Cervical cancer is one of the most common types of carcinomas causing morbidity and mortality in women in all countries of the world. At the moment, the oncology, oncobiology, and oncomorphology of cervical cancer are characterized by the accumulation of new information; various molecular biological, genetic, and immunohistochemical methods of investigation of the mechanisms of cervical carcinogenesis are tested and applied; targeted antitumour drugs and diagnostic, prognostic, and predictive biomarkers are being searched for. Many issues of the etiopathogenesis of cervical cancer have not been sufficiently studied, and the role of many biomarkers characterizing various stages of cervical carcinogenesis remains unclear.
- carcinoma of the cervix
- cervical carcinogenesis
- biomarkers
- immunohistochemistry
1. Introduction
In the structure of morbidity and mortality, cervical cancer (CC) is one of the most common types of carcinomas among women in all countries of the world, in fourth place after breast cancer, colorectal cancer, and lung cancer [1]. According to the data of the International Agency for Research on Cancer, in 2018, the number of patients with cervical cancer was 570,000, an increase of 7.8% over the past decade, and the number of deaths from this disease amounted to 311,000 [1,2][1][2]. In 2020, 604,100 new cases of cervical cancer were detected around the world, and the number of deaths was 341,800 [1,2,3,4][1][2][3][4]. Therefore, despite the recommended screening programs for cervical cancer, morbidity and mortality rates from this oncopathology have been steadily growing in recent years, both worldwide and within the Russian Federation [4,5,6,7][4][5][6][7]. Indicators of later diagnosis of cervical cancer in stages III-IV of the disease in Russia have also reached high numbers—they made up 32.6% in 2018 [4]. Currently, the oncology, oncobiology, and oncomorphology of cervical cancer are characterized by the accumulation of new information; various molecular biological research methods aimed at studying the mechanisms of cervical carcinogenesis are being tested and applied; targeted anticancer drugs and biomarkers which are useful for the diagnosis of various stages of the tumor process in cervical cancer are being sought [8,9,10,11,12,13,14,15,16,17,18,19][8][9][10][11][12][13][14][15][16][17][18][19]. The search for reliable genetic, molecular and immunohistochemical markers for early diagnosis of precancerous and neoplastic processes in the cervix is also an important task of current interest in modern oncology. Biomarkers in oncology include certain genes, DNA and RNA molecules, proteins, enzymes, antigens, and other cellular and biological products that can be detected at various stages of carcinogenesis, under the influence of therapy, and are described in many modern reviews and articles concerning carcinogenesis, precancerous lesions, and cervical cancer ( Table 1 ).
| Croups of Markers | Markers | References | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomarkers of HPV infection and carcinogenesis | HPV DNA, E6/E7 mRNA p53, Rb, p16INK4a, telomerase RNA gene (TERC), serum SCC-Ag, OCVA1 |
[8,10,11,12,13,15,20,21,] | [8] | 22, | [10] | 23, | [ | 24,25, | 11][12][13][15][20][21][22][23][24][25] | 26 | [26] | ||||
| Markers of cell cycle and proliferation | Ki-67, cyclin D1, p53, p63 | [12,26,27,28,29,30,31, | ][28][29][ | 32, | 30 | 33,34, | ][31 | 35,36] | [12][26][27][32][33][34][35][36] | ||||||
| Markers of apoptosis | P53, BCL-2, BCL-XL, BAX | [10,27,37, | 27][ | 38, | 37][ | 39, | 38][ | 40, | 39][ | 41, | 40][ | 42, | 41][ | 43,44] | [10][42][43][44] |
| Expression of cytokeratins–markers of differentiation | CK7, CK8, CK17, CK19 | [27,36,37,45,46,47,48,49] | [27][36][37][45][46][47][48][49] | ||||||||||||
| Markers of cell adhesion, invasion and metastasis | E-cadherin, P-cadherin, CD44, ADAM9, MT1-MMP, TIMP-1, TIMP-2, MT1-MMP, MMP-2, MMP-1, MMP-9, MMP-14, proMMP-14 furin, gelatinase, TIMP-1 and TIMP-2 | [27,50,51,52,53,54,55] | [27][50][51][52][53][54][55] | ||||||||||||
| Biomarkers of cancer stem cells | Nanog, nucleostemin (NS), musashi1 (Msi1), SOX2, KLF4, CD133, Cd44, ALDH1, CD49f, ABCG2, BMI1, PIWIL2, LGR5, OCT4, CD117 | [48,56,57,58,59,60,61,62,63,64] | [48][56][57][58][59][60][61][62][63][64] | ||||||||||||
| Markers of angiogenesis | VEGF, podoplanin (PDPN), thrombospondin-1 (TSP-1, antiangiogenesis factor), CD31 (a nonspecific endothelial marker), CD34, CD105 (a tumor-specific endothelial marker) | [27,65, | 27 | 66,67, | ][65 | 68, | ][66 | 69, | ] | 70,71] | [[67][68][69][70][71] | ||||
| Vaginal microbiome, inflammation and immune homeostasis | Evaluation of the diversity of cervicovaginal microbiome | [56,72,73,74,75,76,77,78,79,80,81] | [56][72][73][74][75][76][77][78][79][80][81] |
According to the type of biomolecules and methods of their detection, genomic, transcriptomic, and metabolic factors (immunohistochemical, biochemical and others) are distinguished. Biomarkers can be divided into diagnostic markers and prognostic and predictive clinical factors ( Figure 1 ).
