor, F.B. Complexes of copper(II) with o-nitrosophenols (monoximes of ortho-benzoquinones). J. Chem. Soc. A Inorg. Phys. Theor. 1969, 2787.
- Al-Obaidi, U.; Moodie, R.B. The nitrous acid-catalysed nitration of phenol. J. Chem. Soc. Perkin Trans. 2 1985, 467.
- Hayton, T.W.; Legzdins, P.; Sharp, W.B. Coordination and Organometallic Chemistry of Metal − NO Complexes. Chem. Rev. 2002, 102, 935–991.
- Zhang, M. Production Device of Rubber Colorant Pigment Green B 2017. Available online: https://patents.google.com/patent/CN108239422A/en?oq=CN108239422A (accessed on 28 July 2019).
- Wegener, J.W.; Klamer, J.C.; Govers, H.; Brinkman, U.A.T. Determination of organic colorants in cosmetic products by high-performance liquid chromatography. Chromatographia 1987, 24, 865–875.
- American Association of Textile Chemists and Colorists (AATCC). Available online: https://www.standardsportal.org/usa_en/trade_associations/aatcc.aspx (accessed on 5 November 2019).
- Shi, H.; Zhang, T.; An, T.; Li, B.; Wang, X. Photocatalytic Hydroxylation of Phenol to Catechol and Hydroquinone by Using Organic Pigment as Selective Photocatalyst. Curr. Org. Chem. 2012, 16, 3002–3007.
- Chain, E.B.; Tonolo, A.; Carilli, A. Ferroverdin, a Green Pigment containing Iron produced by a Streptomycete. Nature 1955, 176, 645.
- Candeloro, S.; Grdenic, D.; Taylor, N.; Thompson, B.; Viswamitra, M.; Hodgkin, D.C. Structure of Ferroverdin. Nature 1969, 224, 589–591.
- Maciejewska, M.; Pessi, I.S.; Arguelles-Arias, A.; Noirfalise, P.; Luis, G.; Ongena, M.; Barton, H.; Carnol, M.; Rigali, S. Streptomyces lunaelactis sp. nov., a novel ferroverdin A-producing Streptomyces species isolated from a moonmilk speleothem. Antonie Van Leeuwenhoek 2015, 107, 519–531.
- Tomoda, H.; Tabata, N.; Shinose, M.; Takahashi, Y.; Woodruff, H.B.; Omura, S. Ferroverdins, Inhibitors of Cholesteryl Ester Transfer Protein Produccd by Streptomyces sp. WK-5344. I. Production, Isolation and Biological Properties. J. Antibiot. (Tokyo) 1999, 52, 1101–1107.
- KUROBANE, I.; DALE, P.L.; VINING, L.C. Characterization of new viridomycins and requirements for production in cultures of Streptomyces griseus. J. Antibiot. (Tokyo) 1987, 40, 1131–1139.
- Yang, C.C.; Leong, J. Mode of antibiotic action of 4-hydroxy-3-nitrosobenzaldehyde from Streptomyces viridans. Antimicrob. Agents Chemother. 1981, 20, 558–562.
- Basu, P.; Pal, S.; Chakravorty, A. Redox in a ferroverdin analogue: Recognition of isomeric co-ordination spheres by Fe II and Fe III. J. Chem. Soc. Chem. Commun. 1989, 977.
- Cronheim, G. O-Nitrosophenols. J. Org. Chem. 1947, 12, 1–29.
- Millon, E. Sur un Réacrif Propre aux composés proteiques. Compt. Rend. Acad. 1849, 132, 156.
- Vaubel, W. Zur Kenntniss der Millon’schen Reaction. Zeitschrift für Angew. Chemie 1900, 13, 1125–1130.
- Kido, Y.; Tamura, Z. An improved method for the millon reaction. Chem. Pharm. Bull. (Tokyo) 1981, 29, 2296–2302.
- Gibbs, H.D. PHENOL TESTS.* II. Nitrous Acid Tests. The Millon and Similar Spectrophotometric Iinvestigations. J. Biol. Chem. 1926, 71, 445–459.
- Baudisch, O. A New Chemical Reaction With the Nitrosyl Radical Noh. Science 1940, 92, 336–337.
- Baudisch, O.; Smith, S. Ftir die kurzen Originalmitteilungen ist ausschlieBlich der Verfasser verantwortlich. Kurze Orig. 1939, 17, 768–769.
- Deka, H.; Ghosh, S.; Gogoi, K.; Saha, S.; Mondal, B. Nitric Oxide Reactivity of a Cu(II) Complex of an Imidazole-Based Ligand: Aromatic C-Nitrosation Followed by the Formation of N-Nitrosohydroxylaminato Complex. Inorg. Chem. 2017, 56, 5034–5040.
