Spray congealing is an emerging technology for the production of solid dispersion to enhance the bioavailability of poorly soluble drugs by using low-melting hydrophilic excipients. Since the '90s, spray congealing technology has been used to obtain microparticles (MPs) for controlled release, taste masking and stability enhancement of APIs. The main advantages are the absence of solvents and the possibility to obtain free-flowing MPs by a relatively inexpensive, simple and one step process. This entry focused on the application of spary congealing as an amerging technology for the production of solid dispersions to enhance the bioavailability of poorly water soluble drugs by using low-melting hydrophilic excipients.
1. Introduction
Oral route is the most common way of drug administration because of its simplicity and ease of administration. About 80% of the dosage forms in the worldwide market are administered orally
[1]. Compared to oral liquid medicines (e.g., oral solution or suspensions), solid oral dosage forms have many benefits for the patient, such as convenience and simplicity of use, accurate dosing of the active ingredient, and no need of preparation/manipulation of the medicine before administration. From the industrial perspective, solid forms also offer the advantages of good stability and generally moderate production costs. For these reasons, most of the new chemical entities (NCEs) under development are intended to be marketed as solid dosage forms for oral administration
[2]. Because of the recent advances in combinatory chemistry and high-throughput screening methods, nowadays the NCEs are frequently large-molecular-weight lipophilic compounds. Therefore, poorly water soluble drugs constitute the 70% of the new drug candidates
[3]. Various physical-chemical properties contribute to make a compound poorly soluble and those include its complex structure, size, high molecular weight, high lipophilicity (log P), compound H-bonding to solvent, intramolecular H-bonding, intermolecular H-bonding (crystal packing), polymorphic forms, ionic charge status, pKa, and salt form
[4]. According to the Biopharmaceutical Classification System (BCS), a drug compound is poorly soluble if the highest dose strength is not soluble in 250 ml aqueous media over a pH range of 1–7.5 at 37.5 °C
[5]. Thus, poor aqueous solubility represents a serious concern if the clinical dose of drug cannot dissolve in the available volume of gastrointestinal fluids
[6].
Different strategies have been proposed over the years to address the issue of poorly water soluble drugs and they include particle size reduction, formation of nanocrystals and co-crystals, pH adjustment, use of cosolvents, self-emulsifying drug delivery systems (SEDDS), inclusion complexes, nanosuspensions, and solid dispersions. Among these methods, the development of solid dispersion (SD) has demonstrated to be one of the most promising approaches
[7]. The term SD refers to a system in which the drug is dispersed in a solid inert matrix and it consists of at least two different components which are commonly a hydrophilic matrix and a hydrophobic drug
[8]. Generally, SD can enhance oral bioavailability by allowing a rapid release of the drug into the solution in a supersaturated state above its equilibrium solubility and maintaining this state for as long as possible
[9].
Numerous technologies can be used for the manufacturing of SD. The methods for the preparation of SD are classified in solvent-based methods, melting-based methods, and mechanochemical activation. All kinds of high energy milling are considered as mechanochemical activation
[10]: high levels of mechanical energy may result in the crystal lattice disruption of materials, causing polymorphic transformations, crystal defect generation, amorphization, and other additional physical and chemical changes in a crystalline drug
[11]. Spray-drying represents the main widespread solvent-based method to obtain SD; it involves the evaporation of a solvent used to solubilize the SD components, with the formation of solid particles. Spray drying is a versatile technique to make SD. In addition, it is widely used to prepare microparticles, nanoparticles, self-emulsifying delivery systems, eutectic mixtures, and may use either organic or inorganic polymeric carriers
[12]. The main disadvantages are the relatively high process temperature, use of solvents, small particle size distribution, and high maintenance cost. Melting-based methods involve either the melting of the carrier or most frequently the softening of the thermoplastic polymeric carrier. The key feature of the melting-based technologies are the absence of solvent, either aqueous or organic, with related advantages of no toxicity related to the presence of organic solvents and the possibility to be applied for the loading of hygroscopic and water-sensitive drugs. Hot melt extrusion is the most established melting-based technique and it has been extensively explored for the manufacturing of SD
[13]. Beside to the numerous advantages, hot melt extrusion presents some drawbacks, such as the need of downstream processes to obtain the final product. Spray congealing (SC) has been recently proposed as an emerging technology for the manufacturing of SD in form of microparticles (MPs).
