Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Raffaella Mancuso and Version 2 by Bruce Ren.
Coumarin (2H-chromen-2-one) derivatives have important uses in medicinal and synthetic chemistry, for example, as fluorescent probes. These properties have prompted chemists to develop efficient synthetic methods to synthesize the coumarin core and/or to functionalize it. In this context, many metal-catalyzed syntheses of coumarins have been introduced; among them, copper-catalyzed reactions appear to be very promising owing to the non-toxicity and cheapness of copper complexes.
- coumarin
- copper compounds
- metal catalysis
1. Introduction
The synthesis of coumarins and their derivatives has attracted considerable attention from organic and medicinal chemists. In fact, coumarins display pharmacological properties. For instance, methoxsalen, warfarin (coumadin), and tioclomarol are commercially available, coumarin-derived drugs [1][2][3][4][5][6][7]. Moreover, they are also employed as fluorescent probes, laser dyes, fluorescent chemosensors, light absorbers for solar cells, optical brighteners, and organic light-emitting diodes (Figure 1) [8][9].
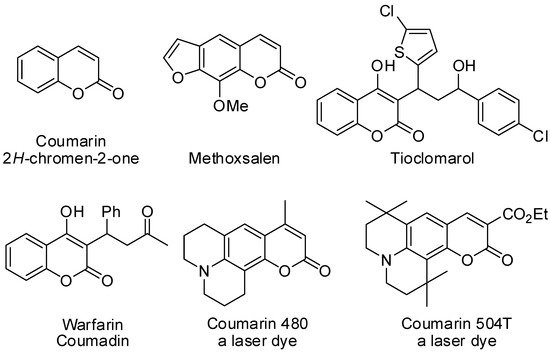
Figure 1. Some commercially available coumarin derivatives.
Obviously, the synthesis of these compounds has received much attention. When searching for the word “coumarins” in the most common literature databases, and filtering by year “2020–2021”, a thousand references appear. Thus, only some of the most significant papers are referenced in this introduction.
Coumarins can be synthesized by Pechmann, Perkin, Knoevenagel, Reformatsky, and Wittig reactions [10][11][12][13][14][15][16][17][18][19]. In particular, eco-friendly [20][21] and asymmetric syntheses [22][23][24] have become paramount in the field of organic synthesis in recent years. Catalysis is of great importance for this purpose [25][26], especially when carried out with palladium complexes [27]. In some reviews, many metal catalysts are discussed: Pt, Au, Co, Fe, Ni, Rh, Ru, Zn. However, our literature screening showed that copper salts are scarcely used in the synthesis of coumarins and often they are only a co-catalyst (see for example [28][29][30]). Yet copper salts and complexes are very cheap and abundant catalysts, widely employed in transition metal-catalyzed synthesis [31][32][33]. The synthesis of coumarins, using Cu-catalyzed cyclization reaction or Cu-catalyzed functionalization of the coumarin core, on the other hand, are selective, versatile, and provide high yields of the final product, which can be easily recovered from the reaction crude. Moreover, among the thousand papers on the synthesis of these compounds, there is not a single review on the Cu-catalyzed synthesis of coumarins.
2. Synthesis of the Coumarin Core
The oldest known synthesis of coumarin core involving copper derivatives dates back to 2006. Pechmann condensation is the acid-catalyzed reaction of acetoacetate and phenols, with the process leading to the synthesis of coumarins. Dipyridine copper chloride was found to be an efficient stoichiometric Lewis acid for this reaction (Scheme 1). The reason why stoichiometric instead of catalytic amounts of such Lewis acid was needed was not explained in the paper [34]. Electron-donating substituents and the use of microwave irradiation increased yields and shortened reaction times.
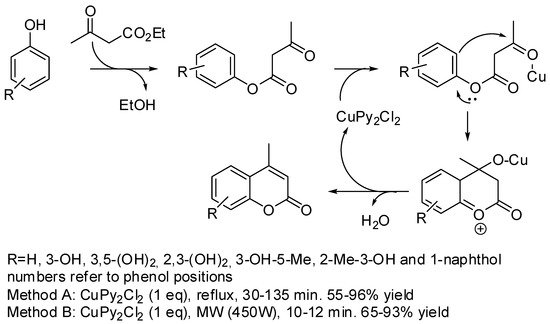

Scheme 1. Copper-promoted Pechmann condensation.
Later, Čejka and co-workers tested the applicability of Cu-benzene-1,3,5-tricarboxylate as a solid acid MOF for use in a Pechmann reaction. In fact, Cu-benzene-1,3,5-tricarboxylate has two active centers in close proximity to one another and the optimum pore size. However, resorcinol and pyrogallol did not produce coumarins, because they were very strongly adsorbed at the active sites of the MOFs, thus inhibiting the catalytic activity. On the other hand, naphthol produced the expected benzo-coumarin in a 94–98% conversion after 20 h, because it was less bound to the active sites owing to its higher bulkiness [35].