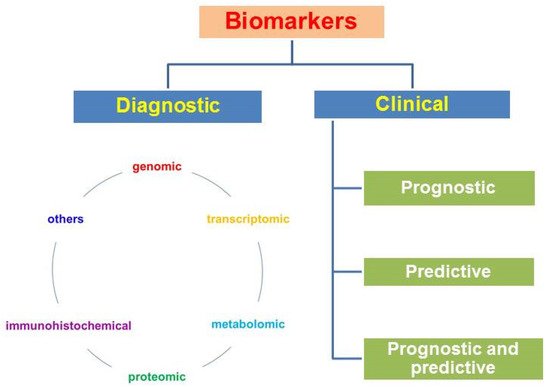
Diagnostic markers make diagnosis possible; clinical biomarkers include the following groups: (1) prognostic ones, which allow one to predict the course of the disease and survival without antitumor treatment; these are factors associated with tumor proliferation, differentiation, angiogenesis, invasion, or metastasis; (2) predictive ones, which allow prediction of the clinical effect, the relapse-free period, survival, sensitivity to treatment, and the toxicity of the planned treatment; they are associated with molecular targets for targeted drugs and a cascade of intracellular signals in a tumor cell; (3) there are factors that can simultaneously have both prognostic and predictive properties [82].
2. Mechanisms of Cervical Carcinogenesis
A complex genomic study of cervical cancer identified new mutated genes and gene amplifications in endometrioid-like cancers, consisting mainly of HPV-negative tumors with a high frequency of the mutations KRAS, ARID1A, and PTEN; it established that in more than 70% of cases of cervical cancer, genomic changes take place in one or both of the PI3K/MAPK and TGFβ signaling pathways [16]. These studies support the existence of various molecular subtypes of cervical cancer [16].
According to different research, in HPV infection and cervical cancer there are various epigenetic changes and DNA methylation processes, and the participation of microRNAs in these processes has been described [18,19,65,83,85,86,87,88][18][19][65][83][84][85][86][87]. At present, many of these processes have been studied insufficiently, and their interpretation is ambiguous, so the molecular mechanisms underlying these pathological changes are being investigated [11,14,85][11][14][84]. Recently, Wang et al. (2021) described the epigenetic regulation of the p16 tumor suppressor by miR 29a, which can justify the search for miRNA-based treatment options for cervical cancer [85][84]. It has been found that during the development of carcinomas, including various stages of cervical cancer, numerous epigenetic changes, such as hyper- and hypomethylation of DNA and tumor suppressor genes and modification of histones associated with the promoter regions of regulatory genes are observed. The results of meta-analysis confirm the hypermethylation of p16INK4a as an epigenetic marker for the progression of carcinogenesis in cervical cancer [65]. Expression of tumor suppressor genes, oncogenes, and growth factors is regulated by many histone-modifying enzymes, such as deacetylases and histone acetyltransferases; changes in the expression of histone modifiers also lead to epigenetic control of gene expression [16]. For example, it was found that DNA methylation is much higher in CIN2 and CIN3 compared to CIN1 [18]; the DNA methylation test is characterized by high specificity and sensitivity as compared to the ASCUS + cytological study and HPV16/18 genotyping [18]. Understanding the role of epigenetic changes in cervical carcinogenesis will help to develop effective methods for the detection and treatment of cervical cancer [8,18,85][8][18][84].
At the moment, the possible role of circular RNAs (circRNAs) in oncogenesis in the development of carcinomas of the organs of the female reproductive system is being investigated. It is believed that in cancer of the ovaries, endometrium, cervix, and breast carcinoma, circRNAs have prognostic, diagnostic, and therapeutic potential [86,87,88,89][85][86][87][88]. Circular RNAs are a new class of non-coding RNAs. In several types of cancer, abnormal expression of these biomolecules happens, for example, in virus-associated malignant neoplasms—anogenital carcinoma associated with HPV, oropharyngeal and oral cancer [89][88]. It has been shown that circRNAs are involved in oncogenesis, cancer progression, and drug resistance, and some of them are useful diagnostic and prognostic markers.
In cervical cancer, aberrant expression of miRNA, circRNA, and IncRNA has been revealed, making a new contribution to the understanding of the molecular mechanisms of oncogenesis, and these molecules may turn out to be potential biomarkers for oncological screening and monitoring of cancer recurrence [87,88,89][86][87][88]. Long non-coding RNAs (LncRNAs) (>200 nucleotides) are non-coding transcripts, and some of them have been associated with the development, progression, and metastasis of certain neoplasia, such as leukemia and breast, colon, and liver cancers. LncRNA can play a role at the transcriptional and post-transcriptional levels, contributing to the development of various diseases, drug resistance, signal modulation, DNA repair, the cell cycle, and apoptosis. Patterns of aberrant expression of some LncRNAs have been found in cervical carcinoma cells and in precancerous lesions of the cervix; nevertheless, molecular mechanisms and biological effects associated with LncRNA require further research [90][89]. The role of long non-coding RNAs associated with HPV and cervical cancer, which can play a role in the processes of cell proliferation, migration, invasion, and apoptosis, is being investigated. LncRNAs can be seen as candidates for prognostic and predictive markers; however, molecular mechanisms involving LncRNAs, ncRNAs, and mRNAs have not been elucidated yet and are subject to further clarification [90][89]. It is known that approximately 20–40% of high-grade intraepithelial neoplasms regress spontaneously; however, an increase in the 3q chromosomal region, in which the human telomerase RNA gene is localized at 3q26, correlates with the degree of disease in CIN and cervical cancer lesions. In a study of an increase in the 3q26 gene as a genetic marker for the prognosis of neoplasia using in situ hybridization, a connection with the prognosis was established; the absence of an increase in this DNA region during a diagnostic biopsy may indicate a high probability of regression of the disease [20].