- Kundu, S.; Kim, W.Y.; Bertke, J.A.; Warren, T.H. Copper(II) activation of nitrite: Nitrosation of nucleophiles and generation of NO by thiols. J. Am. Chem. Soc. 2017, 139, 1045–1048.
- Cone, M.C.; Melville, C.R.; Carney, J.R.; Gore, M.P.; Gould, S.J. 4-Hydroxy-3-nitrosobenzamide and its ferrous chelate from Streptomyces murayamaensis. Tetrahedron 1995, 51, 3095–3102.
- Dessouky, Y.M.; Gal El Rub, L.N. Colorimetric determination of procaine hydrochloride in pharmaceutical preparations. Analyst 1976, 101, 717–719.
- Maruyama, K.; Goto, R.; Tanimoto, I. Studies on the Baudisch Reaction. I. The Synthesis of O-Nitrosophenols. J. Org. Chem. 1967, 32, 2516–2520.
- Masoud, M.S.; Hindawey, A.M.; Mostafa, M.A.; Ramadan, A.M. Synthesis and structural chemistry of 2,4-dinitrosoresorcionl, 1-nitroso-2-naphthol and 4-carboxy-2-nitrosophenol. Spectrosc. Lett. 1997, 30, 1227–1247.
- Porta, F.; Prati, L. Catalytic synthesis of C-nitroso compounds by cis-Mo(O)2(acac)2. J. Mol. Catal. A Chem. 2000, 157, 123–129.
- Williams, D.L.H. Nitrosation Reactions and the Chemistry of Nitric Oxide, 1st ed.; Elsevier B.V: Bodmin, UK, 2004.
- Baluch, D.; Charalambous, J.; Haines, L.I.B. Manganese Complexes of 1,2-Naphthoquinone Mono-oximes (2-Nitrosophenols) as Catalysts for Alkene Epoxidation Dosten. J. Chem. Soc. Chem. Commun. 1988, 1178–1179.
- Aljaar, N.; Malakar, C.C.; Conrad, J.; Beifuss, U. Base-Promoted Domino Reaction of 5-Substituted 2-Nitrosophenols with Bromomethyl Aryl Ketones: A Transition-Metal-Free Approach to 2-Aroylbenzoxazoles. J. Org. Chem. 2015, 80, 10829–10837.
- Li, H.; Pang, M.; Wu, B.; Meng, J. Synthesis, crystal structure and photochromism of a novel spirooxazine derivative. J. Mol. Struct. 2015, 1087, 23–29.
- Atherton, J.H.; Moodie, R.B.; Noble, D.R. Kinetics and mechanism of nitrosation of toluene, o-xylene, and m-xylene in trifluoroacetic acid, or in acetic–sulfuric acid mixtures, under nitric oxide. J. Chem. Soc. Perkin Trans. 2 1999, 699–706.
- Leis, J.R.; Ríos, A.; Rodríguez-Sánchez, L. Reactivity of phenolic nucleophiles towards nitroso compounds. Part 2.1 Reaction with alkyl nitrites (O-nitroso compounds). J. Chem. Soc. Perkin Trans. 2 1998, 2729–2734.
- Charalambous, J.; Kensett, M.J.; Jenkins, J.M. Deoxygenation of 2-nitrosophenols and of their metal complexes with triphenylphosphine. Synthesis of phenazines, dihydrophenazines, triphenyl(o-hydroxyphenylimino)phosphoranes and their metal complexes. J. Chem. Soc. Chem. Commun. 1977, 400–401.
- Atherton, J.H.; Moodie, R.B.; Noble, D.R.; O’Sullivan, B. Nitrosation of m-xylene, anisole, 4-nitrophenyl phenyl ether and toluene in trifluoroacetic acid or in acetic–sulfuric acid mixtures under nitric oxide. J. Chem. Soc. Perkin Trans. 2 1997, 2, 663–664.
- Ramozzi, R.; Chéron, N.; El Kaïm, L.; Grimaud, L.; Fleurat-Lessard, P. Predicting new ugi-smiles couplings: A combined experimental and theoretical study. Chem.-A Eur. J. 2014, 20, 9094–9099.
- Bosch, E.; Kochi, J.K. Direct Nitrosation of Aromatic Hydrocarbons and Ethers with the Electrophilic Nitrosonium Cation. J. Org. Chem. 1994, 59, 5573–5586.
- Crossley, M.L.; Dreisbach, P.F.; Hofmann, C.M.; Parker, R.P. Chemotherapeutic Dyes. I. 5-Aralkylamino-9-alkylaminobenzo phenoxazines 1. J. Am. Chem. Soc. 1952, 74, 573–578.