Yamamoto and Kirai observed the synthesis of 4-arylcoumarins via Cu-catalyzed hydroarylation with arylboronic acids [36]. Where previously they had added boronic acids to alkynoates [37], here they added boronic acids to phenol-derived propiolates. The reaction was to have been successfully carried out if the aromatic hydroxy function was protected as an MOM ether. In fact, the free OH unexpectedly led to the production of 4-methoxycoumarin in 37% yield as a by-product. The authors did not explain how this by-product arose. Thus, the reaction proceeded in two steps: copper-catalyzed hydroarylation of the triple bond, followed by deprotection of the MOM group and cyclization. The best conditions and the prepared products are summarized in Scheme 2. Among them there are seven natural products. Generally, the electron-withdrawing or steric-hindered substituents that were acting on the arylboronic acid required a higher catalyst amount and longer reaction times. Moreover, the functionalization of the 4-aryl moiety was also successfully attempted.
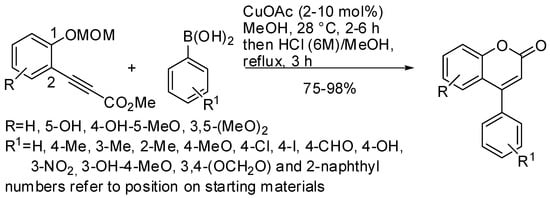

Scheme 2. Synthesis of 4-arylcoumarins via Cu-catalyzed hydroarylation with arylboronic acids.
Another approach is through the reaction between protected salicylaldehydes and acetylenes. These reactions are induced in acetonitrile through exclusive 6-endo-dig cyclization. Reddy and co-workers prepared in situ salicylaldehyde imines with pyrrolidine (25 mol%), and then cyclized them in 50–84% yields (16 examples) with ethoxyacetylene at 100 °C for 2 h, in the presence of CuI (10 mol%). In the proposed mechanism, the iminium ion of salicylaldehyde is attacked by copper ethoxyacetylide, leading to a propargylamine. The coordination of copper around the triple bond activated the cyclization of the phenoxy group to produce vinyl ether. Finally, the addition of water facilitated the generation of the products and restored the catalysts [38].
Later, Hwang and co-workers extended the scope of this reaction to every terminal alkyne, starting with salicylaldehyde N-tosylhydrazones. The oxo group of the coumarin nucleus was furnished by molecular oxygen. The reaction worked at room temperature and therefore it was very function tolerable. In fact, 41 different coumarins were prepared in 62–89% yields after 12–13 h; among them, diethynylbenzenes produced only mono substitution and 1-ethynyl-4-(phenylethynyl)benzene reacted only on the terminal alkyne. Furthermore, 2-hydroxyacetophenone-N-tosylhydrazone and 2-hydroxybenzophenone-N-tosylhydrazone generated products in 27–56% (three examples) and aliphatic terminal alkynes formed products in 26–42% yields (five examples). It should be noted that aliphatic terminal alkynes are generally unreactive in the reactions catalyzed by other metal catalysts. The reaction was scaled up to gram scale with comparable yields. The authors performed further experiments to elucidate the mechanism and then proposed the pathway shown in Scheme 3 [39].
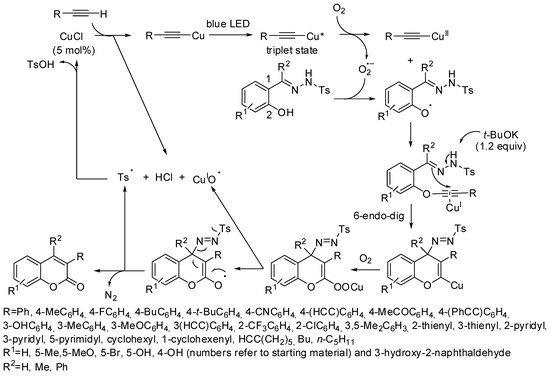

Scheme 3. Copper-catalyzed synthesis of 3-substituted coumarins. Cu* = light excited copper atom.
Polyhydroxy benzenes reacted with dimethyl and diethyl but-2-ynedioate, leading to the production of coumarins in 62–79% yields (eight examples) (CuO (5 mol%), in toluene, at 110 °C, for 2–4 h). In the proposed mechanism, the highly activated benzene ring gave a Michael addition to the Cu-complexed alkyne ester, followed by its lactonization with the ortho phenolic moiety [40].
A trifluoromethyl group could generally improve the physical, chemical, and biological properties of an already important organic molecule. Thus, the copper-catalyzed synthesis of coumarins was extended with direct trifluoromethylation. The reaction started with aryl propiolates and Togni’s reagent I, and was tolerant of many activating functions, but did not take place with inactivated 1-methoxy-4-(3-phenoxyprop-1-yn-1-yl)benzene. The substrates that bore a meta-methyl group on the phenoxy moiety led to a mixture of 7- and 5-methyl isomers (ratio = 5:2), while an ortho-methyl group produced only traces of the product for steric reasons. The authors proposed a radical mechanism for the reaction, and supported their assertion with controlled experiments (Scheme 4) [41].
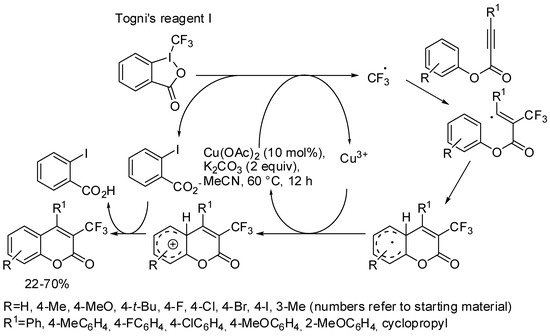

Scheme 4. Synthesis of trifluorocoumarins.
