Liver fibrosis is a wound healing response that degenerates, and is characterized by excessive accumulation of extracellular matrix (ECM) components that form scar tissue. Liver transplantation is often needed as a course of treatment for patients in critical conditions, but limitations associated with transplantation prompted the continuous search for alternative therapeutic strategies. Cell therapy with stem cells has emerged as an attractive option in order to stimulate tissue regeneration and liver repair. Transplanted mesenchymal stem cells (MSCs) could trans-differentiate into hepatocyte-like cells and, moreover, show anti-fibrotic and immunomodulatory effects.
- liver fibrosis
- cell-free therapy
- mesenchymal stem cells
- secretome
- extracellular vesicles
1. Introduction

2. Composition of MSCs’ Secretome (Derived Soluble Factors CM/EV)
3. Role of MSCs-Sourced Secretome in Liver Regeneration
3.1. MSCs in Liver Disease Treatment
3.2. Effect of MSC-Derived Conditioned Medium (MSC-CM)
The conditioned medium (CM) from MSCs contains all the components of the secretome, and is easily obtainable from cultured MSCs by centrifugation and filtration [27]. CM was frequently used in studies to evaluate the effects of MSC-sourced secretome in liver regeneration, without the risks associated with cell transplantation. UC-MSCs were isolated and differentiated in vitro into hepatocyte-like cells, and their secretome was obtained in a study by An et al. [80][46]. CM-MSCs, from differentiated and undifferentiated UC-MSCs, were distributed to TGF-β1-activated HSCs and mice with thioacetamide (TAA) and CCl4-induced liver fibrosis. CM-MSCs inhibited HSCs activation and reduced the expression of fibrotic factors such as α-SMA, collagens, metalloproteinases, TGFβ, and Smad proteins in the TGFβ signaling pathway. One highly expressed protein was identified in the UC-MSCs secretome, milk fat globule-EGF factor 8 (MFGE8), and it was proven, in the study, to show anti-fibrotic action similar to the effect of CM-MSC.3.3. Pre-Treatment of MSCs for Improved Secretome Content
3.4. Effect of EVs
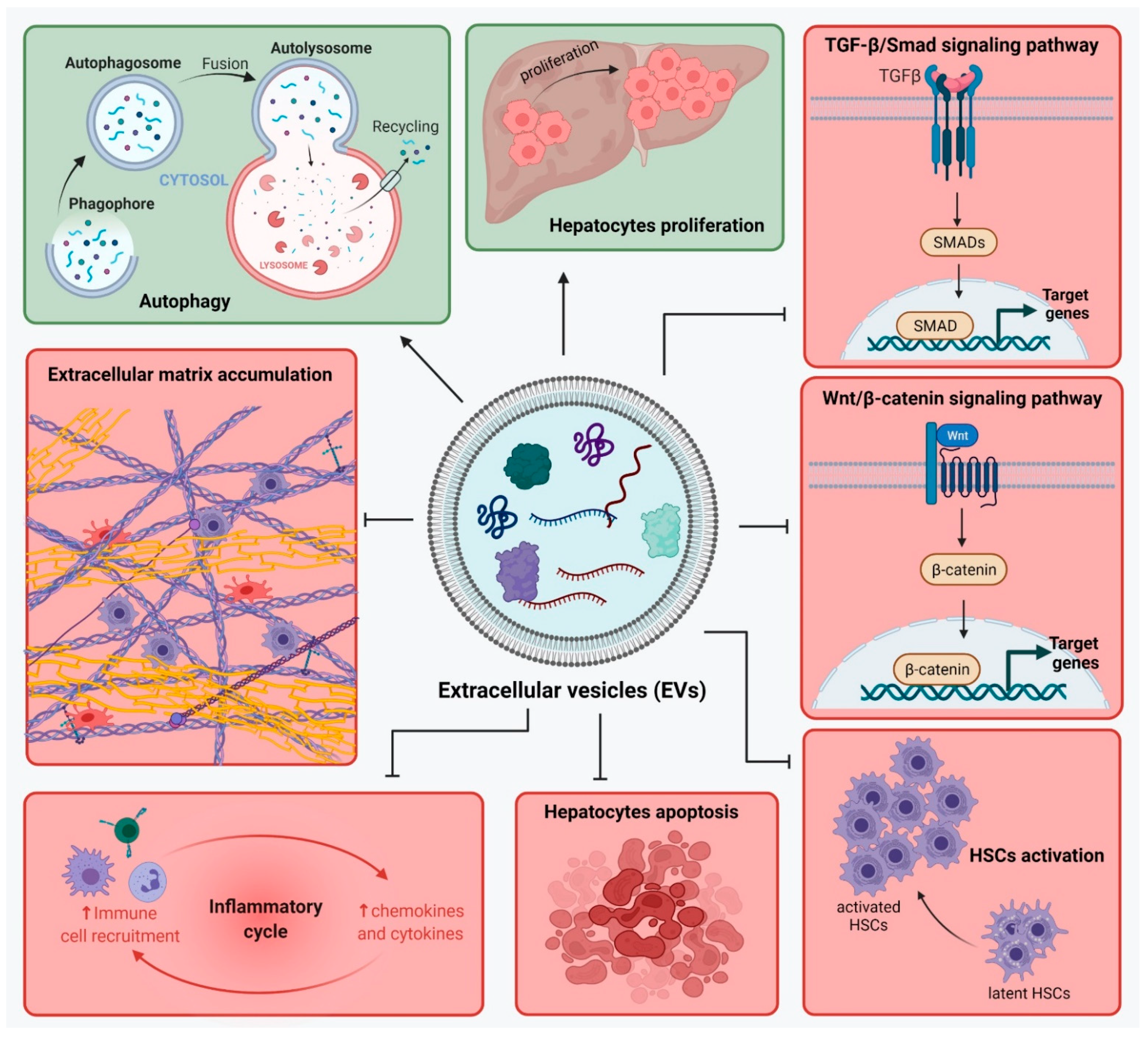
3.5. Exosomes from MSCs with Specific Overexpressed Cargo Such as miRNAs
4. Conclusions
