Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Alan Prem Kumar and Version 2 by Catherine Yang.
Pancreatic cancer (PC) is one of the leading causes of death and is the fourth most malignant tumor in men. The epigenetic and genetic alterations appear to be responsible for development of PC. Small interfering RNA (siRNA) is a powerful genetic tool that can bind to its target and reduces expression level of a specific gene. The various critical genes involved in PC progression can be effectively targeted using diverse siRNAs.
- pancreatic cancer
- small interfering RNA (siRNA)
1. Proliferation and Growth
The uncontrolled growth of PC cells makes it difficult to manage this life-threatening disease. Several different factors and pathways are responsible for rapid proliferation of PC cells. A number of recent studies have focused on various oncogenic signaling networks involved in the progression of PC cells. For instance, pancreatic stellate cells are able to secrete exosomes containing miRNA-5703 to induce PI3K/Akt signaling and promote PC growth rate [1][87]. The β-catenin is another pathway that its up-regulation by Mind Bomb 1 leads to PC growth [2][88]. On the other hand, tumor-suppressor factors such as miRNA-573 suppress PC growth via TSPAN1 down-regulation [3][89]. Hence, the process of PC growth seems to be complicated and each gene can target various downstream targets to modulate PC progression [4][90]. The aim of current section is to show that how siRNAs can be applied in targeting pathways related to the aberrant growth of PC cells.
Precursor of nerve growth factor (proNGF) is a new and potential therapeutic target in cancer therapy. ProNGF expression undergoes up-regulation in PC and mediates metastasis of tumor cells. The lncRNA OIP5-AS1 enhances ProNGF expression via down-regulating miRNA-186-5p expression to increase PC invasion [5][91]. On the other hand, anoikis is a kind of apoptotic response stimulated by loss of adhesion to substrate. Reversing anoikis resistance is of importance in PC therapy [6][92]. Furthermore, proNGF can induce anoikis resistance [7][8][93,94]. In another study, it was found that proNGF-siRNA promotes anoikis induction in PC cells, and significantly reduces their proliferation. Following proNGF down-regulation by siRNA, autophagy inducers including autophagy-related gene 5 (ATG5) and Beclin-1 can undergo inhibition, thereby showing that apoptosis induction and autophagy inhibition can occur by proNGF-siRNA [9][95]. This study provides new insight about siRNA capacity in affecting interaction among programmed cell death (PCD) pathways. The apoptosis and autophagy interaction can be considered a determining factor in cancer. Inhibiting pro-survival autophagy can sensitize tumor cells to apoptosis [10][11][12][96,97,98]. The previous study clearly revealed that proNGF-siRNA could be beneficial in apoptosis induction via preventing autophagy [9][95].
NUF2 (NUF2 Component of NDC80 Kinetochore Complex) is a linker between kinetochore attachment site and tubulin subunits [13][99]. It has been reported that NUF2 down-regulation by RNAi leads to an impairment in attachment of kinetochore to spindle microtubules and can effectively suppress cell proliferation at prometaphase [14][100]. In vitro and in vivo experiments have shown that NUF2 down-regulation by siRNA leads to decreased PC cell growth. Colony formation was stopped and cell cycle arrest at G0/G1 phase occurs due to down-regulation of cyclin B1, Cdc2 and Cdc25A [15][101].
Receptor-associated protein 80 (RAP80) shows overexpression in PC and can mediate its progression as well as proliferation [16][102]. RAP80 is involved in DNA repair process via binding to BRCA1 and recruiting it to DNA damage sites [17][18][19][20][21][22][103,104,105,106,107,108]. Therefore, RAP80-siRNA can be of significant importance in reducing PC proliferation. In this case, RAP80 down-regulation by siRNA can lead to apoptosis induction via Bax up-regulation and Bcl-2 down-regulation. Caspase-8 as an executor of apoptosis was stimulated, while no changes were observed in survivin levels [23][109]. One of the features of cancer cells is their hypoxic microenvironment that facilitates their progression and malignancy. Hypoxia inducible factor-1α (HIF-1α) up-regulation is responsible for immunosuppression [24][110], radio-resistance [24][110], chemoresistance (gemcitabine) [25][111] and proliferation (Warburg effect) [26][112] in PC. HIF-1α-siRNA leads to a decrease in mRNA and protein levels of HIF-1α that can remarkably diminish the proliferation and induce apoptosis in PC cells [27][113]. Therefore, using siRNA can act as a potential strategy in suppressing PC proliferation [28][114]. Cell cycle arrest and apoptosis induction are major outcomes of targeting tumor-promoting factors by siRNA in PC therapy [29][115].