- Maleski, R.J.; Kluge, M.; Sicker, D. A facile access to substituted 2-nitrosophenols and 2-nitrophenols via regioselective nitrosation of resorcinol monoethers. Synth. Commun. 1995, 25, 2327–2335.
- Nicholls, A.J.; Batsanov, A.S.; Baxendale, I.R. Copper-Mediated Nitrosation: 2-Nitrosophenolato Complexes and their use in the Synthesis of Heterocycles. Molecules 2019. under review.
- Maruyama, K.; Tanimoto, I. Studies on the Baudisch Reaction. IV. The Reaction Mechanism. Bull. Chem. Soc. Jpn. 1971, 44, 3120–3123.
- Konecny, J.O. Hydroxylation of Benzene in Aqueous Solution in the Presence of Hydroxylamine Hydrochloride. J. Am. Chem. Soc. 1955, 77, 5748–5750.
- 1-Nitroso-2-Naphthol (2019). Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/114693?lang=en®ion=GB (accessed on 1 August 2019).
- Patil, S.V.; Mohite, B.S. Palladium (II) Chelates of some o-Nitrosophenols. J. Mol. Catal. 1977, 46, 638.
- Ka-Hong Lee, K.; Wong, W.-T. Syntheses and molecular structures of ruthenium carbonyl complexes containing 1,2-naphthoquinone-1-oximate ligands. J. Chem. Soc. Dalt. Trans. 1997, 2987–2996.
- Lyubchenko, S.N.; Ionov, A.M.; Shcherbakov, I.N.; Aleksandrov, G.G.; Kogan, V.A.; Tsivadze, A.Y. Coordination compounds based on 4,6-di-tert-butyl-2-nitrosophenol. Russ. J. Coord. Chem. 2006, 32, 539–544.
- Rout, K.C.; Mondal, B. Aromatic C-nitrosation by a copper(ii)–nitrosyl complex. Dalt. Trans. 2015, 44, 1829–1835.
- Baluch, D. Studies of 1,2-Quinone Mono-Oximato Complexes and Their Redox Reactions; The Polytechnic of North London: London, UK, 1987. Available online: https://core.ac.uk/display/161337781 (accessed on 5 November 2019).
- Castellani, C.B.; Carugo, O. Studies on copper(II) complexes of o-quinone monooximes. 8. Penta and hexacoordinated adducts of bis(4-chloro-1,2-benzaquinone 2-oximato)copper(II), , with imidazole and N-methylimidazole. Inorg. Chim. Acta 1988, 150, 119–123.
- The Cambridge Crystallographic Data Centre Home—The Cambridge Crystallographic Data Centre (CCDC). Available online: https://www.ccdc.cam.ac.uk (accessed on 5 November 2019).
- Basu, P. Cobalt analogue of ferroverdin: Synthesis and geometric structure. Polyhedron 1992, 11, 3037–3040.
- Castellani, C.B.; Buttafava, A.; Carugo, O.; Poggi, A. Studies on metal complexes of ortho-quinone mono-oximes. Part 6. Redox behaviour of bis(4-chloro-1,2-benzoquinone 2-oximato)-copper(II) and -nickel(II); synthesis and characterization of reduced species containing a paramagnetic ligand. J. Chem. Soc. Dalt. Trans. 1988, 1497.
- Masoud, M.S.; Haggag, S.S.; Ramadan, A.M.; Mahmoud, S. a Nitrosophenol complexes of transition metal salts. Transit. Met. Chem. 1998, 23, 343–347.
- Castellani, C.B.; Gatti, G.; Millini, R. Studies on copper(II) Complexes of o-quinone monooximes. adducts of bis(4-chioro-1 -benzoquinone 2-oximato) copper(II) with Some heterocyclic bases. structure of the 2,2’-bipyridine adduct. Inorg. Chem. 1984, 23, 4004–4008.
- Castellani, C.B.; Carugo, O.; Coda, A. Studies on copper(II) complexes of o-quinone monooximes. 3. Reactivity of bis(4-chloro-1-benzoquinone 2-oximato)copper(II) with halides. Crystal structure of potassium bis(.mu.-iodo)tris. Inorg. Chem. 1987, 26, 671–675.
- Adatia, T.; Chakrabarti, J.; Charalambous, J.; Carugo, O.; Castellani, C.B. Synthesis and structural characterization of lithium and sodium complexes of 5-ethylamino-4-methyl-1,2-benzoquinone 2-oxime. Polyhedron 1996, 15, 1331–1338.
- Li, F.; Liu, Q.; Hu, J.; Feng, Y.; He, P.; Ma, J. Recent advances in cathode materials for rechargeable lithium–sulfur batteries. Nanoscale 2019, 11, 15418–15439.