Liver fibrosis is a result of liver injury and an altered wound healing response, which leads to ECM proteins’ accumulation. There are complex interactions between liver cells in liver fibrosis development and many signaling pathways are involved in maintaining the inflammatory state. Therapies for liver fibrosis are constantly under investigation, and the use of strategies based on MSCs show great promise. MSC transplantation showed to improve liver function as it brings a source of cells with differentiation potential towards hepatocyte-like cells. In addition to contributing in restoring the number of hepatocytes in the injured liver, MSC-sourced secretome is rich in molecules involved in regenerative mechanisms by action on liver cells such as hepatocytes, HSCs and macrophages. MSC-secretome acts upon hepatocytes and promotes their survival and proliferation. Especially when MSC-EVs were used, the expression of apoptotic markers was inhibited. In addition, MSCs show immunomodulatory and anti-inflammatory activity though the action of their secretome. They stimulate the production of M2-type macrophages, which will secrete anti-inflammatory molecules. Moreover, MSC-secretome inhibits the infiltration of inflammatory cells and suppresses the expression of inflammatory cytokines in KCs. Activated HSCs represent another important cellular target of the MSC-sourced secretome. Not only does it help reduce their number in the fibrotic liver by stimulating their apoptosis, but it also further inhibits their activation and proliferation, thus helping control the overall pool of HSCs. HSCs are also responsible for the ECM production in liver fibrosis, and MSC-secretome inhibits the production of most ECM proteins and fibrotic markers. In such a manner, MSCs contribute in different ways (direct MSC transplantation, MSC-sourced secretome, CM from pre-treated MSCs, MSC-EVs) to alleviate liver damage and promote tissue regeneration. Cell-free therapies based on MSC-sourced secretome help overcome a number of limitations and risks associated with MSCs transplantation. The use of secretome could mimic the anti-fibrotic effects observed after MSCs transplantation. MSCs secretome is rich not only in cytokines, chemokines, growth factors, and anti-inflammatory factors, but in EVs as well. The use of MSCs-EVs as a treatment for liver fibrosis may be more effective than MSCs therapy, as they can pass through biological barriers and deliver their anti-fibrotic cargo to specific target cells. MSC-EVs were shown to regulate signaling pathways such as TGF-β1/Smad and Wnt/β-catenin, stimulate autophagy and hepatocyte apoptosis, reduce collagen deposition, inhibit activation of HSCs, and modulate the inflammation. Moreover, MSC-EVs cargo can be modified in order to deliver specific proteins of miRNAs with anti-fibrotic properties. So far, these strategies show great promise, but more research is needed to confirm their efficiency and safety, especially for the use of EVs with modified cargo.
