Capsule endoscopy (CE) has proven to be a valuable diagnostic modality for small bowel diseases over the past 20 years, particularly Crohn’s disease (CD), which can affect the entire gastrointestinal tract from the mouth to the anus. CE is not only used for the diagnosis of patients with suspected small bowel CD, but can also be used to assess disease activity, treat-to-target, and postoperative recurrence in patients with established small bowel CD. As CE can detect even mildly non-specific small bowel lesions, a high diagnostic yield is not necessarily indicative of high diagnostic accuracy. The incidence of inflammatory bowel disease (IBD) is relatively high but rather stable in Western countries; however, the incidence and prevalence of IBD are rapidly increasing in Asia, Eastern Europe, and South America, and this shift in the epidemiology of IBD indicates that it has become a global disease, whose increasing incidence has been burdening health services in recent years. Therefore, the use of CE for the diagnosis and management of IBD is becoming more frequent and its implementation is considered a priority in the field of IBD.
1. Introduction
Capsule endoscopy (CE), which was first used to evaluate the small intestine in 10 healthy volunteers by Iddan et al.
[1], has enabled direct visual observation of small bowel lesions and phenotypes that had been difficult to assess using conventional endoscopy
[2]. As CE allows direct observation of the small intestine, it is able to visualize even mildly inflammatory mucosal lesions, such as erythema, erosion, and small ulcers, which are difficult to detect with radiological imaging modalities such as small bowel follow-through (SBFT), small bowel contrast ultrasound (SBCUS), CT enterography (CTE), and MR enterography (MRE)
[3]. This advantage has aided in precision medicine-based diagnostic and therapeutic decision-making, especially in patients with suspected or established Crohn’s disease (CD) of the small intestine. The scope of use of CE has expanded over the past 20 years, allowing its application in patients with ulcerative colitis (UC), along with pan-enteric CD. This has been made possible with the subsequent development of colon capsule endoscopy (CCE), which enables visualization of both the small and large intestines
[4].
The incidence of inflammatory bowel disease (IBD) is relatively high but rather stable in Western countries; however, the incidence and prevalence of IBD are rapidly increasing in Asia, Eastern Europe, and South America, and this shift in the epidemiology of IBD indicates that it has become a global disease, whose increasing incidence has been burdening health services in recent years
[5][6][7][8][5,6,7,8]. Therefore, the use of CE for the diagnosis and management of IBD is becoming more frequent and its implementation is considered a priority in the field of IBD.
2. Crohn’s Disease
Patients with suspected CD (SCD) who cannot be diagnosed by radiological modalities can be diagnosed by small bowel CE (SBCE), since the latter can visualize even mildly superficial mucosal lesions that are rarely visible using radiological imaging techniques
[9]. SBCE is also useful in assessing disease activity, treat-to-target, and postoperative recurrence in patients with established CD (ECD).
CD mainly involves terminal ileum, and the disease location is limited to the small bowel alone in about 30% of patients
[10][11][10,11]. Ileocolonoscopy with biopsy is considered the first-line diagnostic modality in patients with suspected CD. However, lesions located proximal to the terminal ileum are difficult to diagnose by conventional ileocolonoscopy. Patients with small bowel CD (SBCD) have been diagnosed radiologically by SBFT or small bowel enteroclysis, which are modalities with reasonable diagnostic accuracy
[12][13][12,13]. Recently, however, these modalities have been largely replaced by cross-sectional imaging modalities, which can better classify disease phenotype and behavior. Several meta-analyses have shown that CTE, MRE, and SBCUS have similar accuracy in the diagnosis of SBCD
[14]. SBCE, a safe and painless endoscopic method for the evaluation of the small bowel, is useful diagnostic modality for SBCD. The results of a Spanish national survey showed that SBCE is widely used to manage IBD in patients with SCD (76.3%), to assess inflammatory activity (54.7%) and to evaluate the extent of disease (54.7%)
[15]. In addition, CE has been shown to be useful in assessing mucosal CD activity in selected patients with colonic CD
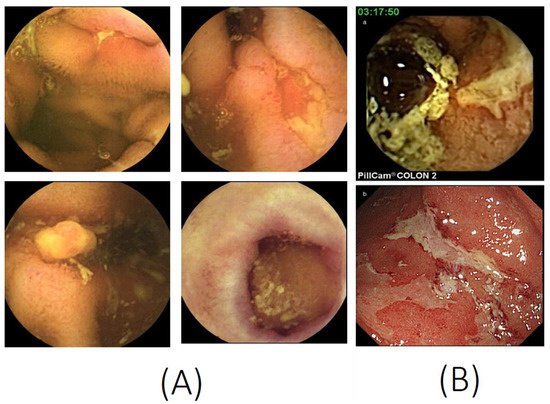
[16][16]. The typical CE findings in SBCD are shown in Figure 1A.