SnoN gene is a key member of Skt family with tumor-promoting role. This gene was first recognized due to its similarity in sequence with v-Ski and further investigation revealed that SnoN could induce growth of chicken and quail embryo fibroblasts [30][31][116,117]. The overexpression of SnoN occurs in human cancers that may result from gene amplification, transcriptional activation and enhanced protein stability [32][33][34][118,119,120]. In respect to the involvement of SnoN in cancer survival, its down-regulation can be considered to be a promising strategy in PC therapy. Therefore, siRNA has been introduced into PC cells for down-regulating SnoN gene expression. Upon SnoN down-regulation, PC cells undergo apoptosis, and their proliferation was interrupted [35][121]. It appears that anti-apoptotic proteins can be directly affected for triggering apoptosis in PC cells, instead of targeting molecular pathways that can promote PC progression. For instance, in mitochondrial pathway of apoptosis, the expression of Bcl-2 as an anti-apoptotic factor decreases. Overexpression of Bcl-2 protects cancer cells against cell death. By introducing Bcl-2-siRNA into PC cells, apoptosis can be induced [36][122]. The interesting point is that a variety of molecular pathways can result in increased proliferation and survival of PC cells. Nek2 is a serine-threonine kinase with potential role in both splitting centrosome and spindle formation in mammalian cells [37][123]. Nek2 up-regulation provides chromosome instability and aneuploidy in cancers [38][124]. The Nek2 inhibition appears to be advantageous in decreasing expression level of PD-L1 to enhance lymphocyte infiltration and promote anti-tumor immunity in PC suppression [39][125]. Hence, targeting Nek2 is of importance in suppressing cancer progression. For this reason, Nek2-siRNA has been applied for PC suppression in vitro and in vivo. Noteworthy, in mouse model of PC, siRNA has been introduced via a catheter. The Nek2-siRNA can impair the proliferation of PC cells and it promotes survival of xenograft mouse model. Furthermore, Nek2-siRNA can prevent liver metastasis of PC cells [40][126].
In addition, it has been reported that RPL1, as a ribosomal protein can be targeted in PC therapy. Moreover, down-regulation of RPL1 by siRNA leads to apoptosis and cell cycle arrest at G1 phase, and suppress DNA replication [41][127]. These studies advocated the fact that first step in PC therapy is identifcation of the various tumor-promoting factors. Thus, siRNAs can be designed for specific targeting of tumor cells to suppress PC proliferation and induce apoptosis [42][128].
Mammalian histone deacetylases (HDAC) are grouped into four different categories (I–IV) [43][44][129,130]. They participate in regulating biological processes including chromatin remodeling, gene repression, regulating cell cycle, and differentiation [43][45][46][47][129,131,132,133]. HDAC dysregulation is associated with transcription repression and inhibiting expression of tumor-suppressor genes [48][49][134,135]. HDAC1 plays a significant role in PC progression. It has been shown that HDAC1 down-regulation can lead to cancer proliferation suppression [50][136]. HDAC1 and HIF-1α can produce a complex in binding to hypoxia response elements (HRE) on the miR-548an promoter, down-regulating its expression and enhancing carcinogenesis in PC [51][137]. HDAC1 recruitment can lead to PC metastasis via reducing E-cadherin levels [52][138]. It has been established that HDAC1 undergoes up-regulation in PC tissues compared to normal ones. The expression of HDAC1 in PC tissues was 56.4%, while this expression was reduced significantly to 6.7% in the normal tissues. HDAC1-siRNA leads to down-regulation of this tumor-promoting factor, therefore paving the way for up-regulation of p21 and Bax in apoptosis induction in PC [53][139].