References
- Sun, M.; Kisseleva, T. Reversibility of liver fibrosis. Clin. Res. Hepatol. Gastroenterol. 2015, 39, S60–S63.
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171.
- Ignat, S.-R.; Dinescu, S.; Hermenean, A.; Costache, M. Cellular Interplay as a Consequence of Inflammatory Signals Leading to Liver Fibrosis Development. Cells 2020, 9, 461.
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Invest. 2005, 115, 209–218.
- Al-Dhamin, Z.; Liu, L.-D.; Li, D.-D.; Zhang, S.-Y.; Dong, S.-M.; Nan, Y.-M. Therapeutic efficiency of bone marrow-derived mesenchymal stem cells for liver fibrosis: A systematic review of in vivo studies. World J. Gastroenterol. 2020, 26, 7444–7469.
- Forbes, S.J.; Alison, M.R. Knocking on the door to successful hepatocyte transplantation. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 277–278.
- Wang, J.; Sun, M.; Liu, W.; Li, Y.; Li, M. Stem Cell-Based Therapies for Liver Diseases: An Overview and Update. Tissue Eng. Regen. Med. 2019, 16, 107–118.
- Tsolaki, E. Stem cell-based regenerative opportunities for the liver: State of the art and beyond. World J. Gastroenterol. 2015, 21, 12334.
- Beer, L.; Mildner, M.; Ankersmit, H.J. Cell secretome based drug substances in regenerative medicine: When regulatory affairs meet basic science. Ann. Transl. Med. 2017, 5, 170–170.
- Fierabracci, A.; Fattore, A.; Muraca, M. The Immunoregulatory Activity of Mesenchymal Stem Cells: ‘State of Art’ and ‘Future Avenues. ’ Curr. Med. Chem. 2016, 23, 3014–3024.
- Wang, M.; Yang, X.; Zhang, P.; Cai, L.; Yang, X.; Chen, Y.; Jing, Y.; Kong, J.; Yang, X.; Sun, F. Sustained Delivery Growth Factors with Polyethyleneimine-Modified Nanoparticles Promote Embryonic Stem Cells Differentiation and Liver Regeneration. Adv. Sci. 2016, 3, 1500393.
- Assoni, A.; Coatti, G.; Valadares, M.C.; Beccari, M.; Gomes, J.; Pelatti, M.; Mitne-Neto, M.; Carvalho, V.M.; Zatz, M. Different Donors Mesenchymal Stromal Cells Secretomes Reveal Heterogeneous Profile of Relevance for Therapeutic Use. Stem Cells Dev. 2017, 26, 206–214.
- Lu, W.-Y.; Bird, T.G.; Boulter, L.; Tsuchiya, A.; Cole, A.M.; Hay, T.; Guest, R.V.; Wojtacha, D.; Man, T.Y.; Mackinnon, A.; et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 2015, 17, 971–983.
- Carraro, A.; Flaibani, M.; Cillo, U.; Michelotto, L.; Magrofuoco, E.; Buggio, M.; Abatangelo, G.; Cortivo, R.; Herrera, M.B.; Tetta, C.; et al. A Combining Method to Enhance the In Vitro Differentiation of Hepatic Precursor Cells. Tissue Eng. Part C Methods 2010, 16, 1543–1551.
- Liu, Z.-J.; Zhuge, Y.; Velazquez, O.C. Trafficking and differentiation of mesenchymal stem cells. J. Cell. Biochem. 2009, 106, 984–991.
- Kobolak, J.; Dinnyes, A.; Memic, A.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 2016, 99, 62–68.
