Virus-like particles (VLPs) are a versatile, safe, and highly immunogenic vaccine platform. The use of a very flexible vaccine platform in COVID-19 vaccine development is an important feature that cannot be ignored. Incorporating the spike protein and its variations into VLP vaccines is a desirable strategy as the morphology and size of VLPs allows for better presentation of several different antigens.
- virus-like particles
- vaccines
- COVID-19
- SARS-CoV-2
1. Introduction
| Name | Platform | Adjuvant | Dosage | Efficacy * | References |
|---|---|---|---|---|---|
| Coronavac (Sinovac) |
Inactivated | Alum | 2 doses | 83.5% (95% CI, 65.4–92.1) | [8][9][10][11] |
| BBIBP-CorV (Sinopharm) |
Inactivated | Alum | 2 doses | 72.8% (95% CI, 58.1–82.4) | [12][13] |
| BBV152-Covaxin (Bharat Biotech) |
Inactivated | Alum | 2 doses | 77.8% (95% CI, 65.2–86.4) | [14][15] |
| AZD1222–Vaxzevria (Oxford/AstraZeneca) |
Viral vector | No | 2 doses | 74.0% (95% CI, 65.3–80.5) | [16][17][18] |
| Covishield (Oxford/AstraZeneca formulation) |
Viral vector | No | 2 doses | 74.0% (95% CI, 65.3–80.5) | [16][17][18] |
| Ad26.COV2.S (Johnson &Johnson-Janssen) |
