Please note this is a comparison between Version 1 by Erika Cione and Version 2 by Rita Xu.
Exosomes are nano-sized extracellular vesicles produced and released by almost all cell types. They play an essential role in cell-cell communications by delivering cellular bioactive compounds such as functional proteins, metabolites, and nucleic acids, including microRNA, to recipient cells.
- diabetes
- exosomes
- insulin resistance
1. Introduction
Diabetes is a metabolic disorder stemming from defective insulin secretion and the occurrence of insulin resistance in peripheral tissues. Obesity, dietary fat intake, and physical inactivity are recognized as the main risk factors [1][2][1,2]. The close link between obesity and the development of this metabolic disorder has led to the creation of the new term “diabesity”, which combines the burden of obesity and diabetes [3]. This condition will affect more than 650 million people by 2045, with a concomitant increase in sanitary health costs concerning diabesity-related diseases [4][5][6][4,5,6]. Despite the considerable advancement in the understanding and treatment of diabetes, the correlated morbidity and mortality rates have continued to increase. Therefore, there is an urgent need for biomarkers to improve the clinical diagnostic process and the therapeutic approach of diabetes [7].
A number of extracellular vesicles (EVs) exist in all human fluids [8][9][8,9]. Their classification is based on their size: (i) large EVs (diameter > 200 nm) and (ii) small EVs (diameter < 200 nm) of which exosomes (30–150 nm) belong [10][11][10,11]. Exosomes are broadly present in human body fluids such as cerebrospinal fluid, urine, semen, saliva, and breast milk [12][13][14][12,13,14]. Exosomes can carry bioactive molecules and are essential for cell-cell communication [15][16][15,16].
With the conclusion of the Human Genome Project and the opening of the postgenomic era, non-coding RNAs (ncRNAs) have gained attention in numerous research fields [17][18][19][17,18,19]. miRNAs are a type of ncRNA with approximately 22 nucleotides encoded by endogenous genes [20]. They act as regulators of post-transcriptional gene expression by directing target mRNA cleavage or translational inhibition. More than one-third of human genes are thought to be regulated by miRNAs, revealing their involvement in various physiological and pathological processes. miRNAs are tissue-specific and more stable as compared with long non-coding RNAs (ln-RNAs) and messenger-RNAs (mRNAs) because of their shorter sequences [21][22][21,22]. miRNAs can be packaged within exosomes, which deliver and release them into target tissue cells. Of note, approximately 100 miRNAs have been identified in the exosomes produced by mast cells [23][24][23,24]. Exosome-miRNAs participate in normal physiological processes and are also involved in the occurrence and development of several diseases [25][26][27][25,26,27]. In this frame, they are emerging as crucial regulators in the onset and development of diabetes. Moreover, exosome-miRNAs released into systemic circulation can be used as diabetes markers because of their specificity and sensitivity [28][29][28,29].
2. Characteristics of Exosome-miRNAs
The first observation of exosomes was by Trams et al. in 1981, who detected “small membranous vesicles in the supernatants of tumor cells cultured in vitro”. Those macrovesicles were called exosomes [30]. At that moment, it was believed that the function of exosomes was only for the waste disposal system for cells. Instead, further research has highlighted exosomes’ role in several biological processes encompassing the immune response, cell differentiation, and cancer [31][32][31,32]. Exosomes are a subtype of extracellular vesicles that can be identified based on their endosomal origin and their size, which ranges from 30 to 150 nm. Their biogenesis initiates with the formation of early endosomes by the inward budding of the cell membrane followed by the second inward budding of the endosomal membrane. The second inward budding results in the formation of late endosome (intraluminal vesicles). Late endosomes comprising intraluminal vesicles (ILVs) are identified as multivesicular bodies (MVBs). During the maturation phase from early endosome to MVBs, the cargoes are incorporated into ILVs through endosomal-sorting complex-dependent or endosomal-sorting complex-independent pathways. MVBs can be transported to the trans-Golgi network for endosome recycling, delivered