- Dowidar, M.; El-Belbasi, H.; Ayoub, A.; Rashed, L.; Elged, D. Biochemical and Molecular Studies on Bone Marrow Derived Stromal Stem Cells on Liver Injuries in Rats. Zagazig Vet. J. 2017, 45, 355–365.
- Lee, S.K.; Lee, S.C.; Kim, S.-J. A novel cell-free strategy for promoting mouse liver regeneration: Utilization of a conditioned medium from adipose-derived stem cells. Hepatol. Int. 2015, 9, 310–320.
- Yin, L.; Zhu, Y.; Yang, J.; Ni, Y.; Zhou, Z.; Chen, Y.; Wen, L. Adipose tissue-derived mesenchymal stem cells differentiated into hepatocyte-like cells in vivo and in vitro. Mol. Med. Rep. 2015, 11, 1722–1732.
- Galateanu, B.; Dinescu, S.; Cimpean, A.; Dinischiotu, A.; Costache, M. Modulation of Adipogenic Conditions for Prospective Use of hADSCs in Adipose Tissue Engineering. Int. J. Mol. Sci. 2012, 13, 15881–15900.
- Kang, S.H.; Kim, M.Y.; Eom, Y.W.; Baik, S.K. Mesenchymal Stem Cells for the Treatment of Liver Disease: Present and Perspectives. Gut Liver 2020, 14, 306–315.
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297.
- Hu, C.; Zhao, L.; Zhang, L.; Bao, Q.; Li, L. Mesenchymal stem cell-based cell-free strategies: Safe and effective treatments for liver injury. Stem Cell Res. Ther. 2020, 11, 377.
- Zhu, M.; Hua, T.; Ouyang, T.; Qian, H.; Yu, B. Applications of Mesenchymal Stem Cells in Liver Fibrosis: Novel Strategies, Mechanisms, and Clinical Practice. Stem Cells Int. 2021, 2021, 6546780.
- Dinescu, S.; Dobranici, A.; Tecucianu, R.; Selaru, A.; Balahura, R.; Ignat, S.; Costache, M. Exosomes as Part of the Human Adipose-Derived Stem Cells Secretome- Opening New Perspectives for Cell-Free Regenerative Applications. 2020, pp. 139–163. Available online: https://link.springer.com/chapter/10.1007/5584_2020_588 (accessed on 5 December 2021).
- Chiabotto, G.; Pasquino, C.; Camussi, G.; Bruno, S. Molecular Pathways Modulated by Mesenchymal Stromal Cells and Their Extracellular Vesicles in Experimental Models of Liver Fibrosis. Front. Cell Dev. Biol. 2020, 8.
- Vizoso, F.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852.
- Dinescu, S.; Hermenean, A.; Costache, M. Human Adipose-Derived Stem Cells for Tissue Engineering Approaches: Current Challenges and Perspectives. In Stem Cells in Clinical Practice and Tissue Engineering; InTech: London, UK, 2018.
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597.
- Dubey, N.; Mishra, V.; Dubey, R.; Deng, Y.-H.; Tsai, F.-C.; Deng, W.-P. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018, 19, 2200.
- Rabani, V.; Shahsavani, M.; Gharavi, M.; Piryaei, A.; Azhdari, Z.; Baharvand, H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol. Int. 2010, 34, 601–605.
- Lee, E.J.; Hwang, I.; Lee, J.Y.; Park, J.N.; Kim, K.C.; Kim, G.-H.; Kang, C.-M.; Kim, I.; Lee, S.-Y.; Kim, H.-S. Hepatocyte Growth Factor Improves the Therapeutic Efficacy of Human Bone Marrow Mesenchymal Stem Cells via RAD51. Mol. Ther. 2018, 26, 845–859.
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444.
- Lombardi, F.; Palumbo, P.; Augello, F.R.; Cifone, M.G.; Cinque, B.; Giuliani, M. Secretome of Adipose Tissue-Derived Stem Cells (ASCs) as a Novel Trend in Chronic Non-Healing Wounds: An Overview of Experimental In Vitro and In Vivo Studies and Methodological Variables. Int. J. Mol. Sci. 2019, 20, 3721.
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312.