Viral vector | No | 1 dose | 66.9% (95% CI, 59.0–73.4) | [19][20][21][22] |
| mRNA-1273 (Moderna) |
mRNA | No | 2 doses | 94.1% (95% CI, 89.3–96.8) | [23][24] |
| BNT162b-Comirnaty (Pfizer/BioNTech) |
mRNA | No | 2 doses | 95% (95% CI, 90.3–97.6) | [25][26] |
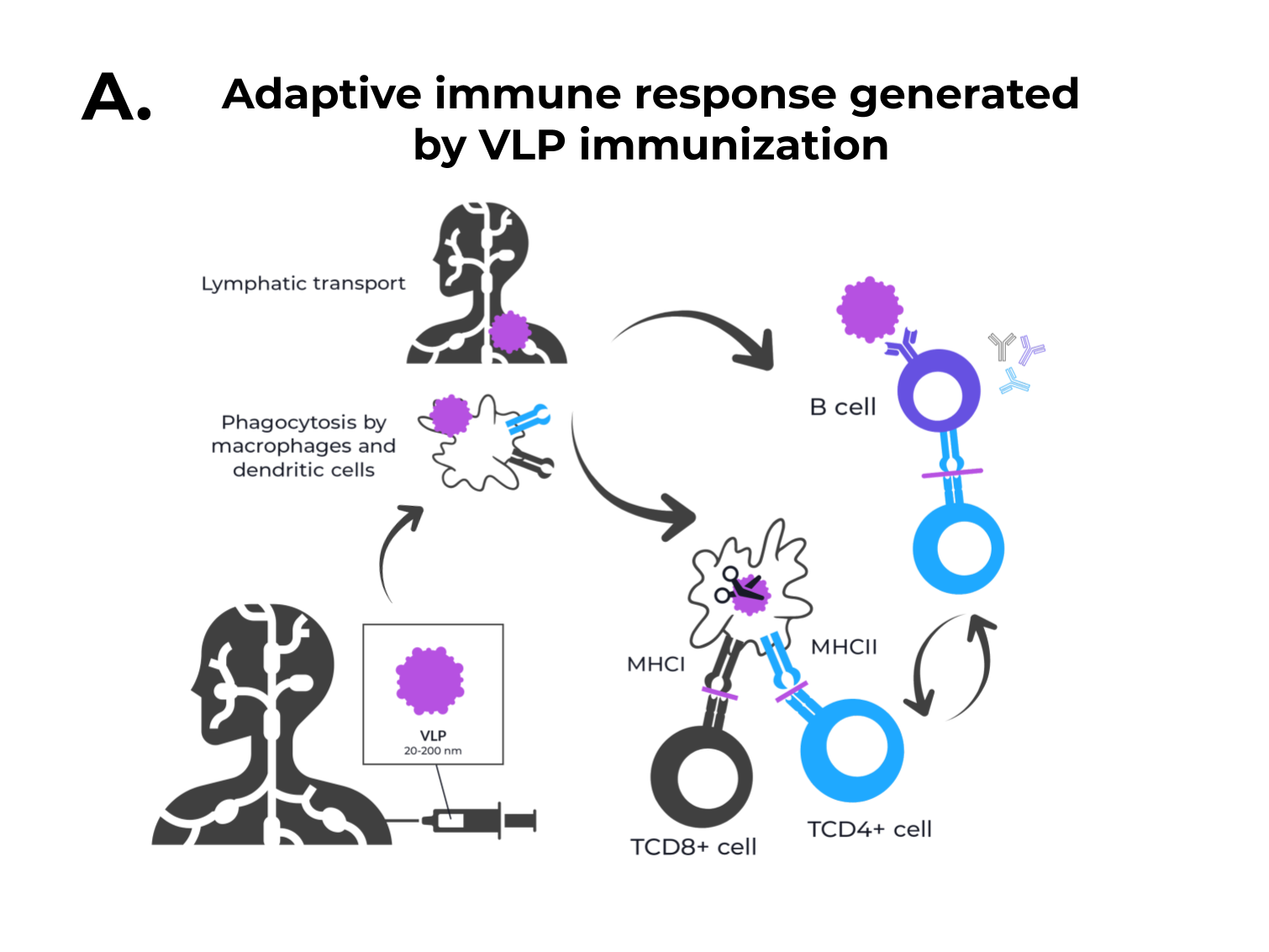
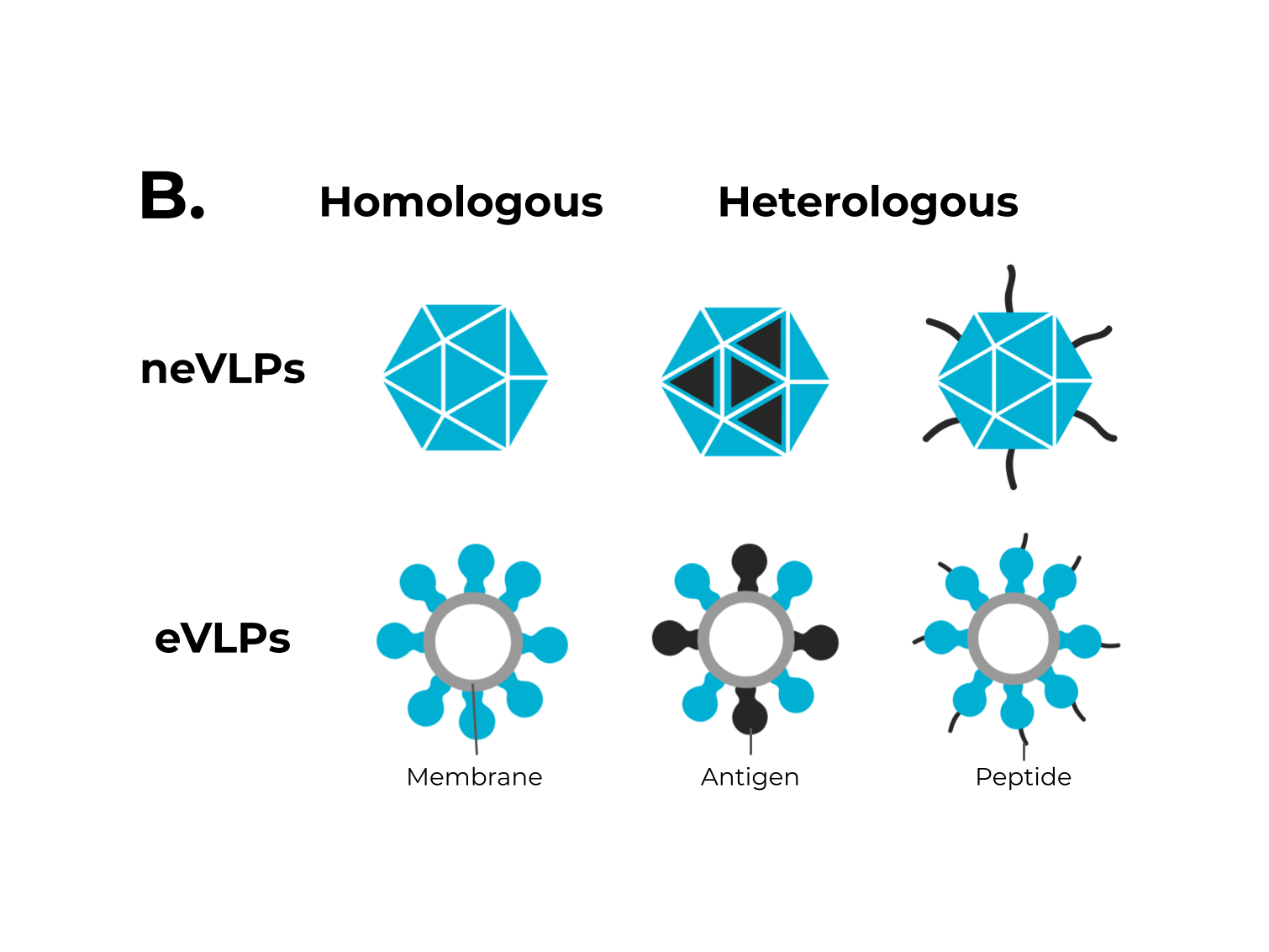 Figure 1. The adaptive immune response generated by VLPs immunization and VLPs classification. (A) After immunization, VLPs are phagocytized by dendritic cells or macrophages. Then, they are carried out to lymphatic vessels, where the antigenic regions will be processed and presented by class II MHC molecules (CD4+ T cells) and, through cross-presentation, by class I (CD8+ T cells). Immunological pathway activation by immunization with VLPs will activate robust cellular (cytokines) and humoral (B cell-antibodies) immune responses. (B) VLPs are classified as nonenveloped (neVLPs) or enveloped VLPs (eVLPs) based on the absence or presence of a lipidic membrane, respectively. These particles can also be classified as homologous or heterologous VLPs according to their composition. Homologous VLPs are assembled using proteins from the native pathogen only (blue), and heterologous VLPs can be assembled using proteins or peptides from different sources (black and blue).