to lysosomes for degradation, or move along microtubules to fuse with the plasma membrane and release exosomes into the extracellular space. MVB fusion with the cellular membrane is a fine-tuned process, which requires several crucial factors. Exosomal cargoes from the source cell can be further delivered to target cells via endocytosis, direct membrane fusion or receptor-ligand interactions [31]. Almost all mammalian cells produce and release exosomes, including the blood cells: (i) B lymphocytes, (ii) T lymphocytes, (iii) platelets, (iv) mast cells, and (v) dendritic cells, but also: epithelial cells, astrocytes, and neurons [33][34][35][36][37][38][39][33,34,35,36,37,38,39]. Exosomes have been reported in all biological fluids, and their composition reflects the metabolic state of the cell of origin. Of note, exosomes can be selectively taken up by neighboring or distant cells far from their release, reprogramming the functional activity of the recipient cells through the delivery of bioactive molecules. Thus, exosomes and their biologically active cargoes may offer potential biomarkers of diagnosis and therapeutic targets in a range of diseases, such as chronic inflammation, cardiovascular and neurodegenerative diseases, cancer, obesity, and metabolic diseases [34]. In addition to specific proteins, exosomes also contain different patterns of RNAs that can be delivered to recipient cells. RNA sequencing analysis demonstrated that miRNAs were the most abundant in human plasma-derived exosomal RNA species [40]. Exosomes-miRNAs undergo unidirectional transfer between cells, leading to an intercellular trafficking network. The latter elicits transient or persistent phenotypic changes in recipient cells [41]. It was proven that after entering a target cell, miRNA released from the exosome can interact with the 3′-UTR region of the targeted mRNA, resulting in inhibition of the specific gene expression [42]. It is worth mentioning that in addition to miRNAs, long RNA species, especially long non-coding RNAs and circular RNAs, have recently been reported to exist in exosomes and affect a variety of biological processes, including the development of cancer [43].
Exosome-miRNAs circulating in body fluid can also act as biomarkers to mirror disease progression. Gathering evidence indicates that exosome-miRNAs are essential in developing diseases; therefore, their use as biomarkers for disease diagnosis, prognosis, and personalized therapy is becoming more apparent [44][45][44,45].
3. Dysregulation of Exosome-miRNAs in Diabetes
The continuous increase in diabetes prevalence and incidence renders this metabolic disorder a global public health emergency [5]. Diabetes can be categorized into: (i) type 1 diabetes, (ii) type 2 diabetes, also known as alimentary diabetes, and (iii) gestational diabetes (genetic types are rare). Chronic hyperglycemia reshapes islet cellular assets with the infiltration of α-cells in the core of β (Figure 1). More serious is the complication that long-term hyperglycemia does in the damage, dysfunction, and failure of multiple organs, particularly blood vessels, nerves, kidneys, heart, and eyes [46][47][46,47]. Therefore, consequences that can be recognized as diabetic are: (i) retinopathy, (ii) macro-vascular complications, (iii) nephropathy, (iv) cardiomyopathy, and (v) foot ulcers [48]. Diabetes-related morbidity and mortality can be reduced by the improvement of preventive care, early clinical diagnosis, and appropriate therapeutic approaches [48][49][48,49]. Hence, identifying effective biomarkers to prevent and treat diabetes earlier, as well as its complications, are needed. Since we are in the precision medicine era, increasing attention is being paid to diagnosing and treating diseases [17][30][17,30].
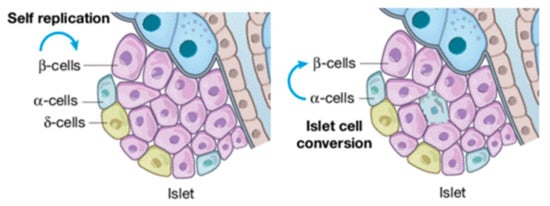

Figure 1. β-cell injuries reshape islet cellular assets.
4. Mechanism of Exosome-miRNAs in Diabetes Progression
About 70% of pancreatic cells are β-cells, which play a fundamental role in sustaining blood glucose homeostasis via insulin secretion into systemic blood circulation [50][59]. β-dysfunction due to cell injury leads to the progression of diabetes [51][60]. This occurs in the early pre-diabetes stage and is characterized by three main mechanisms: (i) the first is hyperglycemia; (ii) the second is elevated free fatty acid levels; (iii) the third is high amylin levels, which is co-secreted with insulin and induces β-cell apoptosis [50][59] (Figure 2).
