Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jetze Tepe and Version 3 by Vicky Zhou.
The proteasome system is a large and complex molecular machinery responsible for the degradation of misfolded, damaged, and redundant cellular proteins. When proteasome function is impaired, unwanted proteins accumulate, which can lead to several diseases including age-related and neurodegenerative diseases. Enhancing proteasome-mediated substrate degradation with small molecules may therefore be a valuable strategy for the treatment of various neurodegenerative diseases such as Parkinson’s, Alzheimer’s, and Huntington’s diseases.
- proteasome
- neurodegeneration
- ubiquitin
- misfolded
- disordered
- degradation
- protein
1. Introduction
The degradation of proteins is a continual process that is highly regulated by the two major proteolysis systems, the lysosomal degradation pathway and the proteasome-mediated pathway. Protein degradation helps maintain biological homeostasis in cells which are needed for all cell functions and for maintaining optimal conditions for enzyme function [1]. The proteasome pathway is the major pathway for the degradation of misfolded, oxidatively damaged, and redundant proteins. Dysregulation of proteasome function has been identified in the pathogenesis of several neurodegenerative diseases including Parkinson’s disease (PD) [2], Alzheimer’s disease (AD), and other neurodegenerative diseases [3]. The proteasome pathway is also involved in the regulation of several other cellular processes such as cell cycle, stress signaling, gene expression regulation, inflammatory response, cell differentiation, and apoptosis, which makes it an appealing target in the treatment of other types of diseases, including cancer [4]. Due to the critical role of the proteasome-mediated degradation pathway in cell regulation, the modulation of proteasome proteolytic activity has become a valuable strategy in the pursuit of new therapeutics to treat several neurodegenerative diseases [5][6][7][8][5,6,7,8].
1.1. The Human Proteasome
The human proteasome is a large complex protein responsible for the intracellular degradation of unwanted and damaged proteins via a ubiquitin-dependent and ubiquitin-independent degradation pathway. The most common proteolytic clearance of proteins proceeds by tagging the protein with polyubiquitin, after which it is degraded into small peptides of seven to eight amino acids by the 26S proteasome [9]. Highly disordered proteins can also be degraded in a ubiquitin-independent manner by the 20S proteasome [10].
1.2. Ubiquitin-Proteasome System
1.2.1. Ubiquitin
Ubiquitin (Ub) is a small protein (approximately 8600 Da) with 76 amino acid residues responsible for tagging a wide range of cellular proteins for proteolytic degradation. In the ubiquitin-proteasome system (UPS) (Figure 1), proteins are tagged for proteolysis by covalent ligation to ubiquitin [11]. Ubiquitination of proteins requires three enzymes in chronological order (see Figure 1a). The E1 ubiquitin-activating enzyme, just like its name, activates the C-terminal glycine residue of the ubiquitin in an ATP-dependent manner. The binding of the ubiquitin to a cysteine residue of E1 forms a Ub-E1 complex via a thioester linkage. The E2 ubiquitin-conjugating enzymes transfer the ubiquitin from the Ub-E1 complex to itself via a trans-thioesterification to form the Ub-E2 complex and release the E1 enzyme from the system. Lastly, the ubiquitin ligases E3s are responsible for selecting proteins for ubiquitin-mediated proteolysis. Humans have two E1 enzymes, about 40 E2 enzymes, and are estimated to have about 500–1000 E3s [12].