Figure 1. Capsule endoscopy images. (A) Small bowel capsule endoscopy features of Crohn’s disease, such as ulcers, longitudinal ulcers, inflammatory polyps, and scars. (B) A colon capsule endoscopy image (a) consistent with the conventional colonoscopy image (b) of ulcerative colitis (photocopies from Hosoe N, et al. J. Gastroenterol. Hepatol. 2013, 28, 1174–1179, with permission from John Wiley and Sons [17]).
2.1. Capsule Retention
Although CE is a simple and safe test, capsule retention (CR) in the gastrointestinal tract is a frequent complication. CE can be retained within the small bowel, usually in patients with stricturing disease. Passage of the CE through the gastrointestinal tract, including the pylorus and ileocecal valve, may be difficult in pediatric patients, but this method can be safely used in children over 9 years of age
[17][18].
A meta-analysis that included 25 studies of 5876 patients with obscure gastrointestinal bleeding, nine studies of 968 patients with SCD, and 11 studies of 558 patients with ECD found that the pooled CR rates were 3.6% (95% confidence interval (CI), 1.7–8.6%) for SCD and 8.2% (95% CI, 6.0–11.0%) for ECD
[18][19]. These CR rates decreased to 2.7% (95% CI, 1.1–6.4%) in subsequent CE after a patency capsule (PC) or CTE to exclude strictures, with the latter comparable to the CR rate for obscure gastrointestinal bleeding (2.1%; 95% CI, 1.5–2.8%)
[18][19]. A more recent meta-analysis found that the CR rates were 2.35% (95% CI, 1.31–4.19%) in 1234 patients with SCD and 4.63% (95% CI, 3.42–6.25%) in 1720 patients with ECD
[19][20]. Although these CR rates are significantly lower than those previously reported, this meta-analysis also found that the CR rate after PC in patients with ECD was 2.88% (95% CI, 1.74–4.74%)
[19][20].
A meta-analysis that included five studies of 203 patients showed that PC had a sensitivity of 97% (95% CI, 93–99%), a specificity of 83% (95% CI, 65–95%), and an accuracy of 0.956 in diagnosing small bowel obstruction
[20][21]. A recent meta-analysis found that the CR rates in patients with ECD were 2.32% (95% CI, 0.87–6.03%) after negative small bowel cross-sectional imaging and 2.88% (95% CI, 1.74–4.74%) after negative PC
[19][20]. MRE showed a high sensitivity (>92%) and negative predictive value (NPV) (>96%) for PC retention in patients with ECD
[21][22]. These results suggest that cross-sectional imaging of the small bowel should be performed prior to CE to determine the presence of strictures in patients with CD. However, a multicenter prospective study showed that CR rates are similar in low-risk (0.7%, 20/2942) and negative PC high-risk (0.7%, 1/151) patients, but are significantly higher in high-risk patients with negative cross-sectional imaging (CTE or MRE; 8.3%, 2/24;
p = 0.049)
[22][23]. Thus, even if cross-sectional imaging is normal, the PC procedure should be offered to patients at increased risk of CR.
As CR is usually asymptomatic, initial periodic monitoring is suggested. Even if CR has occurred in IBD patients, the European Society of Gastrointestinal Endoscopy guidelines recommend observation in patients with asymptomatic CR for the following reasons
[23][24]: (1) 35–50% of CR patients spontaneously excreted the capsules after ≥15 days without further management, (2) a short course of medical therapy may allow capsule excretion, and (3) spontaneous excretion usually occurs 4–12 weeks after ingestion
[24][25]. However, device-assisted enteroscopy or surgical removal should be considered if the capsule is not excreted after 3–6 months or if patients experience symptoms of acute obstruction
[24][25].
2.2. Superior Diagnostic Yield to Other Imaging Modalities
CE provides high-resolution endoluminal images of the small bowel. As there is no standard modality that can be compared with CE to determine its diagnostic accuracy, the ‘diagnostic yield’ is determined in many studies of this modality.