Figure 1. The adaptive immune response generated by VLPs immunization and VLPs classification. (A) After immunization, VLPs are phagocytized by dendritic cells or macrophages. Then, they are carried out to lymphatic vessels, where the antigenic regions will be processed and presented by class II MHC molecules (CD4+ T cells) and, through cross-presentation, by class I (CD8+ T cells). Immunological pathway activation by immunization with VLPs will activate robust cellular (cytokines) and humoral (B cell-antibodies) immune responses. (B) VLPs are classified as nonenveloped (neVLPs) or enveloped VLPs (eVLPs) based on the absence or presence of a lipidic membrane, respectively. These particles can also be classified as homologous or heterologous VLPs according to their composition. Homologous VLPs are assembled using proteins from the native pathogen only (blue), and heterologous VLPs can be assembled using proteins or peptides from different sources (black and blue).2. SARS-CoV-2, VOCs, and Structural Vaccinology
The SARS-CoV-2 positive-sense single-stranded RNA genome (29 kb in length) encodes four structural and 16 non-structural proteins [3]. The structural proteins are the membrane (M), envelope (E), spike (S), and nucleocapsid (N) proteins, as seen in other coronaviruses (Figure 2A).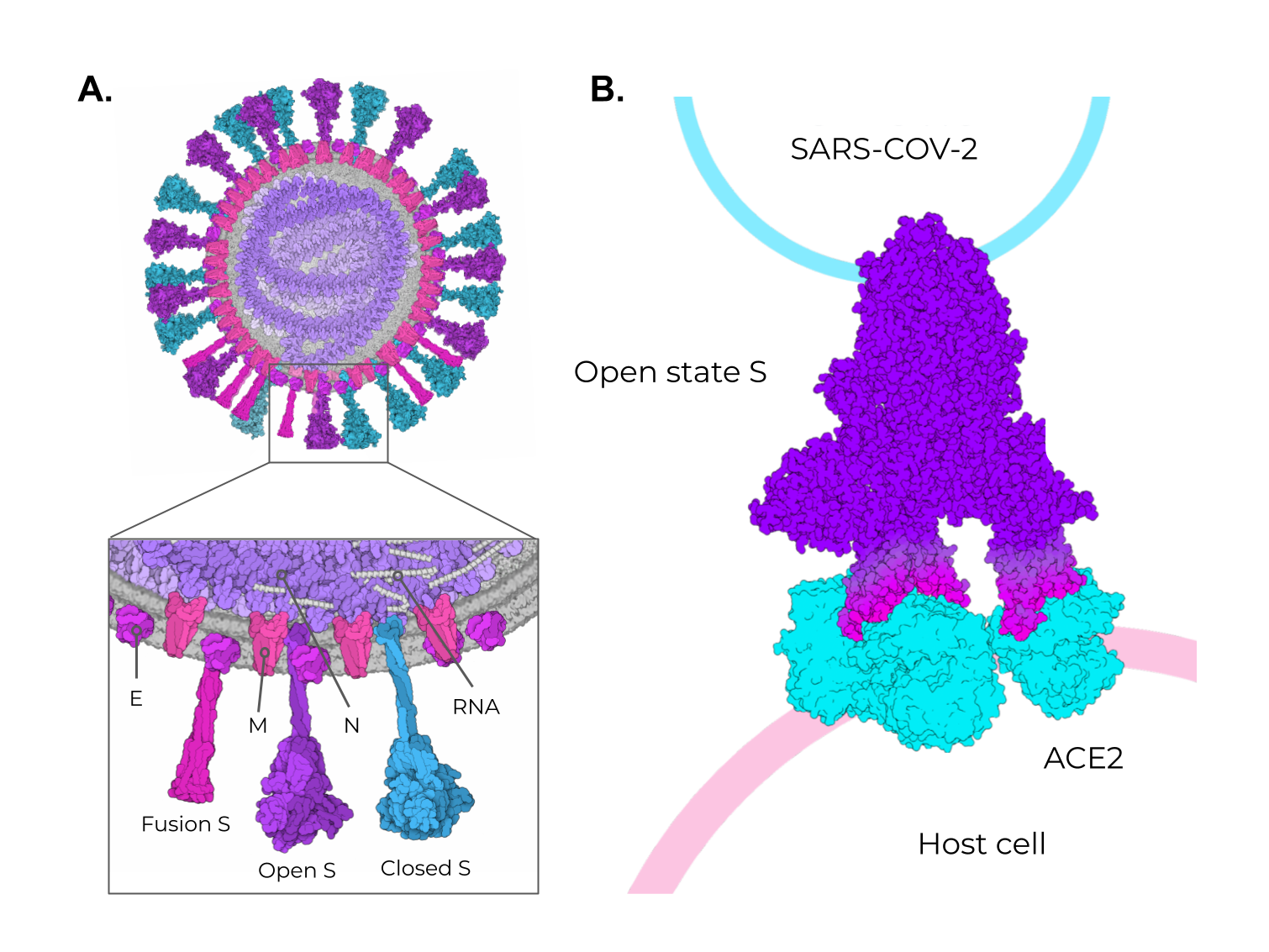 Figure 2. SARS-CoV-2 structural proteins and the different states of the Spike protein. (A) Schematic representation of the SARS-CoV-2 viral particle. The structure of the SARS-CoV-2 viral particle is composed of four structural proteins: Membrane (M), Envelope (E), Nucleocapsid (N), and Spike (S). The S protein is found in two different states on viral particles: open state (minor population) and closed state (major population). In addition, during the membrane fusion process (host cell entry), the S protein can be found in the fusion state (fusion S). (B) Schematic representation of the binding of open-state S (PDB ID 7498) to the ACE2 receptor present in the host cell. The illustrations were made in free software (CellPaint 2.0 [47] [91] and 3D Protein Imager [48][92]). The binding figure was made using the crystal structure of ACE2 bound to Spike available at the Protein Data Bank (PDB ID 7A98).