Autophagy is another important form of PCD that can exhibit both pro-survival and pro-death functions in cancers and inhibiting pro-survival autophagy can boost apoptosis induction in PC cells. The siRNAs have been used for down-regulating expression levels of proNGF, NUF2, RAP80, HIF-1α and SnoN to effectively impair the growth of PC cells and induce apoptosis. There are several other oncogenic pathways involved in PC progression including Wnt/β-catenin, STAT3 and NF-κB that can be focus of future studies.
2. Metastasis and Angiogenesis
The stimulation of MAPK/EscientistsRK axis by A-Raf was found to be vital in elevating migration of PC cells [54][140]. Furthermore, interactions occurring in tumor microenvironment can lead to PC metastasis. The recruitment of macrophages and their M2 polarization can secrete IL-6 that subsequently can induce STAT3 signaling for promoting PC migration and invasion via EMT induction [55][141]. STAT3 signaling can stimulate the growth and proliferation of cancer cells [56][57][58][142,143,144]. Both Akt and ERK1/2 molecular pathways can participate in PC metastasis and their stimulation occurs by FGF19 [59][145]. The metformin administration, as anti-tumor agent, substantially can reduce HNF4G expression via AMPK up-regulation to impair metastasis of PC cells [60][146]. Hence, PC migration is an increasing challenge and can significantly promotes aggressiveness of PC cells [61][147]. This section has been allocated to discuss application of siRNAs in disrupting PC invasion.
Cyclooxygenase-2 (COX-2) is an enzyme of metabolic process of arachidonic acid that can actively participate in carcinogenesis [62][63][64][148,149,150]. COX-2 induces angiogenesis in PC through up-regulating epidermal growth factor receptor (EGFR) [65][151]. COX-2 overexpression demonstrates poor prognosis in PC patients [66][152]. COX-2 inhibitors have been applied in PC therapy due to their efficacy in angiogenesis inhibition via vascular endothelial growth factor (VEGF) down-regulation and suppressing growth [67][68][153,154]. COX-2-siRNA can trigger apoptosis and cell cycle arrest in PC cells. In tumor xenografts, COX-2-siRNA can significantly attenuate volume and weight of tumors, thus showing the efficiency of gene silencing in vivo [69][155].
One of the potential therapeutic targets in cancer therapy is c-Src, an important non-receptor protein tyrosine kinase. Increasing evidence demonstrates tumor-promoting role of c-Src in cancer [70][71][72][156,157,158]. It can promote carcinogenesis via glycolysis induction [73][159]. Cell adhesion molecule 1 (CADM1) as a tumor-suppressor, can down-regulate expression of c-Src in suppressing colon tumorigenesis [74][160]. The self-renewal capacity of breast cancer cells is also regulated by c-Src [75][161]. These studies highlight the role of c-Src, as a tumor-promoting factor. It seems that c-Src down-regulation by siRNA impairs progression of PC cells via inhibiting angiogenesis. The activation of angiogenesis can occur by EGFR up-regulation, a process involved in cancer metastasis and migration to the distant sites [76][77][78][162,163,164]. The transfection efficiency of siRNA in PC cells was more than 90% and expression of c-Src was reduced by 86.1%. Following c-Src down-regulation by siRNA, the expression of VEGF was inhibited, thus suppressing angiogenesis and cancer progression [79][165].
Although there have been efforts in using siRNA for reducing migration of PC cells and various factors such as COX-2 and c-Src have been affected, there is still a long way before naked siRNAs can be applied for regulating PC progression. There are several other factors such as EMT and matrix metalloproteinases (MMPs) that can also lead to metastasis of PC cells. However, there are no studies reported about using siRNAs for modulating their upstream regulators such as ZEB1/2, TGF-β and Snail, among others.