3. Biomarkers of HPV Infection and Carcinogenesis in Cervical Cancer
Potential diagnostic and prognostic biomarkers in most neoplastic lesions of the cervix are based on molecular mechanisms associated with HPV infection [10,11,13][10][11][13]. Clinically significant markers of HPV-mediated oncogenesis are used in the following methods: determination of virus by PCR methods, assessment of viral load, detection of E6/E7 mRNA, p16INK4a methylation, p53, Rb and p16INK4a, other cell cycle biomarkers, proliferation and apoptosis, determined by immunohistochemistry [11,21,22,27][11][21][22][27]. The most widely used and well-studied biomarkers are HPV DNA in cervical epithelial cells and the immunohistochemically detectable expression of the p16INK4a protein, which is directly related to HPV oncogenic risk and Ki-67, which is a proliferation marker [10,11,12][10][11][12]. The p16 protein takes part in the regulation of the cell cycle and is an inhibitor of the activity of the cyclin-dependent kinase CDK4/6; normally its concentration in the cell is extremely low. Overexpression of p16 indicates active expression of the HPV E7 viral oncogene and inactivation of the retinoblastoma gene by the E7 virus protein; this is observed in malignant neoplasms caused by HPV of high oncogenic risk [10,11,12][10][11][12]. From 10% to 25% of cervical carcinomas are adenocarcinomas of various histotypes; high-risk HPV DNA is found in 94% of lesions in situ, 85% of adenosquamous carcinomas, and 76% of adenocarcinomas [23]. HPV DNA is most often found in endocervical adenocarcinoma of the usual type (90%), less often in serous (30%), clear cell (27%) and endometrioid carcinoma (13%). A surrogate marker of HPV is overexpression of p16, detected immunohistochemically; p16 is a sensitive and specific marker not only in the diagnosis of squamous cell neoplasms and squamous cell carcinoma, but also in identifying several histotypes of glandular neoplasms of the cervix [23].
HPV infection can lead to aneuploidy, chromosomal aberrations, and DNA hypermethylation. Potential biomarkers in cervical neoplasia are methylation factors and chromosomal abnormalities in cervical carcinoma detected by fluorescence in situ hybridization (FISH), in particular in the region of the telomerase RNA gene (TERC) located in the 3q26 region [20]. It has been established that E6 proteins of oncogenic HPV promote transcription of telomerase reverse transcriptase (TERT), which restores DNA at the telomeric end of chromosomes and therefore increases the cell division limit [11]. Increases of chromosome 3q, which contains the telomerase sequence (TERC), and chromosome 5p, which contains the TERT gene, are associated with intraepithelial neoplasia, which is a useful marker for detecting progressive lesions [8,11,15][8][11][15]. Telomerase is an enzyme which with an increase of its activity, compensates for the shortening of telomeres during sequential replications, which is observed during cell division; an increase in telomerase activity is characteristic of stem and tumor cells, and telomerase activity is not detected in most somatic cells [15]. It was found that the telomerase RNA gene in the 3q26 region is found in CIN lesions and cervical carcinoma and correlates with the degree of the disease. It was demonstrated by fluorescent in situ hybridization that patients without an increase in 3q26 showed regression of the disease, i.e., the lack of 3q26 increase on diagnostic biopsy can potentially be used to identify high-grade CIN lesions with a high probability of disease regression, preferably as part of a wider biomarker panel [20]. The authors suppose that 3q26 FISH may help select women with low-grade cervical lesions (LSIL) who do not need immediate colposcopy and patients with severe lesions (HSIL) who do not need immediate treatment. Such approach can help to reduce treatment costs and reduce the side effects of surgery treatment; however, further studies on larger sample groups are needed to confirm this hypothesis [20].
Serum biomarkers play an important role in the monitoring of many malignant neoplasms, including cervical cancer [24,25][24][25]. A well-known biomarker is the squamous cell carcinoma antigen SCC-Ag, which was discovered in 1977. The squamous cell carcinoma antigen (SCCa) is the most widely used and most reliable tumor marker for squamous cell carcinomas [24]. During the analysis of the literature devoted to the study of the level of SCCa in the blood serum as a predictor of cervical cancer metastases to lymph nodes, it was found that SCCa can serve as a reliable prognostic factor; the meta-analysis confirmed the value of the predicting property of SCCa, even though the predictive value was moderate. Elevated SCCa is clinically relevant information in addition to CT scan or magnetic resonance imaging (MRI) supporting the need for laparoscopic evaluation of the pelvic and/or para-aortic lymph nodes [24]. In a meta-analysis of the association of serum SCC-Ag with relapse and mortality in patients with squamous cell carcinoma of the cervix, it was found (in 61 articles) that SCC-Ag in serum was invariably associated with relapse and mortality in newly diagnosed cervical cancer; this marker may be useful for monitoring disease progression in patients with cervical cancer [25].
Despite the huge progress that has been made in the screening and management of women with HPV-associated cervical pathology, there is still a need for clinically reliable biomarkers to further improve the screening, diagnosis, and prognosis of cervical carcinomas [8,10,21,22][8][10][21][22]. An important role in clinical pathology and oncologic morphology is given to immunohistochemical methods aimed at solving diagnostic problems and searching for new prognostic and predictive factors. Taking into account the role of individual biomolecules in the cell during the development of physiological and pathological processes, the following categories of immunohistochemical biomarkers are distinguished –markers of the cell cycle, proliferation, apoptosis, adhesion, invasion, angiogenesis, microenvironment, immune factors, and others—which are currently being tested for verification of “cancer stem cells” [56].
4. Biomarkers of Cancer Stem Cells in Cervical Carcinomas
In recent years, the concepts of tumor (cancer) stem cells (CSCs) and tumor microenvironments (niches) have been suggested and developed [57,91][57][90]; various biomarkers of tumor stem cells, including immunohistochemical ones, are being investigated. According to the modern concept, the CSC population is a small fraction of the total quantity of tumor cells, characterized by the expression of certain surface markers that allow it to be identified and isolated, support the growth of a heterogeneous population of tumor cells, form a separate pool of cells, be verified by biological and physicochemical methods, and have an unlimited capability of self-renewal and differentiation in various directions with the formation of a heterogeneous population of tumor cells consisting of different clones, and it is characterized by high resistance to therapy [57]. Resistance to therapy may occur due to the selective expression of some members of the multidrug resistance transporter family, an increase in the expression of anti-apoptotic molecules, an increased ability to repair DNA, or activation of stem cell-specific survival signals (pro-survival signaling), namely Notch, Hedgehog (Hh), Wnt, JAK/STAT, and others [57].