- Psaraki, A.; Ntari, L.; Karakostas, C.; Korrou-Karava, D.; Roubelakis, M.G. Extracellular vesicles derived from Mesenchymal Stem/Stromal Cells: The regenerative impact in liver diseases. Hepatology 2021.
- Brigstock, D.R. Extracellular Vesicles in Organ Fibrosis: Mechanisms, Therapies, and Diagnostics. Cells 2021, 10, 1596.
- Rautiainen, S.; Laaksonen, T.; Koivuniemi, R. Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 10890.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383.
- Eom, Y.W.; Shim, K.Y.; Baik, S.K. Mesenchymal stem cell therapy for liver fibrosis. Korean J. Intern. Med. 2015, 30, 580–589.
- Afshari, A.; Shamdani, S.; Uzan, G.; Naserian, S.; Azarpira, N. Different approaches for transformation of mesenchymal stem cells into hepatocyte-like cells. Stem Cell Res. Ther. 2020, 11, 54.
- Salehinejad, P.; Alitheen, N.B.; Mandegary, A.; Nematollahi-mahani, S.N.; Janzamin, E. Effect of EGF and FGF on the expansion properties of human umbilical cord mesenchymal cells. Vitr. Cell. Dev. Biol. Anim. 2013, 49, 515–523.
- Neuss, S.; Becher, E.; Wöltje, M.; Tietze, L.; Jahnen-Dechent, W. Functional Expression of HGF and HGF Receptor/c-met in Adult Human Mesenchymal Stem Cells Suggests a Role in Cell Mobilization, Tissue Repair, and Wound Healing. Stem Cells 2004, 22, 405–414.
- Hong, S.H.; Gang, E.J.; Jeong, J.A.; Ahn, C.; Hwang, S.H.; Yang, I.H.; Park, H.K.; Han, H.; Kim, H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem. Biophys. Res. Commun. 2005, 330, 1153–1161.
- Miyajima, A.; Kinoshita, T.; Tanaka, M.; Kamiya, A.; Mukouyama, Y.; Hara, T. Role of Oncostatin M in hematopoiesis and liver development. Cytokine Growth Factor Rev. 2000, 11, 177–183.
- An, S.Y.; Jang, Y.J.; Lim, H.-J.; Han, J.; Lee, J.; Lee, G.; Park, J.Y.; Park, S.-Y.; Kim, J.H.; Do, B.-R.; et al. Milk Fat Globule-EGF Factor 8, Secreted by Mesenchymal Stem Cells, Protects Against Liver Fibrosis in Mice. Gastroenterology 2017, 152, 1174–1186.
- Yu, S.P.; Wei, Z.; Wei, L. Preconditioning Strategy in Stem Cell Transplantation Therapy. Transl. Stroke Res. 2013, 4, 76–88.
- Feng, Y.; Huang, W.; Meng, W.; Jegga, A.G.; Wang, Y.; Cai, W.; Kim, H.W.; Pasha, Z.; Wen, Z.; Rao, F.; et al. Heat Shock Improves Sca-1 + Stem Cell Survival and Directs Ischemic Cardiomyocytes Toward a Prosurvival Phenotype Via Exosomal Transfer: A Critical Role for HSF1/miR-34a/HSP70 Pathway. Stem Cells 2014, 32, 462–472.
- Hawkins, K.E.; Sharp, T.V.; McKay, T.R. The role of hypoxia in stem cell potency and differentiation. Regen. Med. 2013, 8, 771–782.
- Das, R.; Jahr, H.; van Osch, G.J.V.M.; Farrell, E. The Role of Hypoxia in Bone Marrow–Derived Mesenchymal Stem Cells: Considerations for Regenerative Medicine Approaches. Tissue Eng. Part B Rev. 2010, 16, 159–168.
- Bunnell, B.A.; Betancourt, A.M.; Sullivan, D.E. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res. Ther. 2010, 1, 34.
- Ha, T.; Hua, F.; Liu, X.; Ma, J.; McMullen, J.R.; Shioi, T.; Izumo, S.; Kelley, J.; Gao, X.; Browder, W.; et al. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc. Res. 2008, 78, 546–553.