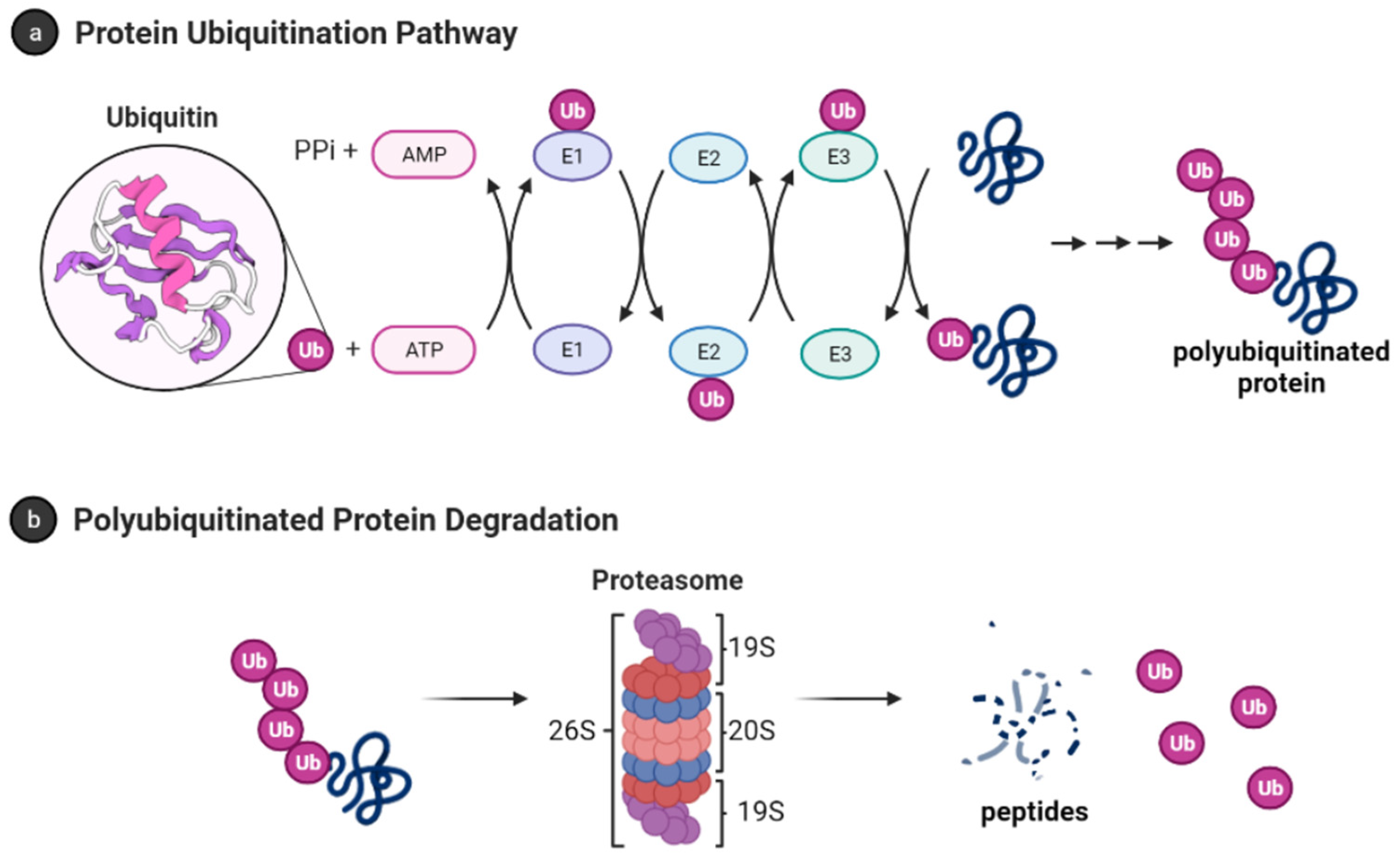
Figure 1. Ubiquitin-proteasome system [13]. (a) Protein polyubiquitination process using the ubiquitin-activating enzyme E1, conjugating enzymes E2 and the E3 ligase; (b) Polyubiquitinated proteins are degraded by 26S proteasome into small peptides following its deubiquitination.
After monoubiquitination of the targeted protein, the C-terminus of each ubiquitin molecule can be linked to any of the other seven lysine residues (K6, K11, K27, K29, K33, K48, and K63) on the previous ubiquitin to extend the ubiquitin chain and form the polyubiquitinated tagged protein [14][15][14,15]. However, the signal for protein degradation by the proteasome usually involves the linking of Ub to the K48 of the previous Ub on the protein [16][17][16,17]. In addition, K11, K29, and K63 linked chains have also been shown to play a role in proteasomal degradation [17][18][17,18]. The 26S proteasome degrades polyubiquitinated proteins (see Figure 1b), and a previous study shows that proteins marked for degradation must be tagged with at least four ubiquitin molecules to be recognized by the 26S proteasome [16][19][16,19]. However, shorter chains, monoubiquitinated and multiple monoubiquitinated proteins can also be targeted for degradation by the proteasome [20][21][22][23][20,21,22,23]. It is also important to note that the ubiquitination process is reversible, and the deubiquitinating enzymes (see Section 3.1.1) are present in the cell to remove ubiquitin-tagged proteins [24].