2. Spray Congealing Technology: General Aspects
In the past decades SC, also called spray chilling or spray cooling, has attracted increasing attention because it is a simple technique, less time and energy consuming compared to other methods to obtain SD
[14][15][14,15]. Other advantages include the ability to obtain spherical free-flowing MPs suitable for tableting or capsule filling without the need of other downstream processes (e.g., secondary drying, milling, granulation) and high encapsulation efficiency values (90–100%). From the industrial perspective, SC is easily scalable and can be adapted to the “continuous manufacturing”. As regards the equipment and mechanism of atomization, SC is closely associated with spray drying. Generally, spray drier apparatus can be used for SC processes with modifications. Although both techniques are based on the generation of droplets through atomization of a fluid, the mechanisms of MPs formation in spray drying and spray congealing are basically different, as centered on the evaporation of solvent and on the hardening of a molten material, respectively. Essentially, in the spray drying process a liquid solution (or suspension) is converted to a powdered solid by pumping the liquid feed into a drying chamber via a nozzle where fine droplets rapidly encounter a hot drying gas. The wet gas and dry particles are then separated by a cyclone and/or bag filter and the powdered product is dispensed into a collection vessel and/or bag filter
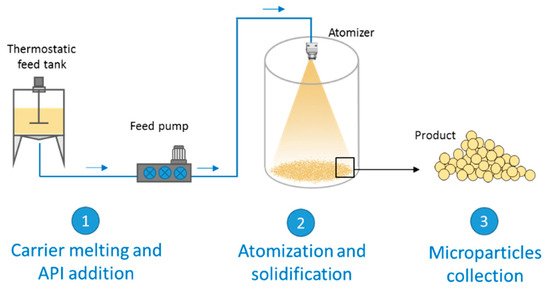
[12]. On the other hand, SC is based on the atomization of a fluid (solution or a suspension of a drug in a melted carrier) into a chamber maintained at a temperature below the carrier melting point. The steps of the process are schematized in
Figure 1. The first step involves the preparation of the fluid consisting of the molten carrier, kept at a temperature above its melting point, and the active ingredient. Excipients suitable for SC are substances with melting temperatures generally ranging from 35 to 90 °C. In the second step, the molten fluid stream is broken into small droplets by means of an atomizer. Subsequently the molten droplets solidify upon cooling in the chamber, producing the final solid spherical MPs. The performance of the spray congealing process strictly depends on the atomization efficiency of the molten fluid. Thus, the most important step is the atomization phase, which can be performed using various type of atomizers: pressure, two-fluids (also called pneumatic or air nozzles), rotary and ultrasonic nozzles. The reader interested in details on the different equipment and nozzle utilized in the spray congealing is referred to a recent review, where they are presented and fully discussed
[16].
Figure 1.
Scheme of a general spray congealing apparatus with the different steps for the production of microparticles.
SC has been used in a wide range of applications, including food and pharmaceutical area. The applications of SC in the production of different oral and non-oral drug delivery systems have been described in a recent review
[15]. Specifically, the pharmaceutical applications of this technology cover a wide area, including modified release systems, taste masking, bioavailability enhancement, API protection from different causes of degradation, and encapsulation of biologic drugs.
Spray congealed MPs are a multiparticulate dosage form as they consist of a multiplicity of small discrete units, each containing a fraction of the total amount of the administered API. Multiparticulate oral drug delivery systems are preferred over single unit dosage forms because of their comparatively greater dispersibility in the GIT, reduced-risk of dose dumping and systemic toxicity, decreased dosing frequency, increased patient compliance, lesser variability in concern to absorption, and accurate dosing
[17]. Moreover, because of the small size, multiparticulates are less dependent on gastric emptying, resulting in less inter and intra-subject variability in gastrointestinal transit time. Above all, they represent a flexible dosage form and, therefore, they are particularly attractive for developing patient-tailored pharmaceutical products.
3. Structure and Composition of Spray Congealed SD
Spray congealed SD for bioavailability enhancement of poorly water soluble drug can be considered SD in form of hydrophilic MPs, consisting in spherical particles with a size in the range of 10 μm–500 μm. More specifically, the spray congealed product consists on microspheres. Opposite to microcapsules, where the API is confined within the microcapsule by a surrounding layer of excipient, spray-congealed microspheres are a matrix system where the active compound is evenly distributed throughout the carrier. In particular, the carriers employed in SC for bioavailability enhancement application include hydrophilic low-melting materials such as polyethylene glycol (PEG) and its surface active derivatives poloxamers and polyoxylglycerides (commercially known as Gelucires
®). They are all semicrystalline carriers, as they contain both crystalline and amorphous domains
[18][19][20][18,19,20].
