Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Robert Szabo.
Nanoparticles (NPs) improve delivery of ions and confer targeting capabilities, with the potential for use in treatment and diagnosis. Iron deficiency, cancer, and sepsis are persisting major issues. While targeted delivery using Fe NPs can be used as food fortifiers, chemotherapeutic agents against cancer cells and microbes have been developed using both Fe and Cu NPs. A fast and accurate means of diagnosis is a major impacting factor on outcome of patients, especially when critically ill. Good quality imaging and bed side diagnostic tools are possible using NPs, which may positively impact outcome.
- nanoparticles
- copper
- zinc
- Iron
1. Introduction
Divalent metal ions are part of the physiology of complex cellular organisms such as humans but are also essential for the simpler organisms such as bacteria and fungi. The most abundant ion in humans is iron, a divalent element essential for many processes. Like iron, both copper and zinc are divalent metals possessing the ability to partake in Fenton reaction [1]. Byproducts of reduction and oxidation produce cell damage by denaturing proteins such as enzymes and DNA but also phospholipid structures such as cell membranes. These processes are a double-edged sword because, on one hand, they damage the self-organism, and on the other, these ions can act as defense mechanisms when aimed against foreign organisms. The fine balance is maintained by a complex network of homeostatic mechanisms.
Fe, Cu, and Zn are cofactors in processes of protein synthesis. This explains why their role is essential in the functioning of many organs and systems (Figure 1) [2]. Some proteins act as enzymes while others play a structural role; for example, the hemoglobin and DNA. In simpler organisms such as bacteria, divalent ions are involved in enzyme production, transcription, and signal transduction, which confer them the ability to be virulent and to replicate [3]. The shared need for these elements creates competition between invader (bacteria) and host (human). When withheld by the host, it represents a natural defense against the invader.
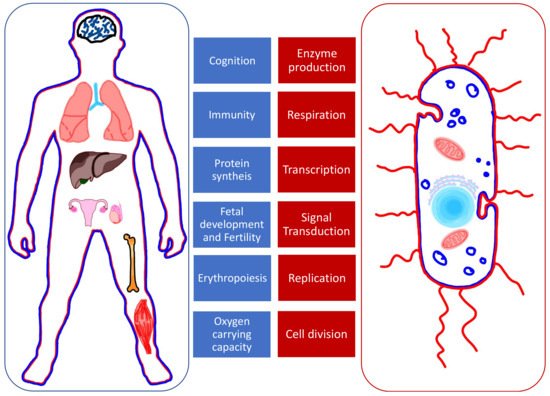
Figure 1. The roles of Fe, Cu, and Zn in human organs (left, in blue) and bacteria (right, in red).
2. Medical Uses of Iron, Copper, and Zinc Nanoparticles
Understanding the metabolism of these ions in normal and abnormal conditions allows for the use of nanoparticles in treatment and diagnosis (Figure 52). NPs can be used to alter the levels of the ions already present in organisms or, when produced as ion-NPs, to take advantage of homeostatic pathways present in the human body. Nanoparticles are produced by physicochemical processes, which use heat, ultrasound, or microwaves. Various geometrical shapes and sizes can be obtained by altering the reactants’ concentrations or the conditions of the reaction. When arranged in more complex structures, nanoparticles gain superior properties such as improved bioavailability, targeting capabilities, and theragnostic properties [37][4]. Spherical and octopod particles, apart from their imaging properties, also possess the ability to produce heat [38][5]. Size also influences their properties. One-pot synthetic procedure, as described by Lu Z. et al. and later modified by Ledda M. et al., has been used to produce ultra-small particles. Ultra-small iron oxide nanoparticles (USIONPs), due to their size of less than 5 nm, penetrate cells more readily while retaining their paramagnetic properties. Moreover, increasing evidence supports vascular as well as blood homeostasis applications of iron oxide nanoparticles, including thrombolysis, vascular grafts and stents, atherosclerosis treatment, or cardiovascular regeneration [39][6].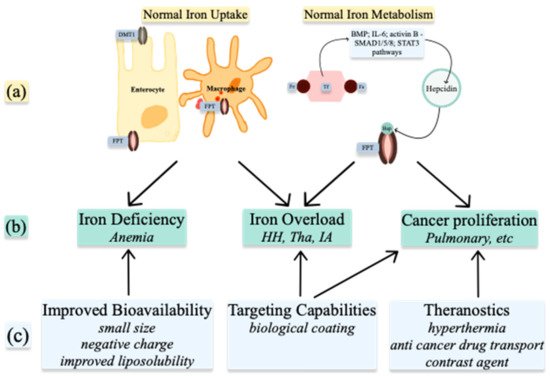
Figure 2. Medical uses of Iron nanoparticles: (a) normal Iron homeostasis; (b) pathologies resulting from abnormal Iron homeostasis; (c) properties of nanoparticles. DMT1—divalent metal transporter 1; FPT—ferroportin; Fe—iron; Hep—hepcidin; BMP—bone morphogenic protein; IL-6—interleukin 6; HH—hemochromatosis; Tha—Thalassemia; IA—inflammatory anemia.