3. Coumarin Functionalization
The functionalization of coumarins is also important, in particular when another heterocycle is formed, because pharmaceutically important molecules are always complex molecules, in which a coumarin skeleton is only one of the pharmacophores. In this section, we summarize the few reactions involving copper salts, classified by the type of new function they possess.
In the previous section, many reactions were induced using naphthols, thus leading to the production of benzo[g]coumarins. Moreover, Quayle and co-workers prepared many chlorobenzo-coumarins, starting with the trichloroacetates of allylcoumarins, using their BHQ benzannulation reactions (see Scheme 5 for a representative example of this process). Benzo[c], benzo[f], and benzo[h]coumarins were obtained in 38–94% yields (eight examples) [42].
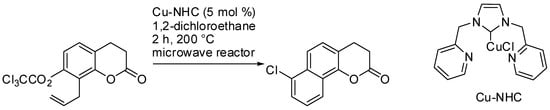

Scheme 5. One typical example of the BHQ synthesis of chlorobenzo-coumarins.
The copper-catalyzed synthesis of furocoumarins has been studied in greater depth because they have fungicidal activity, insecticidal, anti-HIV, and anticancer properties.
In 2007, Cheng and Hu reported the copper-catalyzed cyclization and oxidation of 3-(alk-1-ynyl)-4Hchromen-4-ones (MeSO3H (1.5 equiv), H2O (10 equiv) in DMF, at 90 °C for 1 h, then CuCl2 (2.1 equiv) for 20 h). Furo[3,2-c]coumarins were recovered in 37–89% yields (10 examples). The CuCl2 played the dual role of cyclization promoter and oxidant. However, oxidation also took place in the presence of catalytic amounts of CuCl2 in an open flask with air oxygen [43]. In fact, in a subsequent paper, the same authors modified the reaction conditions (H2O (11 equiv), CuCl (20 mol%)) and obtained the same furocoumarins in 45–81% yields [44]. In one experiment to set up the reaction in the first paper [43], the authors recovered a mixture of 3-chloro-2-phenyl-4H-furo[3,2-c]chromen-4-one (40%) and the expected furocoumarin (35%). Therefore they found the best conditions for the chlorination reaction (CuBr (10 mol%), CuCl2 (4.2 equiv), H2O (10 equiv), in DMF, at 75 °C, for 10 h) [44]. The chlorinated furocoumarins were recovered in 35–86% yields (11 examples). The authors proposed a mechanism whereby, after the Michael addition of water to chromenone in wich the proton or copper acted as a Lewis acid, the alkynyl copper complex underwent cyclization. The protonation or chlorination of the resulting alkenyl copper, and its subsequent oxidation by oxygen, led to the creation of the product.
During the controlled experiments that sought to elucidate the mechanism of a one-pot synthesis of the furocoumarins through sequential Pd/Cu-catalyzed alkynylation and intramolecular hydroalkoxylation of 3-bromo-4-hydroxycoumarins, the authors found that CuI was responsible for the hydroalkoxylation step (CuI (15 mol%), THF, reflux, 18 h, then K2CO3/H2O). In fact, 4-hydroxy-3-(phenylethynyl)coumarin generated the expected furocoumarin in a 91% yield. Unfortunately, authors did not report any other examples of this cyclization [45].
In a paper on the copper-catalyzed oxidative synthesis of benzofurans from phenols and internal alkynes, one reaction was carried out with 4-hydroxycoumarin as the phenol. The reaction with diphenylacetylene produced the corresponding furocoumarin in a 68% yield [46].
The reaction of two molecules of 4-hydroxycoumarins with aldehydes in the presence of CuBr2 as the catalyst produced furo[3,2-c]coumarins derivatives. The authors performed some controlled experiments and then proposed the mechanism depicted in Scheme 6 [47].
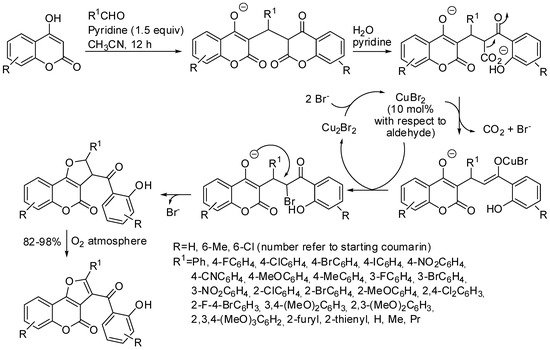

Scheme 6. Copper-catalyzed oxidative synthesis of furocoumarins.
In another study, 2-methylfuro[3,2-c]coumarins (48–82% yields, 13 examples) were prepared through the copper-catalyzed reaction of 4-hydroxycoumarins and terminal arylpropargyl acetates (DIPEA (1 equiv), CuBr (5 mol%), DMSO, microwave irradiation, 100 °C, 20 min). On the other hand, by simply changing the solvent from dimethyl sulfoxide to 1,2-dichloroethane, the isomeric 2-methylene-2,3-dihydrofuro[3,2-c]coumarins were instead recovered (36–85% yields, four examples). Alkylpropargyl acetates generated only a trace of the expected product under the standard reaction conditions, but by increasing the temperature and loading the catalyst, the corresponding methylenecoumarin was recovered in 41% yields and also in DMSO. Moreover, internal alkynes are unreactive. With this experimental evidence, the authors identified the formation of a copper–allenylidene complex [48].