3. Immune Regulation
Transforming growth factor-β (TGF-β) is considered as a novel target in cancer therapy [80][81][166,167]. Under physiological conditions, TGF-β regulates proliferation, differentiation, survival and cell adhesion to preserve tissue homeostasis. In cancer cells, TGF-β acts as a positive factor for metastasis via EMT induction [82][83][84][168,169,170]. On the other hand, retinoic acid-inducible gene I (RIG-I) is affected in cancer immunotherapy by enhancing the levels of interferon and apoptosis induction [85][86][87][171,172,173]. A bifunctional siRNA for down-regulating TGF-β and enhancing RIG-I expression has also been designed. This improvement in function can be obtained by introducing triphosphate group at the 5′ end of siRNA. Following TGF-β down-regulation, an increase occurs in survival time of xenograft models and metastasis and invasion of PC cells undergo down-regulation. Due to RIG-I activation, immune system can be activated that can promote the levels of interferon and RIG-I and induces apoptosis [88][174]. In addition, TGF-β down-regulation and RIG-I up-regulation increase potential of cancer immunotherapy [89][175]. However, an experiment has only investigated role of siRNA in cancer immunotherapy and as immune evasion is a common phenomenon in PC [90][176], more studies are required to show how such genetic tools can be employed in PC treatment and activating anti-tumor immunity. Although only TGF-β signaling has been targeted in improving anti-tumor immunity in PC, there is another well-known molecular pathway, known as PD-L1/PD-1 axis that is involved in triggering immune evasion [91][177]. Hence, further experiments can focus on using siRNA for down-regulating PD-1 expression and promoting anti-tumor immunity against PC cells.
4. Therapy Response and Synergistic Therapy
Another potential of siRNAs lies in improving potential of chemotherapeutic agents in suppressing tumor progression [92][93][178,179]. Therefore, combination cancer therapy with siRNA-anticancer drug could be designed. For instance, L-ascorbate is not capable of preventing PC migration. Co-application of siRNA-HIF-1α and L-ascorbate resulted in ansynergistic effect in suppressing PC invasion [94][180]. siRNA could serve as a potential adjuvant for promoting anti-tumor activity of compounds in PC therapy. Surgery is not considered a successful option in PC therapy due to diagnosis of PC at advanced stages. Therefore, chemotherapy has been primarily used for the treatment of PC patients. However, drug resistance emerges in PC and different molecular pathways including long non-coding RNAs [95][181], SRPX2 [96][182] and USP7 [97][183], among others can participate in the resistance of PC cells to chemotherapy. siRNAs can be potentially beneficial in impairing tumor proliferation as well as metastasis and causing subsequent increase in chemosensitivity of PC cells.
Ribonucleotide reductase (RR) is a rate-limiting enzyme vital for DNA synthesis and replication [98][184]. Celastrol can suppress progression of PC cells via down-regulating RRM2 expression, showing tumor-promoting function of this pathway [99][185]. Exposing PC cells to RRM2-siRNA leads to an increase in apoptosis and cell growth inhibition. Noteworthy, co-administration of RRM2-siRNA and doxorubicin (DOX) can lead to synergistic effect and a four-fold increase in anti-tumor activity [100][186]. Gemcitabine (GEM) is another chemotherapeutic agent that its potential in PC treatment has been reduced due to emergence of drug resistance [101][187]. The hTERT-siRNA can increase the number of PC cells undergoing apoptosis. The hTERT down-regulation induces cell cycle arrest at G0/G1 phase and enhances number of PC cells in S and G2/M phases [102][188]. Following hTERT down-regulation by siRNA, expressions of Bcl-2 and COX-2, as tumor-promoting factors undergo inhibition that is of importance for inducing apoptosis in PC cells [103][189], and enhancing their sensitivity to chemotherapy.
Now, it is obvious that when cancer cells demonstrate malignant behavior in terms of proliferation and migration, they can obtain chemoresistance [104][105][106][107][108][109][110][190,191,192,193,194,195,196]. Therefore, in order to provide effective cancer chemotherapy, it is vital to suppress the various pathways that lead to cancer migration and growth [107][111][193,197]. It appears that an overexpression of heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNP A2/B1) is in favor of PC growth [16][102]. As RNA-binding proteins, hnRNP A2/B1 participates in mRNA processing and telomere biogenesis [112][198]. Clinical study evaluating 42 patients with PC has shown role of hnRNP A2/B1 in PC and its association with E-cadherin, an important epithelial marker [113][199]. The exposure of PC cells to hnRNP A2/B1 can lead to apoptosis induction. A combination of hnRNP A2/B1 and chemotherapeutic agents such as 5-fluorouracil (5-FU), oxaliplatin and GEM can stimulate synergistic effect against PC cells. In fact, by suppressing PC growth, hnRNA A2/B1-siRNA enhances sensitivity of PC cells to chemotherapy. This combination leads to Bcl-2 down-regulation and Bax up-regulation, providing apoptotic cell death. Moreover, expression of P-glycoprotein, as a drug transporter that induces chemoresistance [114][200], decreases following this combination that is of importance in enhancing chemosensitivity [115][201].