4.1. Nanoparticles to Improve Bioavailability
2.1. Nanoparticles to Improve Bioavailability
Iron Administration
Iron deficiency causes anemia with many deleterious systemic effects. Insufficient intake is an issue most prevalent in underdeveloped countries and in cohorts of western countries when a self-restricted diet is adopted. Correcting iron deficits is especially important in surgical patients whereby reversal of iron deficiency lowers transfusion requirements [40][7]. This conduct represents the first pillar of all patient blood management programs [41,42][8][9]. Iron can be administered enterally or intravenously, with the former being most common [43][10]. Food fortification is an option to prevent and treat iron deficiency; however, fortification is limited by bioavailability and side effects. When used for fortification, iron alters the taste of food [44][11]. Notable side effects of oral iron medication are nausea, vomiting, and diarrhea.
Iron NPs have been produced to deliver iron in an elemental form, with improved properties. Absorption occurs in the duodenum by alternate mechanisms to those of DMT1 and duodenal cytochrome B (DCYTB). NPs adhere to the cellular membrane and subsequently undergo endocytosis [45][12]. This was demonstrated in vitro on cellular lines and rat experimental models, where silencing the relevant genes of the classic iron absorption pathway did not inhibit the uptake of iron nanoparticles [46][13]. Nevertheless, DMT1 is essential for overall iron uptake, as it is believed to be the transporter involved in freeing the iron from lysosomes following endocytosis [47][14].
Iron sulfate is the pioneer of NPs and is followed by newer particles [48][15]. Negatively charged or liposoluble particles cross the membrane more easily while mesoporous iron has higher bioavailability compared with iron salts due to the smaller size and larger surface area after dissolution at gastric pH [49,50][16][17].
To improve NPs, different molecules are used to coat the particles. Food fortification is possible using protein-coated iron NPs to increase solubility and absorption. Coating with either albumin or the milk protein’s amyloid fibril component improves hydro solubility and prevents the aggregation and precipitation of iron NPs at gastric pH. This allows for enzymatic degradation without aggregation [44,51][11][18]. Alternatively, the synthetic ferritin FeIII oxo-hydroxide nanoparticles also demonstrate good bioavailability with few side effects when compared with the ferrous iron fortifiers [52,53][19][20].
4.2. Targeting Capabilities
2.2. Targeting Capabilities
NPs can be designed to behave in specific ways depending on their coating, method of synthesis, or morphology. Biological molecules give the NPs cell-targeting capabilities [61][21] via specific receptor pathways. This property is employed in treatment of different human diseases [55][22]. For example, hemin, which is a ferric iron containing protoporphyrin, increases the absorption of iron NPs through the heme-specific transport [62][23], while transferrin-bound NPs interact with the transferrin receptors on different cells and tissues. The nanoparticle and receptor complexes are endocytosed followed by the release of the NP’s cargo [63][24]. Targeting cells and tissues open new frontiers for treatment and diagnostics.42.2.1. Iron Chelation
Targeted gene silencing to reduce iron absorption can be used in the treatment of HH. The disease is characterized by iron overload caused by excessive intestinal absorption of iron and has severe systemic implications. Silencing RNA (siRNA) aimed at the gene responsible for the expression of DMT1 can be transfected via gelatin NPs. This multicompartmental particle demonstrated increased specificity for the duodenal enterocytes, sparing other iron storage organs. Coating the gelatin NP with Eudragit polymer further improved drug stability [64][25]. Another way to deliver siRNA is by lipid-based NPs that are extracted from ginger and similar to exosomes. This naturally occurring compound is less toxic and produces less inflammation compared with synthetic alternatives. Although this method of reducing iron absorption seems promising, humans possess the ability to absorb heme-associated iron through mechanisms independent of DMT1. Since most of the studies have been conducted on murine models that do not possess the capability to absorb heme and the side effects of iron chelators used for the treatment of HH are poorly tolerated by patients, it can be concluded that more studies are required on this issue [65][26].
Targeted gene silencing is also adopted in the treatment of thalassemia. This disease is characterized by a continuous anemic state, which is associated with decreased levels of hepcidin. The resulting iron overload can be addressed by targeting these downstream components of iron metabolism. Blocking the expression of hepcidin’s protease matriptase 2, by silencing the TMPRSS6 gene, results in higher levels of hepcidin, which in turn reduces the absorption of enteral iron. In a murine model experiment, the authors have successfully prevented iron overload. To deliver the genetic load, lipid NP containing antisense oligonucleotides were used [66][27].
Hepcidin targeting is particularly efficient in HH and thalassemia [67][28]. Extrapolating from this idea, future studies could investigate the effects of silencing hepcidin expression in inflammatory anemia, which is a major issue in the intensive care unit (ICU). Increased hepcidin is associated with poor outcome and increased mortality in this population, with iron dysmetabolism and inflammation [68,69][29][30]. The polypeptide synthesis is stimulated by proinflammatory mediators and causes functional iron overload with secondary anemia.