Another approach to the synthesis of furo[3,2-c]coumarins was published by Yan, Lin, and co-workers. They studied the copper-catalyzed reaction between ketoxime carboxylates and 4-hydroxycoumarins. Many examples of linear and cyclic ketoxime carboxylates worked well in this reaction. Phenylacetaldehyde O-acetyl oxime, however, produced the desired 5-phenyl furo[3,2-c]coumarin in only a 7% yield. Moreover, the dehydrogenation of the polycyclic compound from cyclic ketoximes was efficiently performed. The authors conducted some controlled experiments and then proposed the mechanism depicted in Scheme 7 [49].
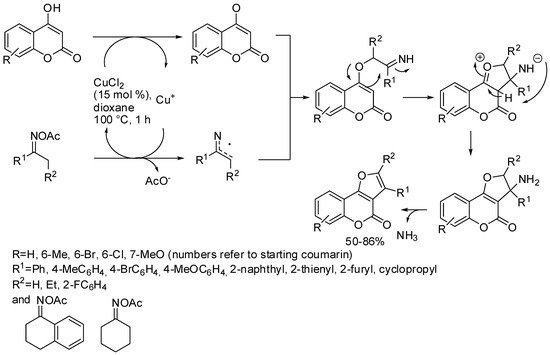

Scheme 7. Synthesis of furo[3,2-c]coumarins from ketoxime carboxylates.
At the same time, Phan and co-workers carried out the same reaction. They used CuBr2 as the catalyst in a much lower amount (1 mol%), with toluene as the solvent at a higher temperature (120 °C). Eighteen products were recovered in 49–89% yields. They proposed the same mechanism depicted in Scheme 6, with the only difference being the involvement of a AcOCuIII species in the first radical step [50].
Coumestans (6H-benzofuro[3,2-c]benzopyran-6-ones) were prepared through C-H activation with copper (II) acetate using 3-(2-hydroxyaryl)coumarins. Despite the high temperature that they employed, the reaction was largely substituent tolerant. The authors carried out some controlled experiments and then proposed the mechanism depicted in Scheme 8 [51].
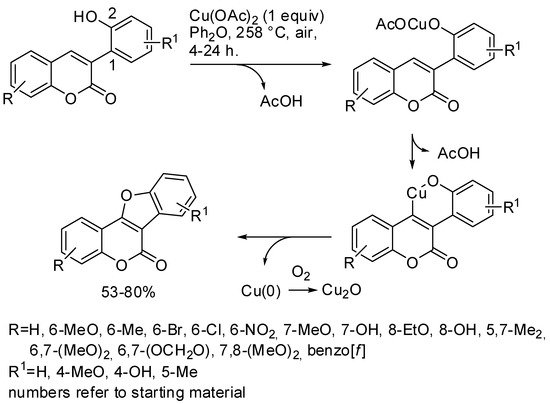

Scheme 8. Copper-mediated synthesis of coumestans.
In the previous section, the reaction of polyhydroxy benzenes with dimethyl and diethyl but-2-ynedioate was described. If 4-hydroxy coumarin was used as the substrate under the same reaction conditions, coumarinopyrones were obtained in 96% and 95% yields from dimethyl and diethyl but-2-ynedioate, respectively, after 2.5 h [40].
Furthermore, compounds with nitrogen heterocycles fused with the coumarin skeleton are important both as dyes and drugs. For instance, in a study on how fluorescent probes can be used for the detection of Cu2+, Wang and co-workers found that aqueous acetonitrile facilitated the cyclization of a coumarin-derived hydrazone, leading to a strong fluorescent product (Scheme 9) [52].
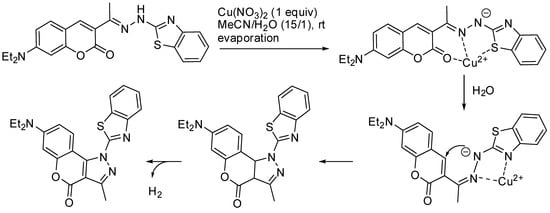

Scheme 9. Cyclization of a coumarin-derived hydrazone.
Pyrrolocoumarins (which are pyrano[3,2-e]indolines) were prepared from 5-(2-trimethylsilylethynyl)6-aminocoumarins in 82%, 98%, and 99% yields for amino, methylamino, and ethylamino derivatives, respectively (CuI (50 mol%) in DMF at reflux, for 1 h). The authors carried out some experiments to try and elucidate the mechanism, but they did not come to a definitive conclusion [53].
In another synthesis by the same research group, copper (II) acetate was found to promote the cyclization of 5-allyl-6-(N-sulfonylamino)coumarins into tetracyclic sultams. The authors envisaged the mechanism depicted in Scheme 10. In that mechanism, copper salts were both the catalyst and the oxidant [54].
