Topical drug delivery has many advantages over other ways of administration, having increased patient compliance, avoiding the first-pass effect following oral drug administration or not requesting multiple doses administration. However, the skin barrier prevents the access of the applied drug, affecting its therapeutic activity. Carriers containing phospholipid soft vesicles are a new approach to enhance drug delivery into the skin and to improve the treatment outcome. These vesicles contain molecules that have the property to fluidize the phospholipid bilayers generating the soft vesicle and allowing it to penetrate into the deep skin layers. Ethosomes, glycerosomes and transethosomes are soft vesicles containing ethanol, glycerol or a mixture of ethanol and a surfactant, respectively.
- soft phospholipid vesicle
- skin disorders
- ethosomes
- glycerosomes
- transethosomes
- skin infection
- skin inflammation
- skin cancer
1. Treatment of Acne Vulgaris
| Treated Disorder | Investigated Vesicular Carrier | Study | Ref. |
|---|---|---|---|
| Acne Vulgaris | Clindamycin and salicylic acid ethosomal system | Clinical study on the reduction of acne vulgaris and skin tolerability of the formulation | [5][31] |
| Azelaic acid ethosomal system | Diffusion study through synthetic membrane | [7][32] | |
| Cryptotanshinone ethosomal system | In vitro skin permeation and skin deposition; in vivo anti-acne activity on rabbits | [8][33] | |
| Karanjin ethosomal system | In vitro skin premeation study on excised rats skin; in vivo skin irritation study on rats; in vivo anti-inflammatory and anti- acne studies on rats | [9][34] | |
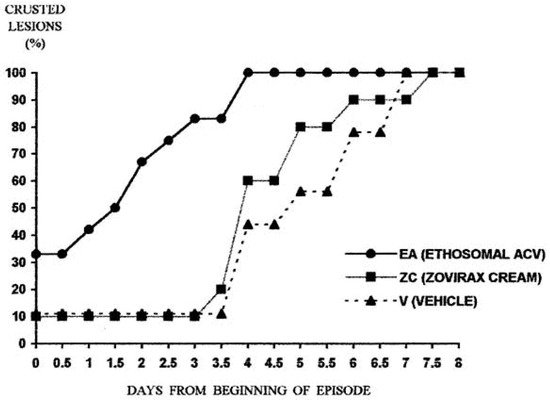
| Viral infections | Acyclovir ethosomal system | Two-armed double-blind clinical study on subjects with recurrent herpes labialis | [10][35] |
| Acyclovir ethosomal system | Antiviral activity against HSV-1 by plaque reduction assay in monolayer cultures of Vero cells | [11][12][36,37] | |
| 9-[(2-hydroxyethoxy) methyl]guanine ethosomal system | Antiviral activity against HSV-1 by plaque reduction assay in monolayer cultures of Vero cells | [12][37] | |
| Ethosomal system of the essential oil of Melissa officinalis L. | In vitro activity against HSV type 1 in mammalian cells | [13][38] | |
| Bacterial infections | Bacitracin ethosomal system | Intracellular penetration and localization in fibroblasts (3T3); in vitro deposition and permeation through human cadaver skin | [14] |
| Erythromycin ethosomal system | In vivo activity in mice model of deep dermal S. aureus infection | [15] | |
| Psoralen ethosomal system | Photodynamic therapy in biofilms formed in Petri dishes | [16][39] | |
| Fungal infections | Voriconazole transethosomal system | In vitro skin permeation and deposition studies through mice skin; in vivo deposition study on mice | [17][12] |
| Griseofulvin ethosomal system | In vitro permeation and deposition study on new-born pig skin | [18][40] | |
| Clove oil ethosomal system | Ex vivo permeation studies on rat skin; antifungal activity in cup plate test against Candida albicans | [19][41] | |
| Voriconazole ethosomal system | In vitro anti-fungal activity against Asperigillus flavus colonies. In vitro skin deposition and permeation through abdominal rat skin |
[20][42] | |
| Econazole nitrate transethosomal system | Ex-vivo skin permeation and retention studies followed by in vitro antifungal activity against C. albicans fungus | [21][43] | |
| Skin inflammation | Diclofenac ethosomal system | In vitro permeation study on rat skin; in vivo anti-inflammatory activity in carrageenan-induced rat paw edema model | [22][23][11,44] |
| Ammonium glycyrrhizinate ethosomal system | In vitro permeation through human skin; clinical study to evaluate the anti-inflammatory activity in volunteers with methyl nicotinate erythema | [24][45] | |