CE showed a higher diagnostic yield than small bowel enteroclysis in the detection of small bowel lesions, in 27 patients with ECD (74.1% vs. 40.7%;
p < 0.05) and in 20 patients with SCD (65% vs. 30%;
p < 0.05)
[25][26]. CE also showed a higher diagnostic yield than CT enterolysis in evaluating jejunal or ileal lesions in 41 patients with small bowel CD (61.0% vs. 29.3%;
p < 0.004) but not in those with terminal/neonatal ileum
[26][27]. CE was superior to MR enteroclysis in detecting inflammatory lesions in the proximal and middle parts of the small bowel (66.7% vs. 5.6%;
p = 0.016) in 18 patients with SCD or ESD
[27][28].
A meta-analysis that included only prospective studies found that the diagnostic yield of CE was significantly superior to that of SBFT, CTE, ileocolonoscopy, and push enteroscopy in the evaluation of SBCD
[28][29]. The results of recent meta-analysis have revealed that the diagnostic yields of CE do not differ significantly from those of CTE and MRE in patients with both SCD and ECD
[29][30]. The different results for CTE in these meta-analyses were due to differences in the inclusion criteria. Another recent meta-analysis found that the diagnostic yield of CE was similar to that of MRE in 10 studies involving 400 patients (odds ratio (OR), 1.17; 95% CI, 0.83–1.67) and SBCUS in five studies involving 142 patients (OR, 0.88; 95% CI, 0.51–1.53) with SCD and ECD
[30][31]. In that analysis, however, CE was superior to MRE in seven studies involving 251 patients with proximal suspected small bowel disease (OR, 2.79; 95% CI, 1.2–6.48)
[30][31]. Subsequent prospective studies comparing CE with MRE found that both modalities independently detected previously unrecognized proximal disease locations in 51% (29/56) and 26% (20/79) (
p < 0.01), respectively, in patients with SBCD in clinical remission or with mild disease
[31][32]. CE was also superior to MRE in detecting small bowel lesions (76.6% vs. 44.7%,
p = 0.001) in SCD and ECD, with CE being superior to MRE in detecting lesions in the jejunum, ileum, and terminal ileum (all,
p < 0.05)
[9].
Although the diagnostic yield of CE was higher than that of other imaging diagnostic modalities, especially in patients with proximal small bowel disease, there are several barriers to the clinical use of CE. The higher diagnostic yield of CE does not directly indicate a higher diagnostic accuracy, as diagnostic yield can be affected by other factors, such as nonsteroidal anti-inflammatory drugs
[32][33]. Minor mucosal injuries and erosions have been detected in up to 20% of healthy volunteers
[32][33]. In addition, cost analyses suggest that the addition of CE as a third test after ileocolonoscopy and negative CTE or SBFT is not cost-effective
[33][34].
2.3. Increasing Diagnostic Capability of Capsule Endoscopy
CE is being developed in several ways, including the development of CE instruments with higher frame rates and increased image resolution, which should increase the possibility of obtaining higher diagnostic yield and accuracy than in the past
[34][35]. For example, the adaptive frame rate (AFR) technology with a movement sensor, which captures images depending on the speed of the capsule’s movement, of the PillCam SB3 (Medtronic, Ltd., Dublin, Ireland; 2–6 images/second) and PillCam Crohn’s capsule (PCC, Medtronic, Ltd.; 4–35 images/second) may increase the diagnostic yield of CD
[35][36][37][36,37,38]. Non-white light imaging has been reported to improve the detection rate and visibility of small intestinal lesions by increasing the visualization of surface patterns and color differences in the presence of bile juice and blood
[38][39]. For example, flexible spectral color enhancement (FICE, Fujifilm Corp., Tokyo, Japan) is a digital processing method of white light imaging that emphasizes specific ranges of wavelengths of light in the red, green, and blue spectrum
[38][39]. The FICE wavelength settings were developed with the aims of reducing blue light interference (FICE1), accentuating blood (FICE 2), and strengthening the differences between bile and blood (FICE3)
[38][39]. A contrast capsule (Olympus Corp., Tokyo, Japan), which increases brightness in the blue wavelength range by using a special instrument equipped with a light-emitting diode
[38][39], enables easy detection of areas of bleeding by selecting green and blue data. However, clinical trials of FICE and the contrast capsule so far have yielded controversial results for the detection of gastrointestinal lesions
[39][40][40,41]. Another optical-digital method similar to contrast capsule, termed narrow-band imaging (Olympus Corp.), which allows better visualization of mucosal surface patterns and superficial capillaries, is being applied to CE in device manufacturing research
[41][42].