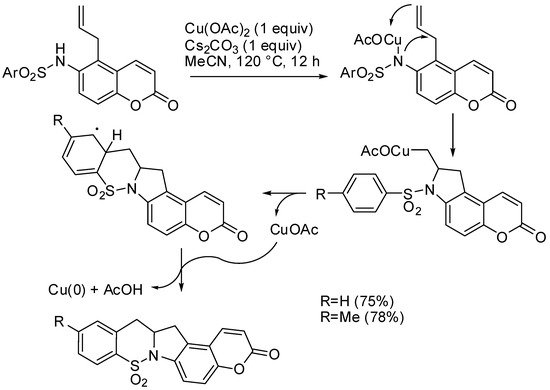

Scheme 10. Copper-catalyzed cyclization of 5-allyl-6-(N-sulfonylamino)coumarins.
In another study, 3-arylcarbonyl coumarins and 3-methylindole were allowed to react in the presence of CuBr2 in order to synthesize coumarin-fused 9H-pyrrolo[1,2-a]indoles. The reaction was scalable, and 1.06 g was prepared in a 97% yield. The reaction did not suffer from the steric hindrance of coumarin substituents, and electron-withdrawing substituents gave higher yields than electron-donating substituents. Unfortunately, both alkylcarbonyl coumarins and every substituted indole, except for 3-methyl, were unreactive. The authors proposed the reaction mechanism depicted in Scheme 11 [55].
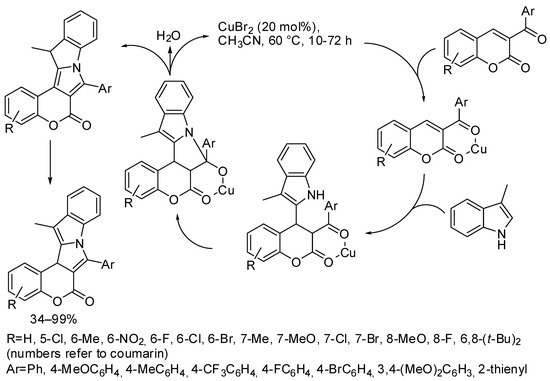

Scheme 11. Copper-catalyzed synthesis of coumarin-fused 9H-pyrrolo[1,2-a]indoles.
The copper-catalyzed cyclization of 4-(phenylamino)-2H-chromen-2-ones to 6H-chromeno[4,3-b]quinolin-6-ones was performed using DMF. Controlled experiments demonstrated that the CH group of the cyclic product arose from one of the DMF methyl groups (Scheme 12). Neither the position nor the electronic effect of the substituents, both on the aniline and coumarin moieties, had much effect on the yields [56].
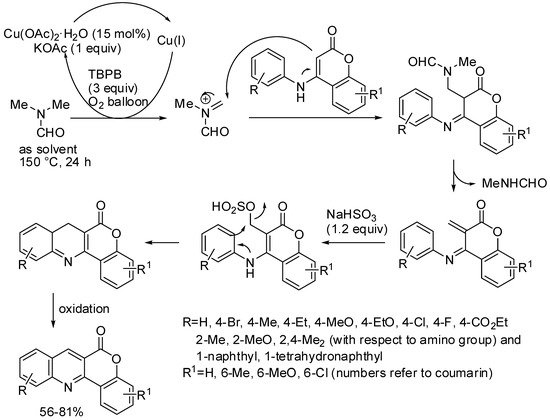

Scheme 12. Copper-catalyzed synthesis of 6H-chromeno[4,3-b]quinolin-6-ones.
The one-pot Cu-catalyzed reaction of 4-chloro-3-formylcoumarins with two benzylamine equivalents led to the generation of 1-benzyl-2-phenyl-1,2-dihydro-5H-chromeno[4,3-d]pyrimidin-5-ones, which showed green or blue fluorescence when irradiated with a UV lamp. The reaction worked well with electron-donating substituted benzylamines, worse with weak electron-withdrawing groups, and did not work at all with strong electron-withdrawing substituents, such as CF3, NO2, or pyridinemethanamine. The reaction was scalable and up to 1.19 g could be obtained (with a 68% yield). The use of two different benzylamines led to a mixture of cross-coupled and homo-coupled products. A plausible mechanism was proposed by the authors after some controlled experiments (Scheme 13) [57].
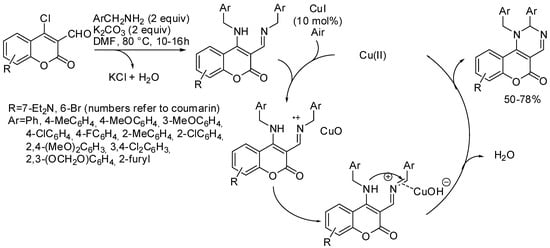

Scheme 13. Copper-catalyzed synthesis of fluorescent chromeno[4,3-d]pyrimidin-5-ones.
Finally, the copper-catalyzed click chemistry between azido derivatives and triple bond-containing compounds was successfully conducted to prepare conjugate products that were between coumarins and nucleotides or nucleosides. One example was the reaction of some modified nucleotides that bore alkynyl side chains with terminal triple bonds (5-(octa-1,7-diynyl)-2′-deoxyuridine, 5-(octa-1,7diynyl)-2′-deoxycytidine, 3H-furo[2,3-d]pyrimidin-2-one nucleoside and 7H-pyrrolo-[2,3-d]pyrimidin-2(3H)-one nucleoside) with 3-azido-7-hydroxycoumarin. In this way, some fluorescent dyes were prepared in 61–85% yields (CuSO4·5H2O, Na-ascorbate, THF–H2O–t-BuOH (3:1:1), at r.t.). The reaction was also successfully induced with small modifications to oligonucleotides [58].