42.2.2. Antimicrobial Treatment
The complex immune response whereby metal ions are tightly controlled in an attempt to fight invading organisms is referred to as nutritional immunity. Iron, copper, and zinc play essential roles during systemic inflammatory response syndrome. Levels of iron and zinc are kept low by the host organism to limit bacterial proliferation; the latter thrives in conditions of iron and zinc overload. Bacterial growth occurs when iron is highly available, often seen after transfusions. The same is also true in genetic disorders with elevated iron [70][31]. Targeted manipulation of ion levels represents a new therapeutic approach in sepsis.
Thanks to their properties, carrier nanoparticle and metal oxide complexes can be used as chemotherapeutic agents for targeting microbes. Sepsis is a leading cause of death, especially in intensive care units. Over the last century, numerous antibiotics have been developed due to the increasing bacterial resistance. Divalent metal NPs have been used as antimicrobial agents and for coating of medical devices. Coatings on devices prevent the formation of biofilm produced by different drug-resistant bacteria. In an experimental model, using a sol-gel process, iron-doped copper NPs successfully inhibited biofilm formation [71,72][32][33].
Divalent elements are catalysts of Fenton reaction producing reactive oxygen species [73][34]. Based on the premise that both iron and copper produce ROS, NPs have been loaded and aimed against microbes, viruses and fungi [74,75][35][36]. Bacteria internalize the nanoparticle complexes that lead to cellular death. Another mechanism is by disruption of DNA, which halts the cellular processes taking place. Such interferences prevent the invading organisms from dividing with some sparing effects on the host [76][37]. Evidence of copper NP supplementation revealed by animal experiments in vivo demonstrates the antioxidant effect on blood with enhanced catalase (CAT) activity resulting in hydrogen-peroxide-optimized detoxification [77][38].
Both the levels of ions and the microenvironments in which they play a role are essential. The antimicrobial effect is not limited to the ions but is also conferred by their ligands. One such ligand is the naturally occurring chitosan found in crustaceans [78][39]. When comparing ligands alone with ligand and metal oxide complexes, the nanosized complexes demonstrate superior efficacy against both bacteria and fungi. Data supporting this showed that Zinc-Cobalt ferrite NPs are efficient against Klebsiella pneumoniae while Copper-Cobalt ferrite NPs have antifungal effects [79][40]. It is worth mentioning that in comparison with existing standard therapy, these NPs have not been superior [80][41] and represent an area of interest for future research. Nevertheless, they may be used as an alternative drug therapy in some drug-resistant microbes.
Targeted delivery and control with NPs is again evident with zinc. While Zn deficiency is detrimental for the host, starvation of bacteria has beneficial effects, as seen in experimental models where Zn chelation re-established antibiotic susceptibility in carbapenem-resistant microorganisms. Supplementing with zinc in an attempt to boost immunity of the host is controversial. It is true that zinc shortens the duration of some viral respiratory tract infections, probably by boosting the immune system; however, the same beneficial effect has not been seen in critically ill patients. Furthermore, when zinc is administered in high doses to support the immune response, it competes with copper for metallothionein, resulting in copper deficiency. High doses result in toxicity, as seen with developing mice experimental models [81][42].
42.2.3. Biological Sample Analysis
A thorough understanding of ion physiology, nanomaterials, and their interactions allowed for the creation of polymers that act as nanoparticle receptors, to determine the titres of specific nanosized molecules, including proteins. As a result of reduced costs of production, high versatility, specificity, and sensitivity, nanosized molecularly imprinted polymer plates are of interest.
Iron status biomarkers are used to predict response to iron therapy and blood transfusions [82][43]. Some markers have been tested to predict response to iron treatment; however, all presented shortcomings such as reference value discordance between manufacturers and published data [31][44]. Conventional tests lack clear cut-off values and must be validated against a gold standard in patients with significant inflammation [83][45].
Hepcidin-specific plates have been developed and tested with success to determine sample levels with ranges as low as 1 nM and may be quantified in as little as three minutes [84,85][46][47]. This technology may prove useful at the point of care in ICU, where marked inflammation is present in critically ill patients. As a result, hepcidin is upregulated and results in the sequestration of iron [25][48]. Hepcidin proved useful in detecting iron deficiency, even in cases of inflammation. Low levels indicate iron depletion while high levels show inflammatory anemia. In ICU patients who initially presented inflammation but later developed iron deficiency, hepcidin followed a decreasing trend as iron loss progressed [86][49]. These patients may benefit from iron supplements to treat anemia. Likewise, ferritin can also be determined with great accuracy. Quantum dots increase the sensitivity of the Western blot technique and yield a 20-fold increase in sensitivity for human ferritin when used together [87][50].