2.4. Clinical Suspicion of Crohn’s Disease with Negative Conventional Modalities
SBCE is a sensitive tool to detect mucosal abnormalities in the small bowel. Approximately 5–10% of patients have isolated small bowel disease that cannot be detected by conventional ileocolonoscopy
[42][43]. The diagnostic yield of SBCE in patients with SCD has been reported to range from 40% to 70%. For example, SBCE diagnosed CD in 12 (71%) of 17 patients with symptoms such as abdominal pain, anemia, and diarrhea of unknown cause with normal appearance on conventional modalities
[43][44]. SBCE in 20 SCD patients suspected of having small bowel lesions diagnosed CD in 13 patients (65%)
[44][45], showing that CE is effective in diagnosing patients with SCD undetected by conventional diagnostic methods. SBCE detected lesions supporting the diagnosis of CD in 9 (43%) of 21 patients with clinical SCD
[45][46]. This method also increased diagnostic yield by 24% in patients with perianal disease and negative conventional work up, including ileocolonoscopy
[46][47]. These results showed that CE is a useful test for the diagnosis of CD in patients who have not been diagnosed by conventional modalities, such as gastroscopy, ileocolonoscopy, and SBFT.
The presence of biochemical markers in patients with SCD symptoms has been reported to increase the diagnostic yield of SBCE
[47][48]. Although two retrospective studies showed the presence of small bowel inflammation in the majority of ECD patients in remission with biomarkers
[48][49][49,50], a meta-analysis showed that the likelihood of a positive diagnosis is very low in SCD patients with fecal calprotectin (FC) < 50 μg/g
[50][51]. A recent retrospective study revealed that FC was positively correlated with significant inflammatory activity (Lewis Score, LS ≥ 135; rank correlation = 0.56;
p < 0.001)
[51][52], further indicating that FC may be a useful marker to select patients with SCD for SBCE. Various FC cut-off values have been reported to be indicative of small bowel CD. For example, 33 (89.2%) of 37 patients with FC ≥ 100 µg/g were found to have an LS ≥ 135
[51][52]. A recent meta-analysis that included 14 studies suggested that FC ≥ 100 ug/g and LS ≥ 135 cut-off values had diagnostic odds ratios of 8.96 and 10.90, respectively
[52][53].
As normal radiological imaging of the small bowel by SBFT, SBCUS, CTE, and MRE cannot entirely exclude small bowel involvement, SCD patients with normal radiological results but elevated FC and/or unexplained anemia should be considered for additional SBCE
[9][13][9,13]. Careful monitoring without additional work up may be sufficient for asymptomatic CD patients with negative FC results
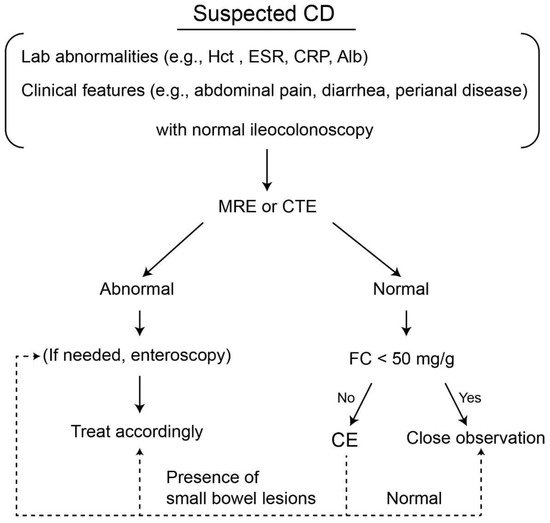
[53][54]. Based on the above results, a diagnostic algorithm using CE in patients with SCD is illustrated in
Figure 12.
Figure 12. Suggested diagnostic algorithm for the use of small bowel capsule endoscopy in patients with suspected Crohn’s disease. CD, Crohn’s disease; Hct, hematocrit; CRP, C-reactive protein; Alb, albumin; MRE, MR enterography; CTE, CT enterography; FC, fecal calprotectin, CE, capsule endoscopy.