Another example was the reaction of 3-[(2-propynylamino)methyl]coumarins with azidothymidine (Na-ascorbate (20 mol%), CuSO4·5H2O (3 mol%), H2O–THF (1:1), at r.t., for 24 h). The resulting triazole derivatives were isolated in 64–76% yields (five examples). These conjugates could be potential drugs against HIV-1, given that modelling studies have shown interesting hydrogen bond interactions of these compounds with the receptor cavity of HIV-1 protease and reverse transcriptase [59].
References
- Carneiro, A.; Matos, M.J.; Uriarte, E.; Santana, L. Trending Topics on Coumarin and Its Derivatives in 2020. Molecules 2021, 26, 501.
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A Review on Anti-Tumor Mechanisms of Coumarins. Front. Oncol. 2020, 10, 592853.
- Qin, H.L.; Zhang, Z.W.; Ravindar, L.; Rakesh, K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020, 207, 112832.
- Prusty, J.S.; Kumar, A. Coumarins: Antifungal effectiveness and future therapeutic scope. Mol. Divers. 2020, 24, 1367–1383.
- Gonçalves, G.A.; Spillere, A.R.; das Neves, G.M.; Kagami, L.P.; von Poser, G.L.; Canto, R.F.S.; Eifler-Lima, V. Natural and synthetic coumarins as antileishmanial agents: A review. Eur. J. Med. Chem. 2020, 203, 112514.
- Pires, C.T.A.; Scodro, R.B.L.; Cortez, D.A.G.; Brenzan, M.A.; Siqueira, V.L.D.; Caleffi-Ferracioli, K.R.; Vieira, L.C.C.; Monteiro, J.L.; Corrêa, A.G.; Cardoso, R.F. Structure–activity relationship of natural and synthetic coumarin derivatives against Mycobacterium tuberculosis. Future Med. Chem. 2020, 12, 1533–1546.
- Goud, N.S.; Kumar, P.; Bharath, R.D. Recent Developments of Target Based Coumarin Derivatives as Potential Anticancer Agents. Mini Rev. Med. Chem. 2020, 20, 1754–1766.
- Kumar, N.; Udayabhanu; Alghamdi, A.A.; Mahadevan, K.M.; Nagaraju, G. Solvent free and green synthesis of efficient solvochromism based coumarin moieties for quick visualization of LFPs and OLEDs applications. J. Mol. Struct. 2021, 1223, 129208.
- Sun, X.Y.; Liu, T.; Sun, J.; Wang, X.J. Synthesis and application of coumarin fluorescence probes. RSC Adv. 2020, 10, 10826–10847.
- Jung, J.W.; Kim, N.J.; Yun, H.; Han, Y.T. Recent Advances in Synthesis of 4-Arylcoumarins. Molecules 2018, 23, 2417.
- Salem, M.A.; Helal, M.H.; Gouda, M.A.; Ammar, Y.A.; El-Gaby, M.S.A.; Abbas, S.Y. An overview on synthetic strategies to coumarins. Synth. Commun. 2018, 48, 1534–1550.
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules 2020, 10, 151.
- Mustafa, Y.F.; Bashir, M.K.; Oglah, M.K. Original and Innovative Advances in the Synthetic Schemes of Coumarin-Based Derivatives: A Review. Syst. Rev. Pharm. 2020, 11, 598–612.
- Gouda, M.A.; Salem, M.A.; Helal, M.H. A Review on Synthesis and Pharmacological Activity of Coumarins and Their Analogs. Curr. Bioact. Compd. 2020, 16, 818–836.
- Zeydi, M.M.; Kalantarian, S.J.; Kazeminejad, Z. Overview on developed synthesis procedures of coumarin heterocycles. J. Iran. Chem. Soc. 2020, 17, 3031–3094.
- Jumal, J.; Norhanis, S. Synthesis, Characterization, and Applications of Coumarin Derivatives: A Short Review. Malays. J. Sci. Health Technol. 2021, 7, 62–68.
- Alwan, E.S.; Mohareb, R.M. Synthesis of biologically active chromene, coumarin, azole, azine and thiophene derivatives from 1,3-diketone. Org. Commun. 2021, 14, 163–227.
- Bouhaoui, A.; Eddahmi, M.; Dib, M.; Khouili, M.; Aires, A.; Catto, M.; Bouissane, L. Synthesis and Biological Properties of Coumarin Derivatives. A Review. ChemistrySelect 2021, 6, 5848–5870.
- Singh, J.; Sharma, A. Visible Light-Induced Synthesis of Functionalized Coumarins. Adv. Synth. Catal. 2021, 363, 3411–3438.
- Molnar, M.; Lončarić, M.; Kovač, M. Green Chemistry Approaches to the Synthesis of Coumarin Derivatives. Curr. Org. Chem. 2020, 24, 4–43.