Urine samples are accessible and contain small proteins that serve as biomarkers used in sepsis and cancer screening. Expanding on treatment options is crucial in sepsis; however, often the culprit is difficult and timely to identify. Bacteria that metabolize Cu can be rapidly identified and targeted in biological products using fluorescent spectroscopy. While E. coli can be detected in urine using iron quantum clusters by interacting with Cu from the bacteria, Zinc-Cobalt ferrite nanoparticles may be used as an antimicrobial agent [79][40]. Establishing the microorganism causing sepsis is often a race against time and can prove invaluable in critically ill patients [88][51]. In oncology, magnetic Concanavalin A-NPs have been used to detect Cathepsin C and transferrin, which may serve as biomarkers in the urine of small-cell lung cancer patients [89][52].
Furthermore, synthesis of dealloyed nanoporous gold (NPG)/ultrathin CuO film nanohybrid has been reported as an efficient detection method of glucose, therefore becoming a promising tool for glucose tests [90][53].
4.3. Theranostics
2.3. Theranostics
By understanding the physiology of iron, copper, and zinc metabolism, and by taking advantage of the pathophysiological changes in diseases such as neoplasms, NPs are concomitantly used for treatment and diagnostics. This concept is termed theragnostics. USIONPs, due to their paramagnetic properties, and when exposed to an external magnetic field, specifically accumulate in intended cells [91][54]. Once in the tissue, the NPs may take up the role of contrast agent, chemotherapeutic agent, or drug mule. The latter provides better bio availability for the cancer treatment with reduced toxicity. The cargo of a drug carrying NP may be released or activated using photo thermal or magnetic fields [39][6]. Concomitantly using magnetic resonance imaging in a theragnostic approach provides personalized treatment in the field of oncology. Small nanoparticles such as octopods, when exposed to a magnetic field, release heat energy. This is the basis for localized hyperthermia. This method allows for precise targeting of neoplastic tissues while preventing any damage from occurring to healthy cells [37,38][4][5].
Iron oxides have the ability to act as contrast agents [57,92][55][56]. Paramagnetic properties are given by the iron core as well as the shells of the NP—for example, polydopamine, gold, or quantum dot coatings [59][57]. An alternative to synthetic contrast agent is ferritin H with ferrous iron-loaded core—termed magneto ferritin, it is used as magnetic resonance imaging (MRI) contrast [93][58]. Further labelling the outer core with radioactive iodine, the newly formed contrast agent is not only magnetic signaling but also positron emitting. This hybrid contrast agent can be used for combined positron emmiting tomography (PET)-computer tomography (CT) or MRI imaging. Due to the specific uptake of ferritin, namely, by transferrin receptor 1, the contrast agent displays specificity for cells with increased numbers of receptors, such as cancer cells [94][59]. This transport pathway not only allows for specific uptake but also solves the problem of multiple injections for this method of imaging [95][60]. Labelling cells and tissues is not limited to MRI. Gold-labeled transferrin is visualized using confocal microscopy, with no cytotoxic effects. The labeled transferrin maintains the ability to recognize and interact with the specific receptor on cell surface, allowing for the visualization of transferrin-receptor-rich cells, with an increased iron metabolism [96][61].
NPs present good penetrability, even crossing the blood-brain barrier [97][62], and target tumor cells, which express high numbers of transferrin receptors [98][63]. Transferrin-bound nanoparticles are adopted in theragnostics [99][64] to achieve improved results. Transferrin is loaded with metallofullerene to inhibit the much-needed iron uptake by the neoplastic cells [100][65]. Moreover, copper oxide nanoparticles have been demonstrated to efficiently target the tumor-initiating cells in pancreatic adenocarcinoma both in vitro and in vivo. The effect was also linked to reverse mitochondrial membrane polarity and excessive ROS production, resulting in enhanced apoptosis in the tumor-initiating cells [101][66]. Several Cu(I) complexes have been designed in a recent study. Among them, several prototypes have demonstrated ROS-generation abilities and cellular apoptosis-necrosis disequilibrium in prostate cancer cells [102][67]. Even more data exist regarding the potential of zinc oxide nanoparticles as anticancer agents. Interestingly, mechanisms such as p53 suppression, bax upregulation, BCL-2 silencing, and DNA fragmentation were documented to appear predominantly in cancer cells and less in normal cell lines, suggesting an intrinsic selectivity in the antitumor effect of zinc oxide nanoparticles [103][68]. Although the precise mechanism responsible for the onset of intrinsic selectivity of divalent ion nanoparticles is still under intensive research, the effect was linked to catalytic activity through Fenton-like reactions and was reported for several tumors, such as human ovarian cancer [104][69], non-small-cell lung cancer [105][70], or breast cancer [106][71].
References
- Tvrda, E.; Peer, R.; Sikka, S.C.; Agarwal, A. Iron and copper in male reproduction: A double-edged sword. J. Assist. Reprod Genet. 2015, 32, 3–16.
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241.
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710.
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307.
- Cotin, G.; Blanco-Andujar, C.; Perton, F.; Asín, L.; de la Fuente, J.M.; Reichardt, W.; Schaffner, D.; Ngyen, D.V.; Mertz, D.; Kiefer, C.; et al. Unveiling the role of surface, size, shape and defects of iron oxide nanoparticles for theranostic applications. Nanoscale 2021, 13, 14552–14571.