An important role in the structure of the tumor is assigned to the CSC microenvironment, the so-called “niches”. Niches are certain anatomical compartments in tissue surrounding stem cells which regulate the participation of these cells in the formation, maintenance, and repair of tissue [48,57][48][57]. The microenvironment includes such structures and components as the extracellular matrix, mesenchymal and endothelial cells, cells of the immune system, adhesion molecules, growth factors, cytokines and their receptors, and blood vessels [57]. The tumor microenvironment is represented by a stroma with cells of various types: fibroblasts, tumor-associated fibroblasts, myofibroblasts, smooth muscle cells, endotheliocytes, pericytes, neutrophilic and eosinophilic leukocytes, basophils, mast cells, T- and B-lymphocytes, macrophages, and dysplasia [91][90]. According to Zibirov and Mozerov (2018), understanding the mechanisms of interaction between tumor cells and the microenvironment is promising for increasing the effectiveness of treatment [49].
Currently, there is a significant number of publications dedicated to different problems associated with CSCs of various tumors. The tasks of verifying CSCs by detecting cell markers (antigens) by immunohistochemical methods are urgent. A large number of experimental works and reviews are devoted to the identification of surface antigens characteristic of CSCs in various tumors, including cervical cancer, and the list of such markers is constantly growing [48,56,58,59,60,61,62,63,64][48][56][58][59][60][61][62][63][64]. Recently, it is believed that cervical cancer develops from stem cells of the transformation zone upon infection with HPV of high oncogenic risk, where the transformation zone is a niche for cells with a unique expression profile and embryonic characteristics [48]. Modern data on CSCs in cervical cancer are, however, incomplete. It has been established that one of the markers of cervical CSC is Nanog, a factor that is expressed mainly in the internal cell mass of blastocysts; its expression is significantly expressed in early embryonic stem cells and decreased in the processes of differentiation. Nanog plays an important role in the regulation of pluripotent cells in embryogenesis, and recently it was found to be closely associated with oncogenesis; it is found in cancer of the stomach and breast, glioblastoma, lung cancer, and various human sarcomas [48,58][48][58]. There is evidence of a pronounced expression of Nanog in the cervix with CIN-II and -III and in cervical carcinoma cells, in contrast to weak expression in CIN I and in normal epithelium [58]. Ye et al. (2008), in an immunohistochemical study of the cervix of 235 patients, showed that the expression of Nanog, nucleostemin (NS) and musashi1 (Msi1) was significantly higher in cervical cancer compared to CIN, and in CIN compared to normal cervical epithelium; the results give evidence that Nanog, NS, and Msi1 may be involved in the carcinogenesis and progression of cervical cancer [58]. Recent studies have demonstrated the expression of CK 17 and CK19 on CSCs; however, further research is required to understand the possible role of CK19 in CSCs, as the study of cervical CSCs is currently at an early stage, so there are no internationally recognized markers of the cells [48].
There is evidence that Sox2-positive SiHa and C33A cells, as compared to Sox2-negative cells, demonstrate more pronounced abilities for self-renewal, differentiation, and tumor formation [62]. In addition, Liu et al. (2014) found that Sox2-positive SiHa and C33A cells expressed higher levels of several genes associated with SCSs, i.e., cells expressing endogenous Sox2 are CSCs in cervical carcinomas [62]. In a review publication devoted to CSCs in cervical cancer, the following factors are included in the group of cancer stem cell markers: ABCG2, ALDH1, CD133, CD49f, OCT4, OPN, and SOX2 [63]. Nevertheless, at present, many issues related to cervical CSCs have not been sufficiently studied, so the specificity of such a general CSC biomarker as CD44, which is used in the diagnosis of many tumors in cervical cancer, is not sufficiently established. The proto-oncogene c-Kit (protein tyrosine kinase KIT or CD117), a transmembrane cytokine receptor, is phosphorylated and activated by binding to a KIT ligand called stem cell factor, which is a marker of SCSs in ovarian and endometrial cancers and osteosarcoma; however, the proposal that c-Kit is a marker of CSCs in cervical cancer is not yet proved. NANOG is a transcription factor that plays an important role in maintaining the pluripotency of CSCs and regulation of proliferation and asymmetric division; it is used widely as a marker of CSCs that regulate renewal and tumorigenesis in many tumors [63]. Therefore, stem cells play a key role in the physiology of the female reproductive system; however, according to Lopez et al. (2013), this field is still in its infancy—definitive markers need to be identified for more selective isolation and enrichment of stem cells, and further research is needed to assess clinical characteristics, prognosis, survival, and population characteristics; these studies will improve the understanding of carcinogenesis in ovarian and uterine cancer and cervical carcinoma and may be useful in the treatment of these neoplasms [64].
References
- Female Genital Tumours. WHO Classification of Tumors, 5th ed.; WHO Press: Geneva, Switzerland, 2020; Volume 4.
- World Health Organization; International Agency for Research on Cancer (IARC); Global Cancer Observatory (GCO). Available online: https://gco.iarc.fr (accessed on 15 May 2021).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789.
- Kaprin, A.D.; Starinskiy, V.V.; Shakhzadova, A.O. The state of cancer care for the population of Russia in 2019. M.: MNIOI named after P.A. Herzen–Branch of the Federal State Budgetary Institution “National Medical Research Center of Radiology” of the Minzdrav of Russia. 2020. Available online: https://glavonco.ru/cancer_register/noMo^b (accessed on 15 May 2021).