- Gulati, S.; Singh, R.; Sangwan, S. A review on convenient synthesis of substituted coumarins using reusable solid acid catalysts. RSC Adv. 2021, 11, 29130–29155.
- Rullo, M.; Pisani, L. 4-Hydroxycoumarins as Michael donors in asymmetric routes to polycyclic coumarins (microreview). Chem. Heterocycl. Compd. 2018, 54, 394–396.
- Moreira, M.; Martelli, L.S.R.; Corrêa, A.G. Asymmetric organocatalyzed synthesis of coumarin derivatives. Beilstein J. Org. Chem. 2021, 17, 1952–1980.
- Dalpozzo, R.; Mancuso, R.; Liu, Y.K. Recent Advances in the Asymmetric Synthesis of Chromane Derivatives. Targets Heterocycl. Syst. 2021, 24, 226–273.
- Priyanka; Sharma, R.K.; Katiyar, D. Recent Advances in Transition-Metal-Catalyzed Synthesis of Coumarins. Synthesis 2016, 48, 2303–2322.
- Bathia, R.; Pathania, S.; Singh, V.; Rawal, R.K. Metal-catalyzed synthetic strategies toward coumarin derivatives. Chem. Heterocycl. Comp. 2018, 54, 280–291.
- Kanchana, U.S.; Diana, E.J.; Mathew, T.V.; Anilkumar, G. Palladium-catalyzed cross-coupling reactions of coumarin derivatives: An overview. Appl. Organomet. Chem. 2020, 34, e5983.
- Seoane, A.; Casanova, N.; Quiñones, N.; Mascareñas, J.L.; Gulías, M. Straightforward Assembly of Benzoxepines by Means of a Rhodium(III)-Catalyzed C−H Functionalization of o-Vinylphenols. J. Am. Chem. Soc. 2014, 136, 834–837.
- Tan, H.; Li, H.; Wang, J.; Wang, L. Ru-Catalyzed Decarboxylative Annulations of a-Keto Acids with Internal Alkynes: Dual Roles of COOH as Directing Group and Leaving Group. Chem. Eur. J. 2015, 21, 1904–1907.
- Li-Jie Cheng, L.J.; Mankad, N.P. C–C and C–X coupling reactions of unactivated alkyl electrophiles using copper catalysis. Chem. Soc. Rev. 2020, 49, 8036–8064.
- Das, A.; Ren, Y.; Hessin, C.; Desage-El Murr, M. Copper catalysis with redox-active ligands. Beilstein J. Org. Chem. 2020, 16, 858–870.
- Xiong, Y.; Li, S.; Xiao, H.; Zhang, G. Recent Advances in Visible-Light-Promoted Copper Catalysis in Organic Reactions. Synthesis 2021, 53. in press.
- Souza de Araújo, F.H.; Rojas de Figueiredo, D.; Auharek, S.A.; Pesarini, J.R.; Meza, A.; da Silva Gomes, R.; Monreal, A.C.D.; Antoniolli-Silva, A.C.M.D.; Pires de Lima, D.; Kassuya, C.A.L.; et al. In vivo chemotherapeutic insight of a novel isocoumarin (3-hexyl-5,7-dimethoxy-isochromen-1-one): Genotoxicity, cell death induction, leukometry and phagocytic evaluation. Genet. Mol. Biol. 2017, 40, 665–675.
- Rajitha, B.; Naveen Kumara, V.; Someshwara, P.; Venu Madhava, J.; Narsimha Reddy, P.; Thirupathi Reddy, Y. Dipyridine copper chloride catalyzed coumarin synthesis via Pechmann condensation under conventional heating and microwave irradiation. ARKIVOC 2006, 2006, 23–27.
- Opanasenko, M.; Shamzhy, M.; Čejka, J. Solid Acid Catalysts for Coumarin Synthesis by the Pechmann Reaction: MOFs versus Zeolites. ChemCatChem 2013, 5, 1024–1031.
- Yamamoto, Y.; Kirai, N. Synthesis of 4-Arylcoumarins via Cu-Catalyzed Hydroarylation with Arylboronic Acids. Org. Lett. 2008, 10, 5513–5516.
- Yamamoto, Y.; Kirai, N.; Harada, Y. Cu-catalyzed stereoselective conjugate addition of arylboronic acids to alkynoates. Chem. Commun. 2008, 2010–2012.
- Reddy, M.S.; Thirupathi, N.; Haribabu, M. Tandem aldehyde–alkyne–amine coupling/cycloisomerization: A new synthesis of coumarins. Beilstein J. Org. Chem. 2013, 9, 180–184.
- Ragupathi, A.; Sagadevan, A.; Charpe, V.P.; Lin, C.C.; Hwu, J.R.; Hwang, K.C. Visible-light-driven copper-catalyzed aerobic oxidative cascade cyclization of N-tosylhydrazones and terminal alkynes: Regioselective synthesis of 3-arylcoumarins. Chem. Commun. 2019, 55, 5151–5154.
- Kayal, U.; Karmakar, R.; Banerjee, D.; Maiti, G. Copper oxide catalyzed domino process for the synthesis of substituted 2H-pyran-2-ones and polyhydroxy coumarin derivatives. Tetrahedron 2014, 70, 7016–7021.