- Friedrich, R.P.; Cicha, I.; Alexiou, C. Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering. Nanomaterials 2021, 11, 2337.
- Gómez-Ramirez, S.; Jericó, C.; Muñoz, M. Perioperative anemia: Prevalence, consequences and pathophysiology. Transfus. Apher. Sci. 2019, 58, 369–374.
- Burton, B.N.; A’Court, A.M.; Brovman, E.Y.; Scott, M.J.; Urman, R.D.; Gabriel, R.A. Optimizing Preoperative Anemia to Improve Patient Outcomes. Anesthesiol. Clin. 2018, 36, 701–713.
- Filipescu, D.; Bănăţeanu, R.; Beuran, M.; Burcoş, T.; Corneci, D.; Cristian, D.; Diculescu, M.; Dobrotă, A.; Droc, G.; Isacoff, D.; et al. Perioperative Patient Blood Management Programme. Multidisciplinary recommendations from the Patient Blood Management Initiative Group. Rom. J. Anaesth. Intensive Care 2017, 24, 139–157.
- Aslam, M.F.; Frazer, D.M.; Faria, N.; Bruggraber, S.F.; Wilkins, S.J.; Mirciov, C.; Powell, J.J.; Anderson, G.J.; Pereira, D.I. Ferroportin mediates the intestinal absorption of iron from a nanoparticulate ferritin core mimetic in mice. FASEB J. 2014, 28, 3671–3678.
- Gu, Y.; Li, Y.; Yang, Y.; Luo, Q.; Zhang, Y.; Zhou, C. One-Pot Facile Fabrication of Bioavailable Iron Nanoparticles with Good Biocompatibility for Anemia Therapy. Med. Sci. Monit. 2018, 24, 6449–6455.
- Pereira, D.I.; Bruggraber, S.F.; Faria, N.; Poots, L.K.; Tagmount, M.A.; Aslam, M.F.; Frazer, D.M.; Vulpe, C.D.; Anderson, G.J.; Powell, J.J. Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomedicine 2014, 10, 1877–1886.
- Latunde-Dada, G.O.; Pereira, D.I.; Tempest, B.; Ilyas, H.; Flynn, A.C.; Aslam, M.F.; Simpson, R.J.; Powell, J.J. A nanoparticulate ferritin-core mimetic is well taken up by HuTu 80 duodenal cells and its absorption in mice is regulated by body iron. J. Nutr. 2014, 144, 1896–1902.
- Pereira, D.I.; Mergler, B.I.; Faria, N.; Bruggraber, S.F.; Aslam, M.F.; Poots, L.K.; Prassmayer, L.; Lönnerdal, B.; Brown, A.P.; Powell, J.J. Caco-2 cell acquisition of dietary iron(III) invokes a nanoparticulate endocytic pathway. PLoS ONE 2013, 8, e81250.
- Perfecto, A.; Elgy, C.; Valsami-Jones, E.; Sharp, P.; Hilty, F.; Fairweather-Tait, S. Mechanisms of Iron Uptake from Ferric Phosphate Nanoparticles in Human Intestinal Caco-2 Cells. Nutrients 2017, 9, 359.
- Lin, J.F.; Wu, C.C.; Liao, Y.J.; Jakfar, S.; Tang, Z.B.; Chen, J.K.; Lin, F.H. In Vitro and In Vivo Evaluations of Mesoporous Iron Particles for Iron Bioavailability. Int. J. Mol. Sci. 2019, 20, 5291.
- Jahn, M.R.; Nawroth, T.; Fütterer, S.; Wolfrum, U.; Kolb, U.; Langguth, P. Iron oxide/hydroxide nanoparticles with negatively charged shells show increased uptake in Caco-2 cells. Mol. Pharm. 2012, 9, 1628–1637.
- Shen, Y.; Posavec, L.; Bolisetty, S.; Hilty, F.M.; Nyström, G.; Kohlbrecher, J.; Hilbe, M.; Rossi, A.; Baumgartner, J.; Zimmermann, M.B.; et al. Amyloid fibril systems reduce, stabilize and deliver bioavailable nanosized iron. Nat. Nanotechnol. 2017, 12, 642–647.
- Jin, Y.; He, J.; Fan, K.; Yan, X. Ferritin variants: Inspirations for rationally designing protein nanocarriers. Nanoscale 2019, 11, 12449–12459.
- Powell, J.J.; Bruggraber, S.F.; Faria, N.; Poots, L.K.; Hondow, N.; Pennycook, T.J.; Latunde-Dada, G.O.; Simpson, R.J.; Brown, A.P.; Pereira, D.I. A nano-disperse ferritin-core mimetic that efficiently corrects anemia without luminal iron redox activity. Nanomedicine 2014, 10, 1529–1538.
- Zhang, H.; Hou, L.; Jiao, X.; Ji, Y.; Zhu, X.; Zhang, Z. Transferrin-mediated fullerenes nanoparticles as Fe(2+)-dependent drug vehicles for synergistic anti-tumor efficacy. Biomaterials 2015, 37, 353–366.