- Comprehensive Cervical Cancer Control: A Guide to Essential Practice, 2nd ed.; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2014.
- Glukhova, Y.K.; Volchenko, N.N. Project of the program of national cytological screening for cervical cancer. Clin. Cytol. News Russ. 2018, 22, 22–30.
- Kiselev, K.V.; Muyzhnek, M.E.; Ashrafyan, A.L.; Sukhikh, S.G. A new paradigm in cervical neoplasia progression: From fundamental knowledge to practical gynecology. Obstet. Gynecol. 2019, 1, 5–12.
- Hwang, S.J.; Shroyer, K.R. Biomarkers of Cervical Dysplasia and Carcinoma. J. Oncol. 2012, 2012, 507286.
- Rachkovskaya, V.I.; Gorbunov, A.; Pashov, A.; Volkova, L.; Kant Baltic Federal University. The use of mass spectrometry in cervical pathology diagnostics literature review. Sib. Med. Rev. 2020, 2020, 30–37.
- Nakamura, M.; Obata, T.; Daikoku, T.; Fujiwara, H. The Association and Significance of p53 in Gynecologic Cancers: The Potential of Targeted Therapy. Int. J. Mol. Sci. 2019, 20, 5482.
- Yeo-Teh, N.S.L.; Ito, Y.; Jha, S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. Int. J. Mol. Sci. 2018, 19, 1706.
- Balasubramaniam, S.D.; Balakrishnan, V.; Oon, C.E.; Kaur, G. Key Molecular Events in Cervical Cancer Development. Medicina 2019, 55, 384.
- Rogovskaya, S.I. Human papillomavirus infection of the genitals. In Cervix, Vagina, Vulva. Physiology, Pathology, Colposcopy, Esthetic Correction: A Guide for Practicing Physicians, 2nd ed.; Rogovskaya, S.I., Lipova, E.V., Eds.; M: Status Praesens; Journal Publishing House Status Praesens: Moscow, Russian, 2016; pp. 305–378.
- Sen, P.; Ganguly, P.; Ganguly, N. Modulation of DNA methylation by human papillomavirus E6 and E7 oncoproteins in cervical cancer (Review). Oncol. Lett. 2017, 15, 11–22.
- Korolenkova, L.I. Cervical Intraepithelial Neoplasms and Early Forms of Cervical Cancer: Clinical and Morphological Concept of Cervical Carcinogenesis. M; 2017; ISBN 978-5-903759-28-6. Available online: http://www.medlib.kuzdrav.ru/articles/26/4361 (accessed on 15 May 2021).
- The Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384.
- Tanaka, T.I.; Alawi, F. Human Papillomavirus and Oropharyngeal Cancer. Dent. Clin. N. Am. 2018, 62, 111–120.
- Kelly, H.; Benavente, Y.; Pavon, M.A.; De Sanjose, S.; Mayaud, P.; Lorincz, A.T. Performance of DNA methylation assays for detection of high-grade cervical intraepithelial neoplasia (CIN2+): A systematic review and meta-analysis. Br. J. Cancer 2019, 121, 954–965.
- Chaiwongkot, A.; Vinokurova, S.; Pientong, C.; Ekalaksananan, T.; Kongyingyoes, B.; Kleebkaow, P.; Chumworathayi, B.; Patarapadungkit, N.; Reuschenbach, M.; Doeberitz, M.V.K. Differential methylation of E2 binding sites in episomal and integrated HPV 16 genomes in preinvasive and invasive cervical lesions. Int. J. Cancer 2013, 132, 2087–2094.
- Koeneman, M.M.; Ovestad, I.T.; Janssen, E.A.M.; Ummelen, M.; Kruitwagen, R.F.P.M.; Hopman, A.H.; Kruse, A.J. Gain of Chromosomal Region 3q26 as a Prognostic Biomarker for High-Grade Cervical Intraepithelial Neoplasia: Literature Overview and Pilot Study. Pathol. Oncol. Res. 2019, 25, 549–557.
- Volkova, T.O.; Kurmyshkina, O.V.; Kovchur, P.I.; Bakhidze, E.V. Biomarkers for theranostics of cervical cancer. J. Biomed. Technol. 2014, 1, 45–51.
- Tornesello, M.L.; Buonaguro, L.; Giorgi-Rossi, P.; Buonaguro, F.M. Viral and Cellular Biomarkers in the Diagnosis of Cervical Intraepithelial Neoplasia and Cancer. BioMed Res. Int. 2013, 2013, 519619.
- Lee, S.; Rose, M.S.; Sahasrabuddhe, V.V.; Zhao, R.; Duggan, M.A.; Mendoza-Cervantes, D.; Eng, B. Tissue-based Immunohistochemical Biomarker Accuracy in the Diagnosis of Malignant Glandular Lesions of the Uterine Cervix. Int. J. Gynecol. Pathol. 2017, 36, 310–322.
- Zhou, Z.; Li, W.; Zhang, F.; Hu, K. The value of squamous cell carcinoma antigen (SCCa) to determine the lymph nodal metasta-sis in cervical cancer: A meta-analysis and literature review. PLoS ONE 2017, 12, e0186165.
- Charakorn, C.; Thadanipon, K.; Chaijindaratana, S.; Rattanasiri, S.; Numthavaj, P.; Thakkinstian, A. The association between serum squamous cell carcinoma antigen and recurrence and survival of patients with cervical squamous cell carcinoma: A systematic review and meta-analysis. Gynecol. Oncol. 2018, 150, 190–200.
- Tong, R.; Yang, Q.; Wang, C.; Bi, F.; Jiang, B. OVCA1 expression and its correlation with the expression levels of cyclin D1 and p16 in cervical cancer and intraepithelial neoplasia. Oncol. Lett. 2017, 13, 2929–2936.