1.2.2. The 26S Proteasome
The 26S proteasome has a molecular weight of approximately 2.5 MDa and it is made up of the 20S core particle (CP), and one or two 19S regulatory particle(s) (RP) attached to one or both end(s) of the CP [25]. The 19S RP (also known as PA700) binds to the 20S CP and facilitates the gate opening of the CP for proteolytic degradation of polyubiquitinated proteins [26]. The 19S RP is also responsible for recognizing, unfolding, and translocating polyubiquitinated protein into the 20S CP [27]. Cryo-EM studies have shown many conformation states of the 26S proteasome when engaged with substrate [28][29][30][31][32][33][34][35][28,29,30,31,32,33,34,35]. Some of these studies showed the processes by which substrate is engaged, deubiquitylated, unfolded, and translocated by the proteasome [28][29][28,29]. The proteasome is also referred to as the 30S proteasome when the 20S CP is capped at both ends with the 19S RP [36]. However, hwerein will refer to the 26S proteasome without distinguishing between the singly or doubly capped CP.
1.3. The 20S Proteasome or Core Particle
The 20S proteasome is a 700 kDa protein with a cylindrical-like structure. The CP contains four heptameric rings stacked on each other in an α1-7β1-7β1-7α1-7 fashion. The outer α-rings form a gate, and they recognize regulatory particles that allow the opening and closing of the gate [37]. The inner β-rings contains six proteolytic sites, three on each β-ring (β1, β2, and β5), and are responsible for the proteolytic activity of the proteasome.
The three different proteolytic sites of the 20S CP exhibit different substrate preferences even though they all use N-terminal nucleophilic threonine to carry out their proteolytic activities. The β1 exhibits a caspase-like (C-L)/PGPH (peptidylglutamyl-peptide hydrolyzing) activity and preferentially cleaves after acidic residues. The β2 and β5 exhibit trypsin-like (T-L) and chymotrypsin-like (CT-L) activities, and they preferentially cleave after basic and hydrophobic residues, respectively [38]. The 20S proteasome on its own degrades unstructured proteins using a ubiquitin-independent pathway.
1.4. Small Molecule Regulation of Proteasome Function
Due to the role of the proteasome in cellular functions, the regulation of proteasome has become a valuable target for the development of therapeutic molecules [39]. Proteasome inhibition is a therapeutic approach for the treatment of cancer. For example, bortezomib, a dipeptide boronate, was approved by the FDA in 2003 as an anticancer drug to treat mantle cell lymphoma and multiple myeloma [40][41][40,41]. Bortezomib inhibits the 26S proteasome by forming a covalent bond between its boron atom and threonine oxygen in the CT-L catalytic site of the 20S CP [40]. Molecules that inhibit the proteasome have also been shown to induce apoptosis in cell cultures and murine models of cancer. One of the proposed mechanisms is that proteasome inhibition prevents the degradation of the IκB, an NF-κB inhibitor, which prevents NF-κB nuclear translocation and consequently NF-κB mediated gene expression [42]. Proteasome inhibition results in the accumulation of IκB [43][44][45][46][47][48][43,44,45,46,47,48], cyclin-dependent kinase (CDK) inhibitor p21 [43][49][50][43,49,50], tumor suppressor p53, and other pro-apoptotic proteins [51][52][53][51,52,53]. The exceptional increase in apoptosis of certain multiple myeloma cells when treated with proteasome inhibitors has also been linked to an increase in protein unfolding and increasing substrate load on the proteasomes [54][55][54,55]. In addition, proteasome inhibition leads to lethal shortage of amino acids in the cells, which are the building blocks for cells to make new proteins. This amino acid scarcity caused by proteasome inhibition results in increasing ER stress and cell apoptosis [56]. Many reviews on proteasome inhibition have recently been published [57][58][59][60][61][62][63][64][65][66][57,58,59,60,61,62,63,64,65,66], including a recent reviework by the by our group on natural products scaffolds as inhibitors of the proteasome [67].
Proteasome activation by small molecules is a proposed strategy for the treatment of age-related diseases and several neurodegenerative diseases such as Parkinson’s disease (PD), Alzheimer’s Disease (AD), Huntington’s Disease (HD), and Amyotrophic Lateral Sclerosis [8][68][69][70][71][72][8,68,69,70,71,72]. Increasing the proteolytic activity of proteasome enhances the degradation of specific intrinsically disordered proteins (IDPs) such as α-synuclein, β-amyloid, and tau, to mention a few, which are associated with the pathogenesis of these neurodegenerative diseases.