- Malhotra, H.; Sheokand, N.; Kumar, S.; Chauhan, A.S.; Kumar, M.; Jakhar, P.; Boradia, V.M.; Raje, C.I.; Raje, M. Exosomes: Tunable Nano Vehicles for Macromolecular Delivery of Transferrin and Lactoferrin to Specific Intracellular Compartment. J. Biomed. Nanotechnol. 2016, 12, 1101–1114.
- Jahn, M.R.; Shukoor, I.; Tremel, W.; Wolfrum, U.; Kolb, U.; Nawroth, T.; Langguth, P. Hemin-coupled iron(III)-hydroxide nanoparticles show increased uptake in Caco-2 cells. J. Pharm. Pharmacol. 2011, 63, 1522–1530.
- Chiu, R.Y.; Tsuji, T.; Wang, S.J.; Wang, J.; Liu, C.T.; Kamei, D.T. Improving the systemic drug delivery efficacy of nanoparticles using a transferrin variant for targeting. J. Control. Release 2014, 180, 33–41.
- Fan, Y.; Dhaliwal, H.K.; Menon, A.V.; Chang, J.; Choi, J.E.; Amiji, M.M.; Kim, J. Site-specific intestinal DMT1 silencing to mitigate iron absorption using pH-sensitive multi-compartmental nanoparticulate oral delivery system. Nanomedicine 2019, 22, 102091.
- Wang, X.; Zhang, M.; Flores, S.R.L.; Woloshun, R.R.; Yang, C.; Yin, L.; Xiang, P.; Xu, X.; Garrick, M.D.; Vidyasagar, S.; et al. Oral Gavage of Ginger Nanoparticle-Derived Lipid Vectors Carrying Dmt1 siRNA Blunts Iron Loading in Murine Hereditary Hemochromatosis. Mol. Ther. 2019, 27, 493–506.
- Schmidt, P.J.; Racie, T.; Westerman, M.; Fitzgerald, K.; Butler, J.S.; Fleming, M.D. Combination therapy with a Tmprss6 RNAi-therapeutic and the oral iron chelator deferiprone additively diminishes secondary iron overload in a mouse model of β-thalassemia intermedia. Am. J. Hematol. 2015, 90, 310–313.
- Schmidt, P.J.; Toudjarska, I.; Sendamarai, A.K.; Racie, T.; Milstein, S.; Bettencourt, B.R.; Hettinger, J.; Bumcrot, D.; Fleming, M.D. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(-/-) mice and ameliorates anemia and iron overload in murine β-thalassemia intermedia. Blood 2013, 121, 1200–1208.
- Minchella, P.A.; Armitage, A.E.; Darboe, B.; Jallow, M.W.; Drakesmith, H.; Jaye, A.; Prentice, A.M.; McDermid, J.M. Elevated Hepcidin Is Part of a Complex Relation That Links Mortality with Iron Homeostasis and Anemia in Men and Women with HIV Infection. J. Nutr. 2015, 145, 1194–1201.
- Hung, M.; Ortmann, E.; Besser, M.; Martin-Cabrera, P.; Richards, T.; Ghosh, M.; Bottrill, F.; Collier, T.; Klein, A.A. A prospective observational cohort study to identify the causes of anaemia and association with outcome in cardiac surgical patients. Heart 2015, 101, 107–112.
- Weiss, G.; Carver, P.L. Role of divalent metals in infectious disease susceptibility and outcome. Clin. Microbiol. Infect. 2018, 24, 16–23.
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies. Int. J. Mol. Sci. 2021, 22, 4595.
- Pugazhendhi, A.; Kumar, S.S.; Manikandan, M.; Saravanan, M. Photocatalytic properties and antimicrobial efficacy of Fe doped CuO nanoparticles against the pathogenic bacteria and fungi. Microb. Pathog. 2018, 122, 84–89.
- Asghar, M.A.; Zahir, E.; Asghar, M.A.; Iqbal, J.; Rehman, A.A. Facile, one-pot biosynthesis and characterization of iron, copper and silver nanoparticles using Syzygium cumini leaf extract: As an effective antimicrobial and aflatoxin B1 adsorption agents. PLoS ONE 2020, 15, e0234964.
- Kim, H.E.; Lee, H.J.; Kim, M.S.; Kim, T.; Lee, H.; Kim, H.H.; Cho, M.; Hong, S.W.; Lee, C. Differential Microbicidal Effects of Bimetallic Iron-Copper Nanoparticles on Escherichia coli and MS2 Coliphage. Environ. Sci. Technol 2019, 53, 2679–2687.
- Antonoglou, O.; Lafazanis, K.; Mourdikoudis, S.; Vourlias, G.; Lialiaris, T.; Pantazaki, A.; Dendrinou-Samara, C. Biological relevance of CuFeO(2) nanoparticles: Antibacterial and anti-inflammatory activity, genotoxicity, DNA and protein interactions. Mater. Sci. Eng C Mater. Biol. Appl. 2019, 99, 264–274.