- Lee, S.C.; Jeong, H.J.; Lee, S.K.; Kim, S.-J. Lipopolysaccharide preconditioning of adipose-derived stem cells improves liver-regenerating activity of the secretome. Stem Cell Res. Ther. 2015, 6, 75.
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Liver Fibrosis. Stem Cells Dev. 2013, 22, 845–854.
- Fiore, E.; Domínguez, L.M.; Bayo, J.; Malvicini, M.; Atorrasagasti, C.; Rodriguez, M.; Cantero, M.J.; García, M.; Yannarelli, G.; Mazzolini, G. Human umbilical cord perivascular cells-derived extracellular vesicles mediate the transfer of IGF-I to the liver and ameliorate hepatic fibrogenesis in mice. Gene Ther. 2020, 27, 62–73.
- Rong, X.; Liu, J.; Yao, X.; Jiang, T.; Wang, Y.; Xie, F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res. Ther. 2019, 10, 98.
- Jun, J.H.; Kim, J.Y.; Choi, J.H.; Lim, J.-Y.; Kim, K.; Kim, G.J. Exosomes from Placenta-Derived Mesenchymal Stem Cells Are Involved in Liver Regeneration in Hepatic Failure Induced by Bile Duct Ligation. Stem Cells Int. 2020, 2020, 5485738.
- Zhang, P.; Gan, Z.; Tang, L.; Zhou, L.; Huang, X.; Wang, J. Exosomes from microRNA-145-5p-modified HUCB-MSCs attenuate CCl4-induced hepatic fibrosis via down-regulating FSCN1 expression. Life Sci. 2021, 119404.
- Jiang, W.; Tan, Y.; Cai, M.; Zhao, T.; Mao, F.; Zhang, X.; Xu, W.; Yan, Z.; Qian, H.; Yan, Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl 4 -Induced Liver Injury through Antioxidant Effect. Stem Cells Int. 2018, 2018, 6079642.
- Rostom, D.M.; Attia, N.; Khalifa, H.M.; Abou Nazel, M.W.; El Sabaawy, E.A. The Therapeutic Potential of Extracellular Vesicles Versus Mesenchymal Stem Cells in Liver Damage. Tissue Eng. Regen. Med. 2020, 17, 537–552.
- Qu, Y.; Zhang, Q.; Cai, X.; Li, F.; Ma, Z.; Xu, M.; Lu, L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J. Cell. Mol. Med. 2017, 21, 2491–2502.
- Ohara, M.; Ohnishi, S.; Hosono, H.; Yamamoto, K.; Yuyama, K.; Nakamura, H.; Fu, Q.; Maehara, O.; Suda, G.; Sakamoto, N. Extracellular Vesicles from Amnion-Derived Mesenchymal Stem Cells Ameliorate Hepatic Inflammation and Fibrosis in Rats. Stem Cells Int. 2018, 2018, 3212643.
- Jin, Y.; Wang, J.; Li, H.; Gao, S.; Shi, R.; Yang, D.; Wang, X.; Wang, X.; Zhu, L.; Wang, X.; et al. Extracellular Vesicles Secreted by Human Adipose-derived Stem Cells (hASCs) Improve Survival Rate of Rats with Acute Liver Failure by Releasing lncRNA H19. EBioMedicine 2018, 34, 231–242.
- Liu, Y.; Lou, G.; Li, A.; Zhang, T.; Qi, J.; Ye, D.; Zheng, M.; Chen, Z. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 2018, 36, 140–150.
- Hsu, S.; Wang, B.; Kota, J.; Yu, J.; Costinean, S.; Kutay, H.; Yu, L.; Bai, S.; La Perle, K.; Chivukula, R.R.; et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J. Clin. Invest. 2012, 122, 2871–2883.
- Bandiera, S.; Pfeffer, S.; Baumert, T.F.; Zeisel, M.B. miR-122—A key factor and therapeutic target in liver disease. J. Hepatol. 2015, 62, 448–457.
- Lou, G.; Yang, Y.; Liu, F.; Ye, B.; Chen, Z.; Zheng, M.; Liu, Y. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J. Cell. Mol. Med. 2017, 21, 2963–2973.