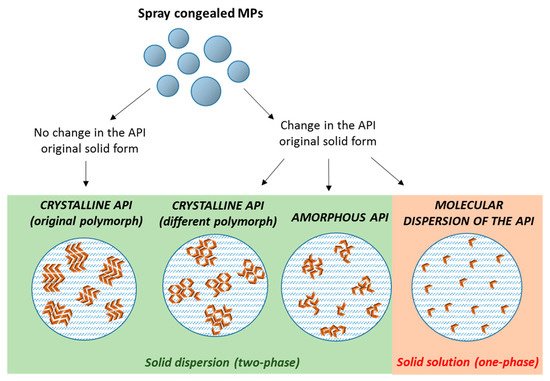
The dispersion of poorly water soluble APIs in these carriers by SC can result in the formation of either
solid dispersions with crystalline or amorphous drug molecules, or
solid solutions of the drug molecularly dispersed within the carrier. Often, an intermediate situation is likely to occur, especially at high drug contents, when there is an excess of drug (superior than its solubility in the carrier). Therefore, MPs with the API in different physical states (crystalline, amorphous, or partially crystalline) can be obtained
[21], according to the drug and carrier chemico-physical characteristics, their miscibility and potential interactions (
Figure 2). The type of the obtained system is strictly dependent on the specific properties of API and carrier (API melting point, API solubility/miscibility in the carrier, API:carrier ratio), and thus it has to be determined on a case-by-case basis.
Figure 2. Scheme depicting the different type of solid dispersions that can be obtained by spray congealing (orange arrows represent individual API molecules).
Among the semicrystalline carriers, PEG has been widely used as a hydrophilic carrier in the preparation of SD formulation, because of its low toxicity and low costs
[22]. It is a polymer of ethylene oxide, available in a range of molecular weights ranging from 200 to millions g/mol. The most commonly used for SD are solid PEGs with molecular weight of 1500–6000 g/mol. PEG exhibits a melting point ranging from ca. 55 °C to 65 °C depending of its molecular weight and it is often used as excipient for melting-based processes. Because of its very low Tg values (from −95°C to −17°C)
[23], the stabilization of the amorphous form it is extremely difficult and therefore most PEG-based SD are characterized by PEG crystallization during the preparation process or during storage. Recent studies
[24] highlight that the API can be located in various domains within the semicrystalline SD and thus multiple microstructures can be obtained, resulting in systems with different properties. For example, aceclofenac and chlorpropamide SD favored the interlamellar incorporation of the drug in the PEG matrix. Haloperidol, a rapidly crystallizing compound, was excluded from the interlamellar region. Loratadine, with a low solubility in PEG, showed more complex phase behavior that depended on the crystallization temperature
[20].
Gelucires
® are semisolid waxy materials with amphiphilic nature widely used for the manufacturing of spray congealed SD. They are composed of a mixture of mono-, di-, and triglycerides, esters of PEG and free PEG. Gelucires
® are characterized by two numbers, the first one referring to the melting point and the second to the theoretical HLB value
[25]. Hydrophilic Gelucires
® are used as solubilization agents for poorly soluble compounds because of their property of forming a dispersed system in water and water/cosolvent mixtures
[26]. The most used compound for spray congealed systems is Gelucire 50/13, a lipid-based excipient with a mean HLB of 11 and a melting temperature around 50 °C
[27].
Poloxamers are ABA-type triblock copolymers composed of polyoxyethylene (A) and polyoxypropylene (B) units with high solubilizing capacity. Because of their chemical structure, they are characterized by an amphiphilic nature, which makes them useful non-ionic surfactants that are employed in many industrial fields. Among these, poloxamer 407 (P407), with an hydrophilic–lipophilic balance (HLB) of 22
[28], is the most commonly used for its low toxicity and its compatibility with numerous biomolecules and excipients
[29]. They have been used for the production spray congealed MPs
[30][31][30,31] although their application as unique carrier may be limited by their high viscosity at the molten state.
4. Properties and Characterization of Spray Congealed SD
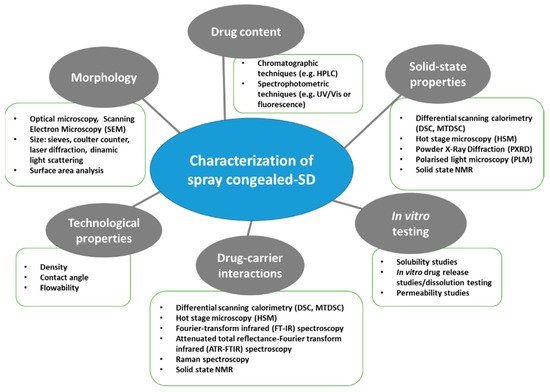
A thorough evaluation of the spray congealed SD properties is essential as they have a direct impact on the technological and biopharmaceutical behavior of the system. Figure 3 shows a schematic classification of the relevant MPs properties and of the techniques used for their analysis.