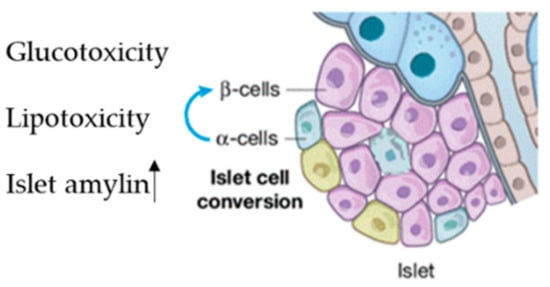

Figure 2. Factor inducing islet reshaping, Lipo and glucotoxicity and increasing of islet amylin.
Several studies have proved that the enrichment of specific exosomal miRNAs can target genes having an essential conservation outcome on pancreatic β-cell function in the initial stages of diabetes. Both high glucose and fatty acid levels negatively regulate this pattern, determining an enrichment of exosome-specific miRNAs involved in β-cell dysfunction in diabetes [52][53][54][61,62,63]. Islet tissue isolation from these mice and exosome-miRNA revealed a significant change in the miR-375-3p expression levels. Furthermore, this microRNA was also found to be higher in diabetes patients versus normoglycemic patients. Therefore, hsa-miR-375-3p could be considered as an early marker of islet injury (Figure 3).
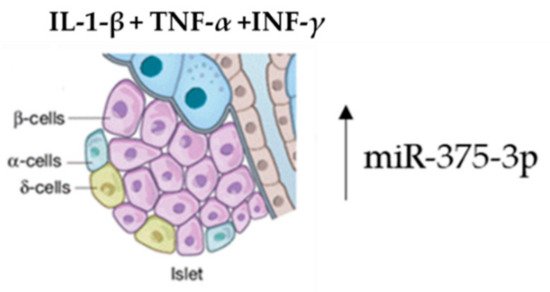

Figure 3. Acute β-cell injury using a mixture of cytokines induces an elevation of miR-375-3p.
The pool of exosome-miRNAs deriving from other cells can act on β-cells. This was demonstrated by treating MIN6B1 pancreatic cells with a mixture of IL-1-β, TNF-α and INF-γ cytokines. The exosome enrichment containing miRNAs, secreted in the medium, can be delivered to contiguous β-cells, inducing cell death [55][54].
In view of possible microRNA-based therapy, agomir miR-106b-5p and miR-222-3p were tail-vein-injected into mice, promoting the proliferation of injured β-cells. It was demonstrated that the injection of both miRNAs leads to the downregulation of the Cip/Kip family, which, in turn, improves hyperglycemia in insulin-deficient diabetes mice. This is evidence that they can function as a therapeutic option to rescue from β-cell dysfunction (Figure 4), so that circulating miRNAs are endocrine factors that facilitate metabolic organ crosstalk.
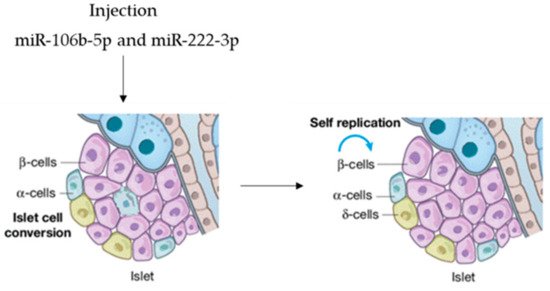

Figure 4. microRNAs as therapeutic option.
Exosome-miRNAs secreted by β-cells can be transferred to other acceptor tissue cells which in turn regulates β-cell activity. For example, when exosome miR-26a [56][66] is transferred to the liver, it improves the insulin sensitivity of the acceptor cells, maintaining metabolic homeostasis. In addition, serum miR-204 is strictly associated with pancreatic β-cell injury, which could be helpful as a novel biomarker for early type 1 diabetes [57][67]. The findings indicate how exosome-miRNAs are strictly related to β-cell damage and dysfunction in diabetes.
 Encyclopedia
Encyclopedia