Among these structural proteins, the main target for vaccine development is the SARS-CoV-2 S protein, which gives the characteristic crown-shaped structure of coronaviruses [49][50][88,89]. S is a highly glycosylated [51][93] homotrimer transmembrane protein (UNIPROT ID P0DTC2) composed of 1273 amino acids per chain. Known human sarbecoviruses (SARS-CoV-2 and SARS-CoV-1) and the alphacoronavirus NL63 invade the host cell through an interaction between the S protein and its receptor, the angiotensin-converting enzyme 2 (ACE2) [51][52][53][54][55][93,94,95,96,97] (Figure 2B). In general, the S protein consists of two major regions in addition to the signal peptide (SP) (1–12): the S1 subunit (13–685), and the S2 subunit (686–1273), which contains the transmembrane region (TM) (1214–1234) followed by the cytoplasmic domain (CD) (1235–1273) (Figure 3A). The S protein and the ACE2 receptor binding are mediated by the receptor-binding motif (RBM; 437–508), located in the receptor-binding domain (RBD; 319–541) [56][98] (Figure 3A, purple and cyan, respectively). The fusion machinery in S2 is composed of two fusion peptides (816–837 and 835–855) and two heptad regions (920–970 and 1163–1202). The first site of cleavage targeted by host proteases, such as furin and TMPRSS2, is located in the S1/S2 interface (685–686) [57][58][59][99,100,101] (Figure 3A, red). Removing the S1/S2 site promotes conformational changes that open the second cleavage site at S2 (815–816). The subsequent cleavage of the S2 site promotes the projection of needle-shaped fusion peptides into the host membrane [60][61][102,103], leading to cell fusion in 60–120 s in feline coronavirus [62][104]. The S protein presents a closed and open conformation [45][63][45,105] (Figure 3B, upper and bottom panel, respectively). With one or more RBDs projected outward, the open state constitutes the major conformation population of viable virions [63][105]. The increased exposure and steric freedom enable stronger interactions with the ACE2 receptor [45][64][45,106]. Therefore, mutations that stabilize this open conformation lead to positive selection, making the virus more transmissible [65][66][67][107,108,109].
Figure 2. SARS-CoV-2 structural proteins and the different states of the Spike protein. (A) Schematic representation of the SARS-CoV-2 viral particle. The structure of the SARS-CoV-2 viral particle is composed of four structural proteins: Membrane (M), Envelope (E), Nucleocapsid (N), and Spike (S). The S protein is found in two different states on viral particles: open state (minor population) and closed state (major population). In addition, during the membrane fusion process (host cell entry), the S protein can be found in the fusion state (fusion S). (B) Schematic representation of the binding of open-state S (PDB ID 7498) to the ACE2 receptor present in the host cell. The illustrations were made in free software (CellPaint 2.0 [47] [91] and 3D Protein Imager [48][92]). The binding figure was made using the crystal structure of ACE2 bound to Spike available at the Protein Data Bank (PDB ID 7A98).
Among these structural proteins, the main target for vaccine development is the SARS-CoV-2 S protein, which gives the characteristic crown-shaped structure of coronaviruses [49][50][88,89]. S is a highly glycosylated [51][93] homotrimer transmembrane protein (UNIPROT ID P0DTC2) composed of 1273 amino acids per chain. Known human sarbecoviruses (SARS-CoV-2 and SARS-CoV-1) and the alphacoronavirus NL63 invade the host cell through an interaction between the S protein and its receptor, the angiotensin-converting enzyme 2 (ACE2) [51][52][53][54][55][93,94,95,96,97] (Figure 2B). In general, the S protein consists of two major regions in addition to the signal peptide (SP) (1–12): the S1 subunit (13–685), and the S2 subunit (686–1273), which contains the transmembrane region (TM) (1214–1234) followed by the cytoplasmic domain (CD) (1235–1273) (Figure 3A). The S protein and the ACE2 receptor binding are mediated by the receptor-binding motif (RBM; 437–508), located in the receptor-binding domain (RBD; 319–541) [56][98] (Figure 3A, purple and cyan, respectively). The fusion machinery in S2 is composed of two fusion peptides (816–837 and 835–855) and two heptad regions (920–970 and 1163–1202). The first site of cleavage targeted by host proteases, such as furin and TMPRSS2, is located in the S1/S2 interface (685–686) [57][58][59][99,100,101] (Figure 3A, red). Removing the S1/S2 site promotes conformational changes that open the second cleavage site at S2 (815–816). The subsequent cleavage of the S2 site promotes the projection of needle-shaped fusion peptides into the host membrane [60][61][102,103], leading to cell fusion in 60–120 s in feline coronavirus [62][104]. The S protein presents a closed and open conformation [45][63][45,105] (Figure 3B, upper and bottom panel, respectively). With one or more RBDs projected outward, the open state constitutes the major conformation population of viable virions [63][105]. The increased exposure and steric freedom enable stronger interactions with the ACE2 receptor [45][64][45,106]. Therefore, mutations that stabilize this open conformation lead to positive selection, making the virus more transmissible [65][66][67][107,108,109].