- Li, Y.; Lu, Y.; Qiu, G.; Ding, Q. Copper-Catalyzed Direct Trifluoromethylation of Propiolates: Construction of Trifluoromethylated Coumarins. Org. Lett. 2014, 16, 4240–4243.
- Bull, J.A.; Luján, C.; Hutchings, M.G.; Quayle, P. Application of the BHQ benzannulation reaction to the synthesis of benzo-fused coumarins. Tetrahedron Lett. 2009, 50, 3617–3620.
- Cheng, G.; Hu, Y. One-pot synthesis of furocoumarins through cascade addition–cyclization–oxidation. Chem. Commun. 2007, 38, 3285–3287.
- Cheng, G.; Hu, Y. Two Efficient Cascade Reactions to Synthesize Substituted Furocoumarins. J. Org. Chem. 2008, 73, 4732–4735.
- Chen, L.; Li, Y.; Xu, M.H. One-pot synthesis of furocoumarins via sequential Pd/Cu-catalyzed alkynylation and intramolecular hydroalkoxylation. Org. Biomol. Chem. 2010, 8, 3073–3077.
- Zhu, R.; Wei, J.; Shi, Z. Benzofuran synthesis via copper-mediated oxidative annulation of phenols and unactivated internal alkynes. Chem. Sci. 2013, 4, 3706–3711.
- Zhang, W.L.; Yue, S.N.; Shen, Y.M.; Hu, H.Y.; Meng, Q.H.; Wu, H.; Liu, Y. Copper(II) bromide-catalyzed intramolecular decarboxylative functionalization to form a C(sp3)–O bond for the synthesis of furo-coumarins. Org. Biomol. Chem. 2015, 13, 3602–3609.
- Zhang, X.Y.; Hu, L.L.; Shen, Z.; Chen, Z.Z.; Xu, Z.G.; Li, S.Q.; Xie, J.W.; Cui, H.L. Copper-Catalyzed Synthesis of Furocoumarins and Dihydrofurocoumarins through a Propargylation/Alkyne Oxacyclization/Isomerization Cascade under Microwave Irradiation. Synlett 2015, 26, 2821–2825.
- He, M.; Yan, Z.; Wang, W.; Zhu, F.; Lin, S. Copper-catalyzed radical/radical cross-coupling of ketoxime carboxylates with 4-hydroxycoumarins: A novel synthesis of furo-coumarins. Tetrahedron Lett. 2018, 59, 3706–3712.
- To, T.A.; Vo, Y.H.; Nguyen, A.T.; Phan, A.N.Q.; Truong, T.; Phan, N.T.S. A new route to substituted furocoumarins via copper-catalyzed cyclization between 4-hydroxycoumarins and ketoximes. Org. Biomol. Chem. 2018, 16, 5086–5089.
- Naik, M.M.; Kamat, V.P.; Tilve, S.G. Copper-mediated synthesis of coumestans via C(sp2)-H functionalization: Protective group free route to coumestrol and 4’-O-methylcoumestrol. Tetrahedron 2017, 73, 5528–5536.
- Zhang, Z.; Liu, Y.; Wang, E. A highly selective “turn-on” fluorescent probe for detecting Cu2+ in two different sensing mechanisms. Dyes Pigments 2019, 163, 533–537.
- Majumdar, K.C.; Mondal, S. A new strategy for the synthesis of coumarin- and quinolone-annulated pyrroles via Pd(0) mediated cross-coupling followed by Cu(I) catalyzed heteroannulation. Tetrahedron Lett. 2008, 49, 2418–2420.
- Majumdar, K.C.; Taher, A.; Nandi, R.K. Copper(II) Acetate Promoted Intramolecular Carboamination of Alkenes: An Efficient Synthesis of Pentacyclic Sultams. Synlett 2010, 21, 1389–1393.
- Jin, S.J.; Guo, J.M.; Zhu, Y.S.; Wang, Q.L.; Bu, Z.W. A copper-catalyzed tandem reaction for the construction of coumarin fused 9H-pyrroloindoles. Org. Biomol. Chem. 2017, 15, 8729–8737.
- Weng, Y.; Zhou, H.; Sun, C.; Xie, Y.; Su, W. Copper-Catalyzed Cyclization for Access to 6H-Chromenoquinolin-6-ones Employing DMF as the Carbon Source. J. Org. Chem. 2017, 82, 9047–9053.
- Kumari, S.; Shakoor, S.M.A.; Khullar, S.; Mandal, S.K.; Sakhuja, R. An unprecedented tandem synthesis of fluorescent coumarin-fused pyrimidines via copper-catalyzed cross-dehydrogenative C(sp3)–N bond coupling. Org. Biomol. Chem. 2018, 16, 3220–3228.
- Seela, F.; Sirivolu, V.R. Pyrrolo-dC oligonucleotides bearing alkynyl side chains with terminal triple bonds: Synthesis, base pairing and fluorescent dye conjugates prepared by the azide–alkyne “click” reaction. Org. Biomol. Chem. 2008, 6, 1674–1687.
- Olomola, T.O.; Klein, R.; Lobb, K.A.; Sayed, Y.; Kaye, P.T. Towards the synthesis of coumarin derivatives as potential dual-action HIV-1 protease and reverse transcriptase inhibitors. Tetrahedron Lett. 2010, 51, 6325–6328.
More