2. Proteasome Activity and Diseases
As humans age, there is a decline in proteasome function [3][73][3,73]. This reduction could be due to the reduction in the expression of proteasome subunits [74], oxidative damage of the protein [75][76][75,76], and disassembly of the 26S proteasome holocomplex [77][78][77,78]. The decrease in proteasome proteolytic function leads to lower rates of unwanted protein degradation which can induce toxic signaling upon accumulation and aggregation (Figure 2). In particular, the accumulation of specific intrinsically disordered proteins (IDPs), such as amyloid-β and α-synuclein, have been identified as a driving cause of many neurodegenerative diseases [79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98]. The exact mechanism by which these oligomers induce neurotoxicity is complex and still debated, but it is widely accepted that dysregulated IDPs accumulate, and the resulting soluble oligomeric forms of these protein aggregates are likely toxic species in disease pathogenesis [79][93][99][100][101][102][79,93,99,100,101,102]. These soluble oligomeric forms are also responsible for impairing proteasome function, which further drives disease progression [94][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][94,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120]. Multiple studies have indicated that enhancing proteasome proteolytic activity prevents the accumulation of these IDPs, reduces brain damage and improves cognitive performance in mouse models, and may be a new therapeutic strategy to treat neurodegenerative diseases [8][68][69][70][72][119][121][122][123][124][125][126][127][128][129][130][131][132][8,68,69,70,72,119,121,122,123,124,125,126,127,128,129,130,131,132]. More recently, it has been recognized that the 20S proteasome of the proteasome plays a critical role in maintaining proteostasis by the direct degradation of oxidatively damaged and highly disordered proteins [10][133][134][135][136][137][138][10,133,134,135,136,137,138]. The 20S proteasome, therefore, serves as the default protease to unremittently maintain low levels of these unwanted IDPs without the need for post-translation modifications, including protein ubiquitination [10][133][10,133]. Highly disordered proteins appear to be the main target of the 20S proteasome [139]. IDPs are also naturally short-lived, but basal levels are secured by forming proteolytically stable structured complexes with “nannies”, chaperones, or other protein complexes [140]. However, when these IDPs production outpaces their degradation, they accumulate, oligomerize, and aggregate, resulting in the induction of downstream cytotoxic signaling events.
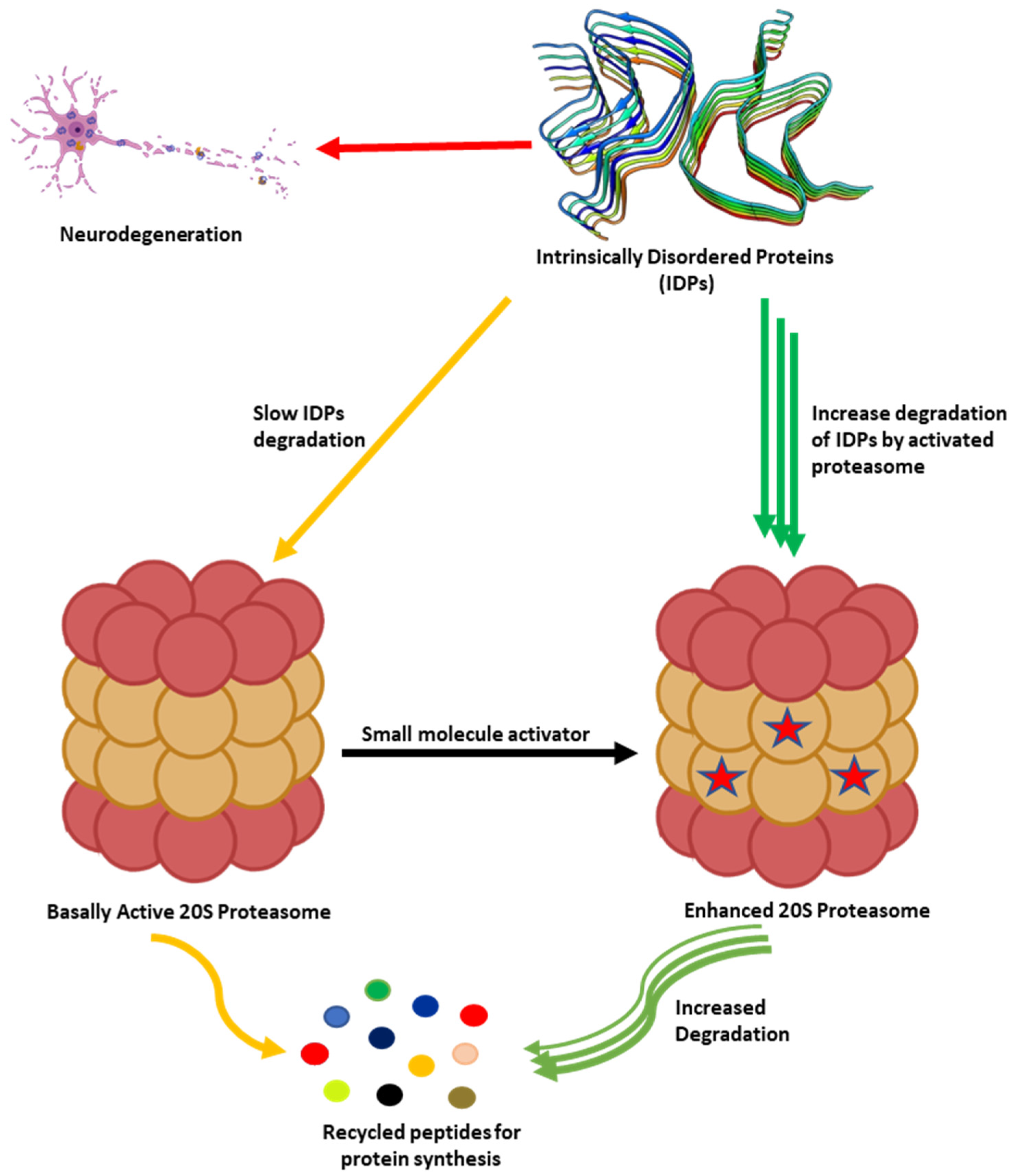
Figure 2. Accumulation of partially unfolded, misfolded, and dysregulated intrinsically disordered proteins (IDPs) such as amyloid-β and α-synuclein leads to neurotoxicity and neuronal cell death. The 20S proteasome degrades unwanted IDPs; however, small molecules can enhance the rate of proteasome-mediated degradation of these IDPs and prevent their accumulation.