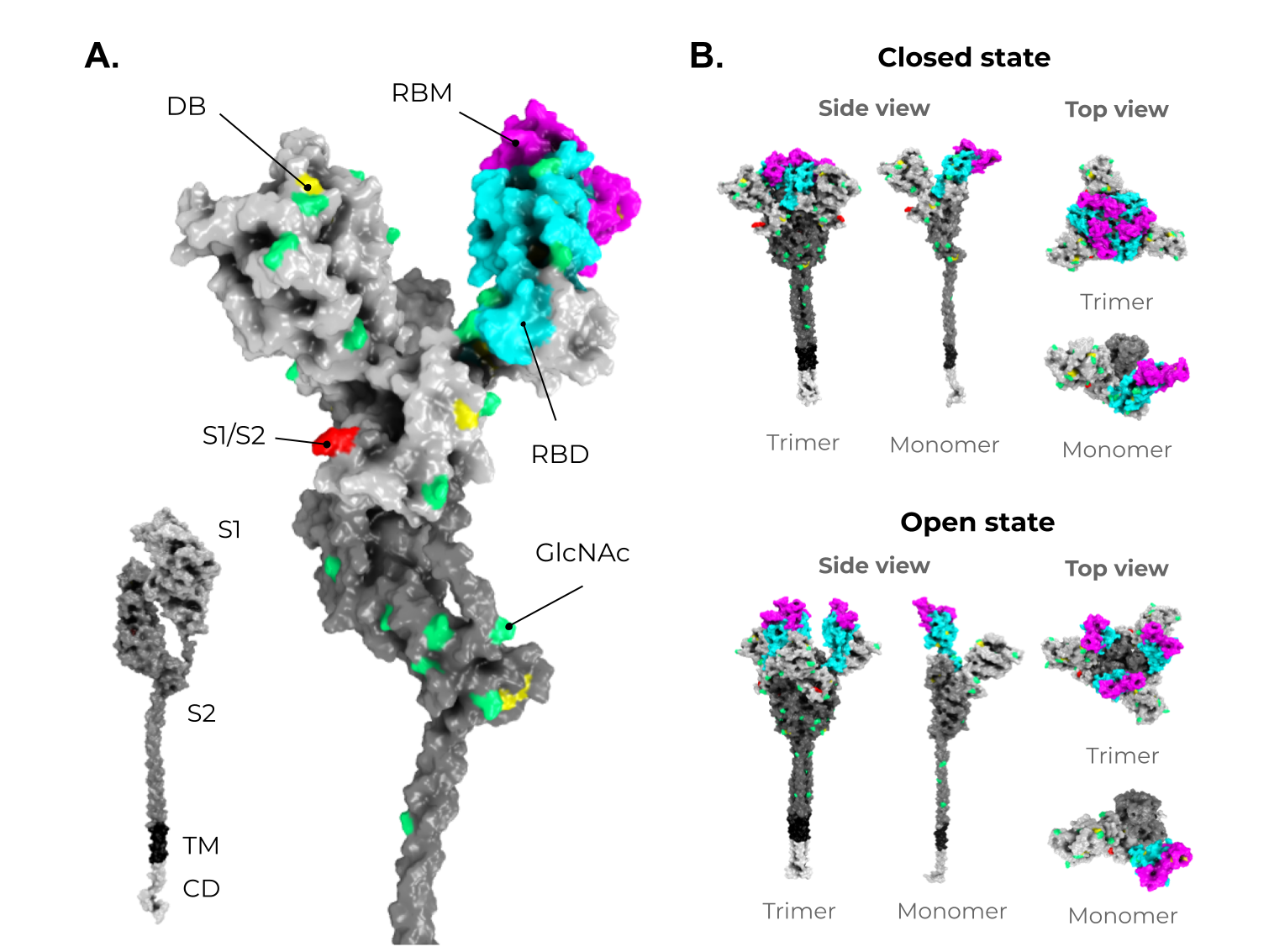 Figure 3. Structure and domain organization of the SARS-CoV-2 Spike (S) protein. (A) The S structure comprises a cytoplasmic domain (CD, white), a transmembrane domain (TM, black), and an ectodomain, which is divided into two subunits, S1 (gray) and S2 (dark gray). The magnification shows the several disulfide bridges (DB, yellow) and the glycosylation sites (GlcNAc, green) through the S protein ectodomain. It is highlighted in red, the S1/S2 interface. The receptor-binding domain (RBD, in cyan) and the receptor-binding motif (RBM, magenta) are also shown in S1. (B) As mentioned in Figure 2, the S protein shows two conformers on viable viruses (closed and open state). The upper panel shows the S protein in the closed state (trimeric and monomeric state). The bottom panel shows the S protein in the open state (trimeric and monomeric state). Illustrations were made in PyMol [68][110] using the wild-type structures available from Zhang et al. [69][70][107,111].