- Henam, S.D.; Ahmad, F.; Shah, M.A.; Parveen, S.; Wani, A.H. Microwave synthesis of nanoparticles and their antifungal activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 213, 337–341.
- Majewski, M.; Ognik, K.; Juśkiewicz, J. Copper nanoparticles modify the blood plasma antioxidant status and modulate the vascular mechanisms with nitric oxide and prostanoids involved in Wistar rats. Pharmacol. Rep. 2019, 71, 509–516.
- Ye, Q.; Chen, W.; Huang, H.; Tang, Y.; Wang, W.; Meng, F.; Wang, H.; Zheng, Y. Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 5213–5227.
- Abdel Maksoud, M.I.A.; El-Sayyad, G.S.; Ashour, A.H.; El-Batal, A.I.; Abd-Elmonem, M.S.; Hendawy, H.A.M.; Abdel-Khalek, E.K.; Labib, S.; Abdeltwab, E.; El-Okr, M.M. Synthesis and characterization of metals-substituted cobalt ferrite nanoparticles as antimicrobial agents and sensors for Anagrelide determination in biological samples. Mater. Sci. Eng C Mater. Biol. Appl. 2018, 92, 644–656.
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; El-Khatib, R.M.; Abdel-Fatah, S.M. Some new nano-sized Fe(II), Cd(II) and Zn(II) Schiff base complexes as precursor for metal oxides: Sonochemical synthesis, characterization, DNA interaction, in vitro antimicrobial and anticancer activities. Bioorg. Chem. 2016, 69, 140–152.
- Wang, C.; Lu, J.; Zhou, L.; Li, J.; Xu, J.; Li, W.; Zhang, L.; Zhong, X.; Wang, T. Effects of Long-Term Exposure to Zinc Oxide Nanoparticles on Development, Zinc Metabolism and Biodistribution of Minerals (Zn, Fe, Cu, Mn) in Mice. PLoS ONE 2016, 11, e0164434.
- Imaeda, T.; Nakada, T.A.; Abe, R.; Oda, S. Decreased total iron binding capacity upon intensive care unit admission predicts red blood cell transfusion in critically ill patients. PLoS ONE 2019, 14, e0210067.
- Lasocki, S.; Longrois, D.; Montravers, P.; Beaumont, C. Hepcidin and anemia of the critically ill patient: Bench to bedside. Anesthesiology 2011, 114, 688–694.
- Shah, A.; Fisher, S.A.; Wong, H.; Roy, N.B.; McKechnie, S.; Doree, C.; Litton, E.; Stanworth, S.J. Safety and efficacy of iron therapy on reducing red blood cell transfusion requirements and treating anaemia in critically ill adults: A systematic review with meta-analysis and trial sequential analysis. J. Crit. Care 2019, 49, 162–171.
- Cenci, L.; Piotto, C.; Bettotti, P.; Maria Bossi, A. Study on molecularly imprinted nanoparticle modified microplates for pseudo-ELISA assays. Talanta 2018, 178, 772–779.
- Cenci, L.; Andreetto, E.; Vestri, A.; Bovi, M.; Barozzi, M.; Iacob, E.; Busato, M.; Castagna, A.; Girelli, D.; Bossi, A.M. Surface plasmon resonance based on molecularly imprinted nanoparticles for the picomolar detection of the iron regulating hormone Hepcidin-25. J. Nanobiotechnol. 2015, 13, 1–15.
- Ruchala, P.; Nemeth, E. The pathophysiology and pharmacology of hepcidin. Trends Pharmacol. Sci. 2014, 35, 155–161.
- Lasocki, S.; Baron, G.; Driss, F.; Westerman, M.; Puy, H.; Boutron, I.; Beaumont, C.; Montravers, P. Diagnostic accuracy of serum hepcidin for iron deficiency in critically ill patients with anemia. Intensive Care Med. 2010, 36, 1044–1048.
- Liu, P.; Na, N.; Liu, T.; Huang, L.; He, D.; Hua, W.; Ouyang, J. Ultrasensitive detection of ferritin in human serum by Western blotting based on quantum dots-labeled avidin-biotin system. Proteomics 2011, 11, 3510–3517.
- Vaezi, Z.; Azizi, M.; Sadeghi Mohammadi, S.; Hashemi, N.; Naderi-Manesh, H. A novel iron quantum cluster confined in hemoglobin as fluorescent sensor for rapid detection of Escherichia coli. Talanta 2020, 218, 121137.
- Zhang, Z.; Cheng, X.; Jiang, H.; Gu, J.; Yin, Y.; Shen, Z.; Xu, C.; Pu, Z.; Li, J.B.; Xu, G. Quantitative proteomic analysis of glycosylated proteins enriched from urine samples with magnetic ConA nanoparticles identifies potential biomarkers for small cell lung cancer. J. Pharm. Biomed. Anal. 2021, 206, 114352.
- Xiao, X.; Li, H.; Pan, Y.; Si, P. Non-enzymatic glucose sensors based on controllable nanoporous gold/copper oxide nanohybrids. Talanta 2014, 125, 366–371.