3. Conclusions
Efficient proteasome function is critical in maintaining healthy cellular homeostasis. Dysregulation of protein or proteasome impairment can result in a toxic accumulation of unwanted proteins, which is observed in the pathogenesis of different neurodegenerative diseases and aging. Enhancing the proteolytic activity of the proteasome by increasing its capacity, accessibility, or the rate at which it degrades has long been hypothesized as a means to prevent the accumulation of dysregulated IDPs. More recently, researchers from various labs have explored the use of small molecules to induce protein proteolysis. Small molecule proteasome agonists can enhance the proteolytic clearance of unwanted proteins and restore homeostasis. Small molecule enhancers of the 26S proteasome mainly induce enhanced 26S-mediated proteolysis of ubiquitinated proteins via an indirect mechanism of proteasome activation.
Small molecule inhibitors of deubiquitinases prevent proteins marked for ubiquitin-dependent degradation from escaping their fate. Even though there are no approved therapies yet based on deubiquitinating enzyme (DUB) inhibitors, this is an emerging field with great significance. Small molecule regulation of upstream signaling pathways, including cAMP-depending protein kinase A and c-GMP-dependent protein kinase G, affect the phosphorylation of the proteasome regulatory particles. As a result, small molecule regulators of phosphodiesterase type-3 (PDE3) can therefore indirectly increase the rate of substrate degradation by the proteasome. Small molecules that directly interact with the 26S proteasome and enhance the rate of 26S proteasome-mediated protein degradation are less known and likely a fruitful field for exploration.
Whereas the 26S proteasome targets ubiquitinylated protein substrates, the 20S proteasome is limited to the degradation of only disordered proteins. Several small molecule enhancers of 20S proteasome-mediated protein degradation have been identified in the literature that induce 20S—mediated degradation of dysregulated intrinsically disordered proteins by direct interaction with the 20S core particle.
The activation of the proteasome by small molecules is a relatively new field in science. Its potential as a therapeutic approach is still unknown and the consequences of chronic exposure to proteasome enhancers are not known. However, considering the possibility of treating multiple disorders for which there are currently no treatment options available, this approach has enormous potential. However, as in all new fields, the approach still needs further validation, in vivo studies in particular, to fully understand its therapeutic potential and limitation. In addition, more studies are needed to elucidate the mechanistic details of small molecule proteasome activation and its overall cellular consequences.