Figure 3. Structure and domain organization of the SARS-CoV-2 Spike (S) protein. (A) The S structure comprises a cytoplasmic domain (CD, white), a transmembrane domain (TM, black), and an ectodomain, which is divided into two subunits, S1 (gray) and S2 (dark gray). The magnification shows the several disulfide bridges (DB, yellow) and the glycosylation sites (GlcNAc, green) through the S protein ectodomain. It is highlighted in red, the S1/S2 interface. The receptor-binding domain (RBD, in cyan) and the receptor-binding motif (RBM, magenta) are also shown in S1. (B) As mentioned in Figure 2, the S protein shows two conformers on viable viruses (closed and open state). The upper panel shows the S protein in the closed state (trimeric and monomeric state). The bottom panel shows the S protein in the open state (trimeric and monomeric state). Illustrations were made in PyMol [68][110] using the wild-type structures available from Zhang et al. [69][70][107,111].
3. Enveloped VLPs against SARS-CoV-2
Some studies have shown that the minimal requirement for the assembly of SARS-CoV-2 VLPs and other coronaviruses is the combination of M and either the E or N proteins. However, most particles include the N protein and the highly immunogenic S protein for better assembly and expression (Figure 5A) [74][75][48,49]. To date, Vero E6 cells presented the highest expression of S-containing VLPs when compared to HEK293 cells [75][49]. All of these initial approaches for SARS-CoV-2 VLP production show a promising use of this platform in vaccine development. However, industrial viability and large-scale production were not considered, and these are crucial features for further development and are still very challenging in the eVLP production field [58][100].
Although homologous VLPs are an attractive strategy for producing these particles, the combination of antigenic SARS-CoV-2 proteins with other highly expressed heterologous proteins (that could be used as alternative VLPs scaffolds) are an exciting strategy to address issues of industrial production. The NDVLP-S2P (Figure 5B) is a heterologous chimeric eVLP vaccine candidate against SARS-CoV-2 that uses the structure of a well-characterized enveloped virus, the Newcastle disease virus (NDV) [41], and is being developed by the National Institute of Allergy and Infectious Diseases (NIAID). The transmembrane domain of the NDV fusion protein was fused to SARS-CoV-2 S2P, allowing the correct display of S2P on the VLP surface [41][45][76][41,45,149]. The NDV-S2P VLPs were more immunogenic than the trimeric S protein alone, showing that the presentation of antigens on the surface of the VLPs is advantageous [41]. Another heterologous SARS-CoV-2 eVLPs vaccine candidate is the CoVLP from Medicago/GSK (Figure 5C) [37][77][37,51]. This vaccine is based on VLPs that display a mutated S2P protein, which comprises a plant signal peptide, GSAS substitutions in the S1/S2 site, and TM/CD regions of the Influenza H5 A/Indonesia/5/2005. The CoVLP vaccine is formulated with AS03 [78][141] and given in a two-dose regimen. After the second dose, immunized volunteers showed higher serum SARS-CoV-2 nAb titers than in convalescent plasma. This vaccine candidate is already in phase 3 clinical trials (NCT04636697). VBI-2902a [76][149] is an MLV-based eVLPs vaccine candidate containing the S protein in the prefusion state fused with the VSV-G transmembrane cytoplasmic domain (VSV-GTC) (Figure 5D). This vaccine is being developed by VBI Vaccines and is in ongoing clinical trials 1/2 (NCT04773665).