Figure 3.
Schematic classification of the most commonly used techniques for the characterization of spray congealed SD.
5. Mechanisms of Bioavailability Enhancement of Spray Congealed Systems
A number of spray congealed systems has been developed for the bioavailability enhancement of different poorly soluble drugs. These formulations can be divided in two categories:
As regard the first group, spray congealed SD of different APIs and excipient have been explored over the years (
Table 1). The mechanisms of bioavailability enhancement of these systems are largely dictated by the structure of the SD formed after the SC process
(see Section 3). Specifically, the increase in oral bioavailability by spray congealed SD depends on multiple effects, which can be divided in two main categories: (i) Effects that are independent on the API physical state inside the MPs and (ii) the effects resulting from the changes in the drug crystalline original state. Whereas the second category is strictly dependent on the specific API/carrier combination should be therefore studied on a case-by-case basis, the first one can be ideally exploited for the bioavailability enhancement of all poorly water soluble drugs.
Table 1. Application of spray congealing to oral bioavailability enhancement of poorly water soluble drugs used without pre-activation.
|
Drug
|
Carrier + Additives
|
Type of SD
|
Achievement
|
Ref.
|
|
Carbamazepine
|
Gelucire® 50/13
|
Crystalline drug (original polymorph)
|
Increased in vitro dissolution rate
|
[32][37]
|
|
Carbamazepine
|
Gelucire® 50/13
|
Crystalline drug (change from β to α)
|
Increased in vitro dissolution rate
|
[33][64]
|
|
Piroxicam
|
Gelucire® 50/13
|
Crystalline drug (original polymorph)
|
Increased in vitro dissolution rate
|
[34][87]
|
|
Praziquantel
|
Gelucire® 50/13
|
Crystalline drug (original polymorph)
|
Increased in vitro dissolution rate
|
[35][36]
|
|
Olanzapine
|
Gelucire® 50/13, Lutrol F68 or Lutrol F127
|
Reduced-sized drug particles
|
Increased in vitro dissolution rate
|
[36][55]
|
|
Acetazolamide
|
Poloxamer-237
|
Conversion to amorphous form/ dispersion on molecular level
|
Increased in vitro dissolution rate and 9-fold solubility enhancement
|
[37][33]
|
|
Glibenclamide
|
Myverol, Myvatex, Gelucire® 50/13, Gelucire® 44/14, Poloxamer 188 and PEG 4000
|
Crystalline drug
|
Five-fold increased drug solubilization (Gelucire 50/13 + PEG 4000)
|
[38][86]
|
|
Metronidazole
|
PEG 3350 + HPMC, Dicalcium phosphate, Magnesium stearate, Methylcellulose, Polyvinylpyrrolidone, Silicon dioxide, Sodium oleate/Citric acid
|
Marked reduction in drug crystallinity
|
Slightly increased in vitro dissolution rate with only carrier (different results depending on the type and amount of additive)
|
[39][49]
|
|
Rifampicin
|
PEG 3350 + HPMC (different grades)
|
No information provided
|
Increased in vitro dissolution rate, with intensity depending on the HPMC grade.
|
[40][89]
|
|
Glimepiride
|
Gelucire® 50/13, poloxamer 188, and PEG 6000
|
Crystalline drug (original polymorph)
|
Increased in vitro dissolution rate (Gelucire the highest increase).
10-fold (Gelucire) and 5-fold (poloxamer, PEG) solubility enhancement
|
[19]
|
|
Diclofenac
|
Gelucire® 50/13
|
Marked reduction in drug crystallinity
|
Increase in the in vitro dissolution rate
|
[41][88]
|
|
Indomethacin
|
Gelucire® 50/13,
Gelucire® 48/16
|
Conversion to amorphous form/dispersion on molecular level
|
Increase in the in vitro dissolution rate and solubility
Increased oral
bioavailability in rats
|
[42][45]
|
|
Bufadienolides (bufalin, cinobufagin, and resibufogenin)
|
Lutrol F127
|
Formation of
molecular dispersions
|
Four-fold increase in vitro dissolution rate
|
[43][50]
|
|
Wild garlic extract
|
Gelucire® 50/13
|
No information provided
|
Increase in the in vitro dissolution rate
|
[44][51]
|