- Babichenko, I.I.; Kovyazin, V.A. New Methods of Immunohistochemical Diagnostics of Tumor Growth. M: RUDN. 2008. Available online: https://sci.house/onkologiya/novye-metody-immunogistohimicheskoy.html (accessed on 15 May 2021).
- Piri, R.; Ghaffari, A.; Gholami, N.; Azami-Aghdash, S.; Pour Ali-Akbar, Y.; Saleh, P.; Naghavi-Behzad, M. Ki-67/MIB-1 as a Prognostic Marker in Cervical Cancer—A Systematic Review with Meta-Analysis. Asian Pac. J. Cancer Prev. 2015, 16, 6997–7002.
- Chen, C.-C.; Huang, L.-W.; Bai, C.-H.; Lee, C.-C. Predictive value of p16/Ki-67 immunocytochemistry for triage of women with abnormal Papanicolaou test in cervical cancer screening: A systematic review and meta-analysis. Ann. Saudi Med. 2016, 36, 245–251.
- Van Zummeren, M.; Leeman, A.; Kremer, W.W.; Bleeker, M.C.; Jenkins, D.; van de Sandt, M.; Heideman, D.A.M.; Steenbergen, R.; Snijders, P.J.F.; Quint, W.G.V.; et al. Three-tiered score for Ki-67 and p16ink4a improves accuracy and repro-ducibility of grading CIN lesions. J. Clin. Pathol. 2018, 71, 981–988.
- Mitildzans, A.; Arechvo, A.; Rezeberga, D.; Isajevs, S. Expression of p63, p53 and ki-67 in patients with cervical intraepithelial neoplasia. Turk. J. Pathol. 2016, 33, 9–16.
- Silva, D.C.; Gonçalves, A.; Cobucci, R.N.; Mendonça, R.C.; Lima, P.H.; Cavalcanti, G. Immunohistochemical expression of p16, Ki-67 and p53 in cervical lesions—A systematic review. Pathol.-Res. Pr. 2017, 213, 723–729.
- Chen, G.; Pan, D.; Wei, K.; Ling, Y.; Su, S.; Zhu, M. The Prognostic Role of Ki-67/MIB-1 in Cervical Cancer: A Systematic Review with Meta-Analysis. Med. Sci. Monit. 2015, 21, 882–889.
- Volkova, V.L.; Paramzin, P.F.; Pashov, A.; Musatov, M.A.; Ashrafyan, A.L.; Hospital, K.C.C.C. Assessment of Ki67 immunohistochemical expression as a prognostic marker in breast carcinoma. Akusherstvo I Ginekol. 2020, 1 (Suppl. S1), 81–86.
- Peres, A.L.; Silva, K.M.P.E.; De Araújo, R.F.F.; Filho, J.L.D.L.; Júnior, M.R.D.M.; Martins, D.B.G.; Filho, N.T.D.P. Immunocytochemical study of TOP2A and Ki-67 in cervical smears from women under routine gynecological care. J. Biomed. Sci. 2016, 23, 42.
- Da Costa, L.B.E.; Triglia, R.D.M.; Andrade, L.A.L.D.A. p16INK4a, Cytokeratin 7, and Ki-67 as Potential Markers for Low-Grade Cervical Intraepithelial Neoplasia Progression. J. Low. Genit. Tract Dis. 2017, 21, 171–176.
- Diagnostic Immunohistochemistry. Theranostic and Genomic Applications, 5th ed.; Dabbs, D.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323477321.
- Qin, C.; Chen, X.; Bai, Q.; Davis, M.R.; Fang, Y. Factor associated with radiosensitivity of cervical cancer (review). Anticancer Res. 2014, 34, 4649–4656.
- Kilic, S.; Cracchiolo, B.; Gabel, M.; Haffty, B.; Mahmoud, O. The relevance of molecular biomarkers in cervical cancer patients treated with radiotherapy. Ann. Transl. Med. 2015, 3, 1–16.
- Srinivasan, R.; Sood, S.; Patel, F.D.; Dhaliwal, L.K. Chemoradiation therapy induces in vivo changes in gene promoter methylation & gene transcript expression in patients with invasive cervical cancer. Indian J. Med. Res. 2018, 147, 151–157.
- Kato, R.; Hasegawa, K.; Torii, Y.; Udagawa, Y.; Fukasawa, I. Factors affecting platinum sensitivity in cervical cancer. Oncol. Lett. 2015, 10, 3591–3598.
- Orun, O.; Tiber, P.M.; Baloglu, L.; Ozden, S.; Ozgen, Z.; Ozyurt, H.; Eren, M. The association of apoptotic protein expressions sensitive to apoptosis gene, p73 and p53 with the prognosis of cervical carcinoma. OncoTargets Ther. 2014, 7, 2161–2168.
- Bosse, T.; Lax, S.; Abu-Rustum, N.; Matias-Guiu, X. The Role of Predictive Biomarkers in Endocervical Adenocarcinoma: Recommendations from the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2021, 40, S102–S110.
- Shukla, S.; Dass, J.; Pujani, M. p53 and bcl2 expression in malignant and premalignant lesions of uterine cervix and their correlation with human papilloma virus 16 and 18. S. Asian J. Cancer 2014, 3, 048–053.
- Lee, H.; Lee, H.; Cho, Y.K. Cytokeratin7 and cytokeratin19 expression in high grade cervical intraepithelial neoplasm and squamous cell carcinoma and their possible association in cervical carcinogenesis. Diagn. Pathol. 2017, 12, 18.