Increasing evidence demonstrates that RR and thymidylate synthase (TS) can induce chemoresistance in cancer cells [116][117][202,203]. It has been reported that RRM2-siRNA, as a subunit of RR can promote GEM sensitivity, in vitro and in vivo [117][118][203,204]. A combination of GEM and RR- and TS-siRNA can stimulate apoptosis in PC cells and reduces their proliferation. This combination inhibits NF-κB activation following GEM administration and enhances TNF-related apoptosis-inducing ligand (TRAIL)-mediated cell death in PC cells [119][205]. In the previous section, it was mentioned that HIF-1α is a desirable factor for progression of PC cells. Moreover, HIF-1α down-regulation by siRNA leads to an increase in chemosensitivity of PC cells [120][206]. A set of tumor-promoting factors such as HIF-1α, ARNT, PFKFB4, and RBKS can be down-regulated by siRNA in inducing apoptosis and enhancing their sensitivity to chemotherapeutic agents including DOX and GEM (Figure 1 2 and Figure 23) [121][207]. The interesting point of this section is that prior studies have considered role of both molecular pathways and drug transporters in triggering drug resistance in PC and these molecular pathways and mechanisms can be markedly suppressed using siRNAs as an effective tool.
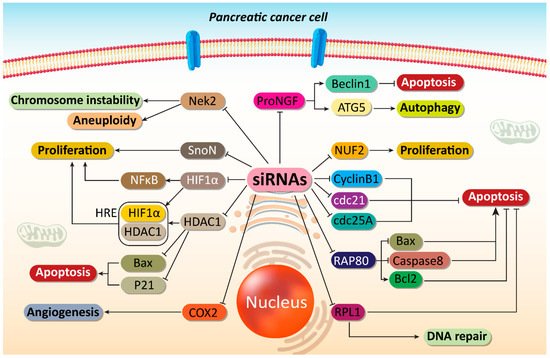
Figure 12. Impairing proliferation and angiogenesis of PC cells. Major molecular pathways can be targeted by siRNAs to induce apoptosis and DNA damage in PC cells. Moreover, angiogenesis responsible for PC progression can be suppressed by siRNAs in PC therapy. ProNGF, precursor of nerve growth factor; ATG, autophagy-related gene; COX2, cyclooxygenase-2; HDAC1, histone deacetylase 1; HIF-1α, hypoxia inducible factor-1α; NF-κB, nuclear factor-kappaB; siRNA, small interfering RNA; PC, prostate cancer.
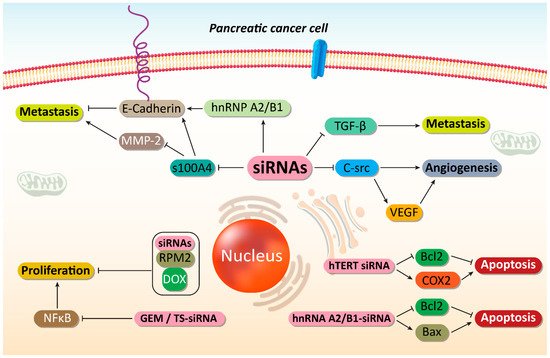
Figure 23. Suppressing PC metastasis and increasing their sensitivity to chemotherapy. By disrupting cancer proliferation and metastasis, as well as triggering apoptotic cell death, an increase occurs in sensitivity of PC cells to chemotherapy. SiRNAs play an important role in mediating these anticancer effects. MMp-2, matrix melloproteinase-2; hnRNP A2/B1, heterogeneous nuclear ribonucleoprotein A2/B1; TGF-β, transforming growth factor-beta; VEGF, vascular endothelial growth factor; COX-2, cyclooxygenase-2; GEM, gemcitabine; NF-κB, nuclear factor-kappaB; DOX, doxorubicin, siRNA, small interfering RNA; PC, prostate cancer.