| Matrine ethosomal system | In vitro percutaneous permeation study on rat skin; in vivo anti-inflammatory activity in rat measured by reflection spectrophotometery | [25][46] | |
| Apigenin ethosomal system | In vitro and in vivo deposition study on rat skin; evaluation of the reduction of cyclooxygenase-2 levels in mouse with skin inflammation | [26][47] | |
| Crocin ethosomal system | Evaluation of the anti-inflammatory activity on healthy volunteers. | [27][48] | |
| Diclofenac ethosomal system and Diclofenac transethosomal system | In vitro permeation and deposition studies on rat skin | [28][49] | |
| Diclofenac glycerosoomal system | In vitro penetration and permeation studies on new-born pig skin | [29][30][31][13,50,51] | |
| Paeoniflorin glycerosomal system | In vitro permeation experiments through excised rat abdominal skin; in vivo deposition in rat synovium | [32][52] | |
| Achillea millefolium L. extract ethosomal system | In vitro permeation strudy through fresh rat skin | [33][53] | |
| Psoriasis | Psoralen ethosomal system | In vitro permeation and penetration study using Franz diffusion cells and excised rat skin | [34][54] |
| Methotrexate and Salicylic acid ethosomal system | In vitro retention and permeation study on pig ear skin; in vivo anti-psoriatic activity in mice model with imiquimod-induced psoriasis | [35][55] | |
| Anthralin ethosomal system | Preparation, comparative evaluation and clinical assessment in psoriatic patients |
[36][56] | |
| Curcumin ethosomal system surface-modified with glycyrrhetinic acid-D-α-tocopherol acid polyethylene glycol succinate | In vitro anti-inflmmatory effect on interleukin-6-induced oxidative stress cell model; in vivo anti-psoriatic activity in mice model with imiquimod-induced psoriasis | [37][57] | |
| Skin cancer | 5- Fluorouracil ethosomal system containing -decyl methyl sulfoxide (Tumorep) | In vitro anti-tumor effect on five cell lines; in vivo anti-tumor effect in mice model of skin cancer | [38][16] |
| Paclitaxel ethosomal system | In vitro permeation study on human SC; in vitro antiproliferative effect in squamous carcinoma cells | [39][58] | |
| Fe-chlorophyllin transethosomal system | In vitro skin permeation and deposition studies through mice skin; in vivo evaluation of the anti-cancer effect in mice | [40][59] | |
| Plumbagin glycerosomal system | Ex vivo permeation study on rats skin | [41][60] | |
| Skin injury (wound healing) | Curcumin ethosomal system | In vivo wound healing effect in rats | [42][61] |
| Curcumin-propylene glycol liposomal system | In vivo wound repair effect is rats with burned skin | [43][62] | |
| Thymosinβ-4(Tβ-4) ethosomal system | In vitro drug release study on mice skin; in vivo pharmacokinetic and skin irritation studies on mice | [44][63] | |
| Skin pigmentation disorders | Linoleic acid ethosomal system | In vitro percutaneous permeation through human stratum corneum and viable epidermis membrane | [45][64] |
| Methoxsalen ethosomal system | Ex vivo release studies and photo- toxicity after exposure to UV light | [46][65] | |
| Hair loss | Minoxidil ethosomal system Minoxidil glycerosomal system |
In vitro penetration and permeation through abdominal nude mice skin | [6][47][48][10,66,67] |
| Ethosomal systems of plant extracts | In vivo effect on hair growth in rats with testosterone induced alopecia | [49][68] | |
| Skin aging | Vitamin E ethosomal system | In vitro permeation studies through skin and cultured fibroblasts | [50][69] |
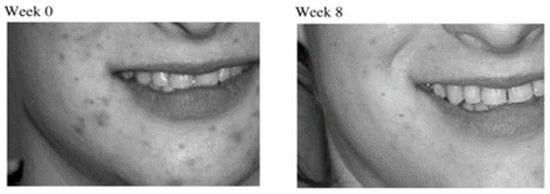
| Curcumin ethosomal system | Clinical trial evaluating skin viscoelasticity, total deformation, biological elasticity and sagginess | [51][70] | |
| Rosmarinic acid ethosomal system | Ex vivo permeation studies using Franz diffusion cells and mice skin; ex vivo antioxidant activity | [52][71] |
2. Treatment of Viral Skin Infections