- Huang, E.C.; Tomic, M.M.; Hanamornroongruang, S.; Meserve, E.E.; Herfs, M.; Crum, C.P. p16ink4 and cytokeratin 7 immunostaining in predicting HSIL outcome for low-grade squamous intraepithelial lesions: A case series, literature review and commentary. Mod. Pathol. 2016, 29, 1501–1510.
- Paquette, C.; Mills, A.; Stoler, M.H. Predictive Value of Cytokeratin 7 Immunohistochemistry in Cervical Low-grade Squamous Intraepithelial Lesion as a Marker for Risk of Progression to a High-grade Lesion. Am. J. Surg. Pathol. 2016, 40, 236–243.
- Yao, T.; Lu, R.; Zhang, Y.; Zhang, Y.; Zhao, C.; Lin, R.; Lin, Z. Cervical cancer stem cells. Cell Prolif. 2015, 48, 611–625.
- Rabban, J.T.; Longacre, T.A. Immunohistology of the female genital tract. In Diagnostic Immunohistochemistry: Theranostic and Genomic Applications, 4th ed.; Dabbs, D.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 653–709.
- Li, B.; Shi, H.; Wang, F.; Hong, D.; Lv, W.; Xie, X.; Cheng, X. Expression of E-, P- and N-Cadherin and Its Clinical Significance in Cervical Squamous Cell Carcinoma and Precancerous Lesions. PLoS ONE 2016, 11, e0155910.
- Zubel, A.; Flechtenmacher, C.; Edler, L.; Alonso, A. Expression of ADAM9 in CIN3 lesions and squamous cell carcinomas of the cervix. Gynecol. Oncol. 2009, 114, 332–336.
- Isa, S.A.M.; Salleh, S.M.; Ismail, M.; Hairon, S.M. ADAM9 Expression in Uterine Cervical Cancer and Its Associated Factors. Asian Pac. J. Cancer Prev. 2019, 20, 1081–1087.
- Timoshenko, O.S.; Gureeva, T.A.; Kugaevskaia, E.V.; Solov’eva, N.I. Membrane type 1 matrix metalloproteinase (MT1-MMP) and the regulators of its activity as invasive factors in squamous cell cervical carci-nomas. Biomed Khim. 2014, 60, 683–688.
- Solovyeva, N.; Timoshenko, O.; Gureeva, T.; Kugaevskaya, E.V. Matrix metalloproteinases and their endogenous regulators in squamous cervical carcinoma (review of the own data). Biomeditsinskaya Khimiya 2015, 61, 694–704.
- Timoshenko, O.S.; Gureeva, T.A.; Kugaevskaya, E.V.; Zavalishina, L.E.; Andreeva, Y.Y.; Solovyeva, N.I. Tissue collagenase MMP-14 and endogenous regulators of its activity in the corpus uteri in squamous cell carcinoma of the cervix. Arkhiv Patol. 2017, 79, 36–42.
- Mendoza, G.; Rocha-Zavaleta, L.; Esparza-Ibarra, E.; Olmos, J. Cervical cancer stem cells and other leading factors associated with cervical cancer development (Review). Oncol. Lett. 2019, 18, 3423–3432.
- Vinogradova, T.V.; Chernov, I.P.; Monastyrskaya, G.S.; Kondratyeva, L.G.; Sverdlov, E.D. Cancer stem cells: Plasticity works against therapy. Acta Nat. 2015, 7, 46–55.
- Ye, F.; Zhou, C.; Cheng, Q.; Shen, J.; Chen, H. Stem-cell-abundant proteins Nanog, Nucleostemin and Musashi1 are highly expressed in malignant cervical epithelial cells. BMC Cancer 2008, 8, 108.
- Tulake, W.; Yuemaier, R.; Sheng, L.; Ru, M.; Lidifu, D.; Abudula, A. Upregulation of stem cell markers ALDH1A1 and OCT4 as potential biomarkers for the early detection of cervical carcinoma. Oncol. Lett. 2018, 16, 5525–5534.
- Fu, H.-C.; Chuang, I.-C.; Yang, Y.-C.; Chuang, P.-C.; Lin, H.; Ou, Y.-C.; Chang Chien, C.-C.; Huang, H.-S.; Kang, H.-Y. Low P16INK4A Expression Associated with High Expression of Cancer Stem Cell Markers Predicts Poor Prognosis in Cervical Cancer after Radiotherapy. Int. J. Mol. Sci. 2018, 19, 2541.
- Hou, T.; Zhang, W.; Tong, C.; Kazobinka, G.; Huang, X.; Huang, Y.; Zhang, Y. Putative stem cell markers in cervical squamous cell carcinoma are correlated with poor clinical outcome. BMC Cancer 2015, 15, 785.
- Liu, X.-F.; Yang, W.-T.; Xu, R.; Liu, J.-T.; Zheng, P.-S. Cervical Cancer Cells with Positive Sox2 Expression Exhibit the Properties of Cancer Stem Cells. PLoS ONE 2014, 9, e87092.
- Huang, R.; Rofstad, E.K. Cancer stem cells (CSCs), cervical CSCs and targeted therapies. Oncotarget 2017, 8, 35351–35367.
- López, J.; Valdez-Morales, F.J.; Benítez-Bribiesca, L.; Cerbón, M.; Carrancá, A.G. Normal and cancer stem cells of the human female reproductive system. Reprod. Biol. Endocrinol. 2013, 11, 53.
- Han, Y.-D.; Wang, X.-B.; Cui, N.-H.; Zhang, S.; Wang, C.; Zheng, F. Associations of P16INK4a promoter hypermethylation with squamous intra-epithelial lesion, cervical cancer and their clinicopathological features: A meta-analysis. Oncotarget 2017, 8, 1871–1883.
- Belfort-Mattos, P.N.; Focchi, G.R.D.A.; Ribalta, J.C.L.; De Lima, T.M.; Carvalho, C.R.N.; Tso, F.K.; Speck, N.M.D.G. Immunohistochemical Expression of VEGF and Podoplanin in Uterine Cervical Squamous Intraepithelial Lesions. Dis. Markers 2016, 2016, 8293196.