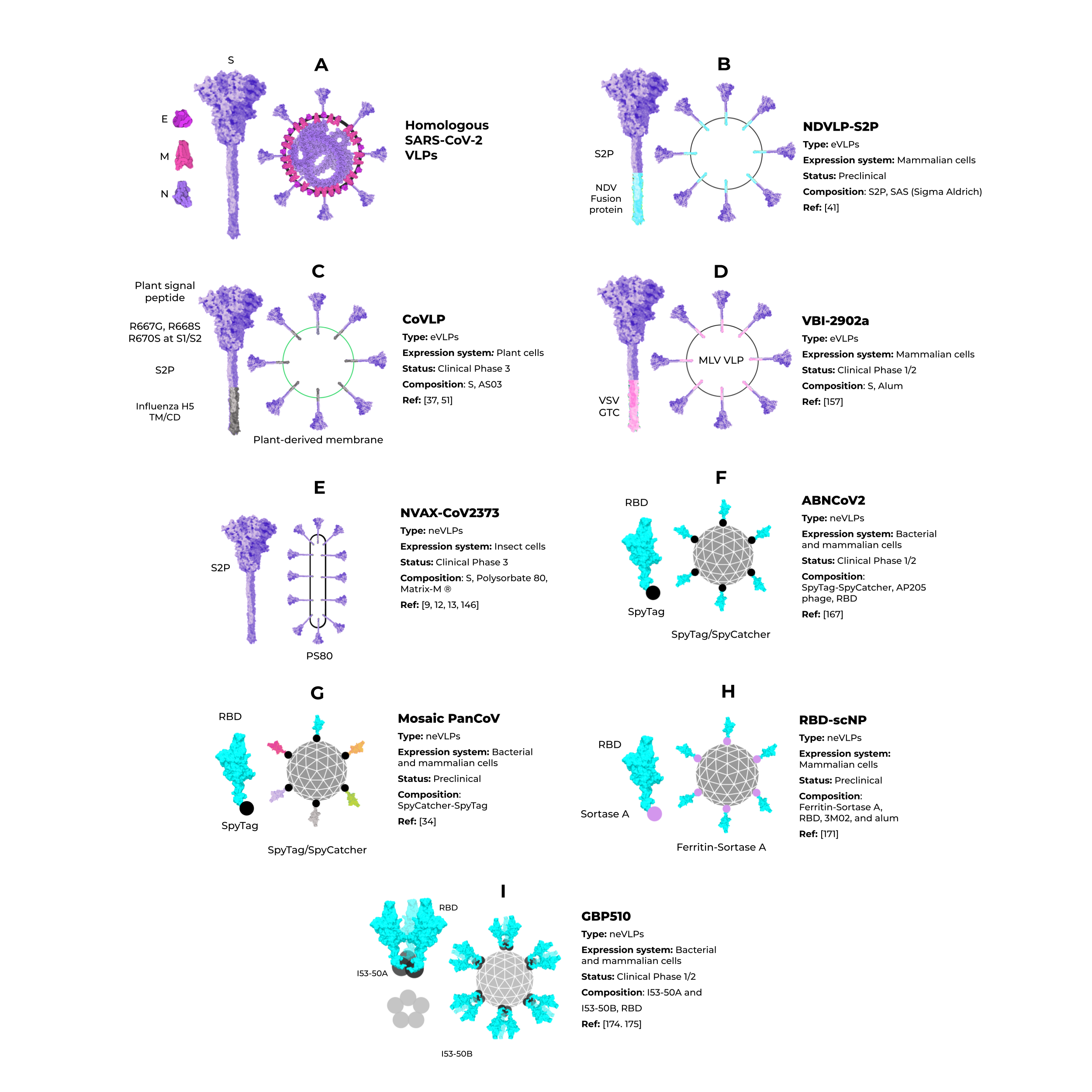
Figure 5.
Enveloped and nonenveloped VLPs against SARS-CoV-2.
4. Non-Enveloped VLPs against SARS-CoV-2
Unlike eVLPs, neVLPs do not contain any lipid membranes. They can be produced in simpler expression systems, such as those using bacteria (i.e., Escherichia coli) and yeast (i.e., Saccharomyces cerevisiae and Pichia Pastoris) cells. The Hepatitis B virus vaccine, Engerix-B® [79][150], and the Human papillomavirus vaccine, Gardasil® and Gardasil 9® [33], are neVLPs-based vaccines approved by the FDA. They are produced in Saccharomyces cerevisiae, an efficient expression system that is industrially scalable and cheaper than mammalian and insect cell systems. Despite these clear advantages, bacteria and yeast cells lack complex post-translational modifications (PTM) needed to produce some proteins, such as the highly glycosylated SARS-CoV-2 S protein [80][81][75,151]. Thus, the choice of the best expression system could be a determinant for protein/VLP production, even considering neVLPs. Cervarix® is another HPV neVLP-based vaccine [82][152] that is highly immunogenic [83][153] and effective [84][85][154,155] against HPV types 16 and 18, which are the main serotypes that cause cervical cancer [86][148]. The Cervarix® vaccine is produced using insect cells infected with recombinant baculovirus [40][87][88][40,156,157].