- Ledda, M.; Fioretti, D.; Lolli, M.G.; Papi, M.; Di Gioia, C.; Carletti, R.; Ciasca, G.; Foglia, S.; Palmieri, V.; Marchese, R.; et al. Biocompatibility assessment of sub-5 nm silica-coated superparamagnetic iron oxide nanoparticles in human stem cells and in mice for potential application in nanomedicine. Nanoscale 2020, 12, 1759–1778.
- Alphandéry, E. Biodistribution and targeting properties of iron oxide nanoparticles for treatments of cancer and iron anemia disease. Nanotoxicology 2019, 13, 573–596.
- Oshtrakh, M.I. Applications of Mössbauer Spectroscopy in Biomedical Research. Cell Biochem. Biophys. 2019, 77, 15–32.
- Wang, K.; Li, L.; Xu, X.; Lu, L.; Wang, J.; Wang, S.; Wang, Y.; Jin, Z.; Zhang, J.Z.; Jiang, Y. Fe(3)O(4)@ Astragalus Polysaccharide Core-Shell Nanoparticles for Iron Deficiency Anemia Therapy and Magnetic Resonance Imaging in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 10452–10461.
- Charlton, J.R.; Pearl, V.M.; Denotti, A.R.; Lee, J.B.; Swaminathan, S.; Scindia, Y.M.; Charlton, N.P.; Baldelomar, E.J.; Beeman, S.C.; Bennett, K.M. Biocompatibility of ferritin-based nanoparticles as targeted MRI contrast agents. Nanomedicine 2016, 12, 1735–1745.
- Aşık, E.; Aslan, T.N.; Güray, N.T.; Volkan, M. Cellular uptake and apoptotic potential of rhenium labeled magnetic protein cages in MDA-MB-231 cells. Environ. Toxicol. Pharmacol. 2018, 63, 127–134.
- Zhao, Y.; Liang, M.; Li, X.; Fan, K.; Xiao, J.; Li, Y.; Shi, H.; Wang, F.; Choi, H.S.; Cheng, D.; et al. Bioengineered Magnetoferritin Nanoprobes for Single-Dose Nuclear-Magnetic Resonance Tumor Imaging. ACS Nano 2016, 10, 4184–4191.
- Le Guével, X.; Daum, N.; Schneider, M. Synthesis and characterization of human transferrin-stabilized gold nanoclusters. Nanotechnology 2011, 22, 275103.
- Kang, T.; Jiang, M.; Jiang, D.; Feng, X.; Yao, J.; Song, Q.; Chen, H.; Gao, X.; Chen, J. Enhancing Glioblastoma-Specific Penetration by Functionalization of Nanoparticles with an Iron-Mimic Peptide Targeting Transferrin/Transferrin Receptor Complex. Mol. Pharm. 2015, 12, 2947–2961.
- Xue, L.; Deng, D.; Sun, J. Magnetoferritin: Process, Prospects, and Their Biomedical Applications. Int. J. Mol. Sci. 2019, 20, 2426.
- Piraux, H.; Hai, J.; Verbeke, P.; Serradji, N.; Ammar, S.; Losno, R.; Ha-Duong, N.T.; Hémadi, M.; El Hage Chahine, J.M. Transferrin receptor-1 iron-acquisition pathway—Synthesis, kinetics, thermodynamics and rapid cellular internalization of a holotransferrin-maghemite nanoparticle construct. Biochim. Biophys. Acta 2013, 1830, 4254–4264.
- Li, J.; Xing, X.; Sun, B.; Zhao, Y.; Wu, Z. Metallofullerenol Inhibits Cellular Iron Uptake by Inducing Transferrin Tetramerization. Chem. Asian J. 2017, 12, 2646–2651.
- Benguigui, M.; Weitz, I.S.; Timaner, M.; Kan, T.; Shechter, D.; Perlman, O.; Sivan, S.; Raviv, Z.; Azhari, H.; Shaked, Y. Copper oxide nanoparticles inhibit pancreatic tumor growth primarily by targeting tumor initiating cells. Sci. Rep. 2019, 9, 1–10.
- Machado, J.F.; Sequeira, D.; Marques, F.; Piedade, M.F.M.; de Brito, M.J.V.; Garcia, M.H.; Fernandes, A.R.; Morais, T.S. New copper (i) complexes selective for prostate cancer cells. Dalton Trans. 2020, 49, 12273–12286.
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857.
- Zhang, Y.; Chen, W.; Wang, S.; Liu, Y.; Pope, C. Phototoxicity of zinc oxide nanoparticle conjugatesin human ovarian cancer NIH: OVCAR-3 cells. J. Biomed. Nanotechnol. 2008, 4, 432–438.
- Wiesmann, N.; Kluenker, M.; Demuth, P.; Brenner, W.; Tremel, W.; Brieger, J. Zinc overload mediated by zinc oxide nanoparticles as innovative anti-tumor agent. J. Trace Elem. Med. Biol. 2019, 51, 226–234.
- Sadhukhan, P.; Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater. Sci. Eng. C 2019, 100, 129–140.
More