- Van Trappen, P.O.; Steele, D.; Lowe, D.G.; Baithun, S.; Beasley, N.; Thiele, W.; Weich, H.; Krishnan, J.; Shepherd, J.H.; Pepper, M.S.; et al. Expression of vascular endothelial growth factor (VEGF)-C and VEGF-D, and their receptor VEGFR-3, during different stages of cervical carcinogenesis. J. Pathol. 2003, 201, 544–554.
- Randall, L.M.; Monk, B.J.; Darcy, K.M.; Tian, C.; Burger, R.A.; Liao, S.-Y.; Peters, W.A.; Stock, R.J.; Fruehauf, J.P. Markers of angiogenesis in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 2009, 112, 583–589.
- Li, Y.; Zhao, S. The expression and underlying angiogenesis effect of DPC4 and VEGF on the progression of cervical carcinoma. Oncol. Lett. 2017, 15, 2534–2540.
- Nagy, V.M.; Buiga, R.P.; Brie, I.; Todor, N.; Tudoran, O.; Ordeanu, C.; Virág, P.; Tarta, O.; Rus, M.; Bălăcescu, O. Expression of VEGF, VEGFR, EGFR, COX-2 and MVD in cervical carcinoma, in relation with the response to radio-chemotherapy. Rom. J. Morphol. Embryol. 2011, 52, 53–59.
- Lin, J.; Lu, J.; Wang, C.; Xue, X. The prognostic values of the expression of Vimentin, TP53, and Podoplanin in patients with cervical cancer. Cancer Cell Int. 2017, 17, 80.
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome 2016, 4, 58.
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 171–180.
- Gillet, E.; Meys, J.F.A.; Verstraelen, H.; Verhelst, R.; De Sutter, P.; Temmerman, M.; Broeck, D.V. Association between Bacterial Vaginosis and Cervical Intraepithelial Neoplasia: Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, e45201.
- Curty, G.; De Carvalho, P.S.; Soares, M.A. The Role of the Cervicovaginal Microbiome on the Genesis and as a Biomarker of Premalignant Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer. Int. J. Mol. Sci. 2019, 21, 222.
- Kyrgiou, M.; Mitra, A.; Moscicki, A.-B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl. Res. 2017, 179, 168–182.
- Usyk, M.; Zolnik, C.P.; Castle, P.E.; Porras, C.; Herrero, R.; Gradissimo, A.; Gonzalez, P.; Safaeian, M.; Schiffman, M.R.; Burk, R.D.; et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathog. 2020, 16, e1008376.
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015, 5, 16865.
- Smola, S. Immunopathogenesis of HPV-Associated Cancers and Prospects for Immunotherapy. Viruses 2017, 9, 254.
- Kozlov, N.N.; Volkova, L.V.; Shatilova, A.A.; Shushval, M.S. Factors associated with cervical neoplastic processes. Mod. Probl. Sci. Educ. 2019, 4, 10.
- Kurmyshkina, O.V.; Kovchur, P.I.; Volkova, T.O. ‘Drawing’ a Molecular Portrait of CIN and Cervical Cancer: A Review of Genome-Wide Molecular Profiling Data. Asian Pac. J. Cancer Prev. 2015, 16, 4477–4487.
- Snegovoy, A.V.; Manzyuk, L.V. The value of biomarkers for deter-mining the tactics of treatment and prognosis of malignant tumors. Pract. Oncol. 2011, 12, 166–170.
- Bhattacharjee, B.; Sengupta, S. CpG methylation of HPV 16 LCR at E2 binding site proximal to P97 is associated with cervical cancer in presence of intact E2. Virology 2006, 354, 280–285.
- Wang, A.; Xu, Q.; Sha, R.; Bao, T.; Xi, X.; Guo, G. MicroRNA-29a inhibits cell proliferation and arrests cell cycle by modulating p16 methylation in cervical cancer. Oncol. Lett. 2021, 21, 272.
- Tran, A.M.; Chalbatani, G.M.; Berland, L.; Santos, M.C.D.L.; Raj, P.; Jalali, S.A.; Gharagouzloo, E.; Ivan, C.; Dragomir, M.P.; Calin, G.A. A New World of Biomarkers and Therapeutics for Female Reproductive System and Breast Cancers: Circular RNAs. Front. Cell Dev. Biol. 2020, 8, 50.
- Tornesello, M.L.; Faraonio, R.; Buonaguro, L.; Annunziata, C.; Starita, N.; Cerasuolo, A.; Pezzuto, F.; Tornesello, A.L.; Buonaguro, F.M. The role of microRNAs, long non-coding RNAs, and circular RNAs in cervical cancer. Front. Oncol. 2020, 10, 150.
- Di Domenico, M.; Giovane, G.; Kouidhi, S.; Iorio, R.; Romano, M.; De Francesco, F.; Feola, A.; Siciliano, C.; Califano, L.; Giordano, A. HPV epigenetic mechanisms related to oropharyngeal and cervix cancers. Cancer Biol. Ther. 2018, 19, 850–857.
- Bonelli, P.; Borrelli, A.; Tuccillo, F.M.; Buonaguro, F.M.; Tornesello, M.L. The Role of circRNAs in Human Papillomavirus (HPV)-Associated Cancers. Cancers 2021, 13, 1173.
- Sun, W.; Shen, N.-M.; Fu, S.-L. Involvement of lncRNA-mediated signaling pathway in the development of cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3672–3687.
- Zibirov, R.F.; Mozerov, S.A. Characterization of the tumor cell microenvironment. Onkol. Zhurnal Im. P.A. Gertsena 2018, 7, 67–72.
