Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Joan Benedicto and Version 2 by Yvaine Wei.
Bladder cancer is the most common malignancy of the urinary system. Nearly 70% of new bladder cancer diagnoses are early stage, and have not yet invaded the muscle layer, whereas the remaining 30% of patients have muscle-invasive bladder cancer (MIBC), including cancer involving the muscularis propria (T2), perivesical tissue (T3) or adjacent pelvic organs/structures (T4) [2]. The treatment of MIBC is complex and is based on a multidisciplinary collaboration between surgery, radiotherapy and medical oncology teams.
- muscle-invasive bladder cancer
- molecular subtypes
- radical cystectomy
- immunotherapy
- checkpoint inhibitors
1. Introduction
Radical cystectomy with lymph node dissection and systemic cisplatin-based combination chemotherapy either before or after radical cystectomy has been considered the standard treatment approach in muscle-invasive bladder cancer (MIBC). However, as many patients are unfit for surgery or are cisplatin-ineligible, bladder-sparing strategies are increasingly recognized as optimal treatment options in selected patients that can be presented at the time of diagnosis [1][2][3,4]. Moreover, apart from chemotherapy, radiation and immunologic therapeutic options, especially checkpoint inhibitors are selective strategies being incorporated in the therapeutic landscape of MIBC [3][4][5,6].
The Genito Urinary Alliance project (12GU) was designed as a space for the integration of innovation progress in the management of patients with bladder cancer. For this purpose, expert members of the Spanish Oncology Genitourinary (SOGUG) Multidisciplinary Working Group discussed some controversial and debatable topics of the current knowledge and approach in the care of patients with localized MIBC. The aim of the project was to summarize practical recommendations on some particular aspects of localized MIBC, including molecular-based analysis for the classification of urothelial carcinoma, bladder-sparing approaches and new molecular classifications, integral evaluation of candidates for radical cystectomy, use of neoadjuvant therapy and integration of immunotherapy, and the role of imaging techniques in staging, assessment of treatment response and follow-up. Challenges and recommendations were reached by agreement of all participants to be applicable in clinical practice to facilitate shared decision making for individual patients diagnosed with MIBC.
2. Subclassification of Urothelial Carcinoma in Different Molecular Groups
The correct morphological classification of urothelial cancer is complex, because there is great tumor heterogeneity and a wide variety of histopathological patterns [5][8]. The current World Health Organization (WHO) classifications clarify terminological issues and provide better definition criteria. Histological variants include: urothelial carcinoma with divergent differentiation, and nested, microcystic, micropapillary, lymphoepithelioma-like, plasmacytoid/signet ring cell/diffuse, giant cell, lipid-rich, clear cell (glycogen-rich), and poorly differentiated urothelial carcinomas. The urothelial carcinoma with divergent differentiation includes squamous, glandular, trophoblastic and other types of differentiation [5][8]. The College of American Pathologists recommends that the percentage of morphological subtype differentiation should be specified in the pathology report [6][9]. Experimental studies using cytogenetic, molecular genetics and immunohistochemical methods have provided new insights into the molecular mechanisms and pathways involved in bladder cancer. Frequent genetic abnormalities include CYP1A and GSTM1 polymorphisms, methylation of GpC sites, and mutations with FGRF3 and p53 as the most common [7][10]. On the other hand, carcinogenicity may result in pan-urothelial and multifocality of urothelial cancer. It is also accepted that human bladder cancer develops via two distinct, but sometimes overlapping pathways, papillary and non-papillary. In the papillary pathway of superficial or low-grade lesions (80% of cases), there is activation of proliferative factors (FGFR3 and HRAS), whereas in the non-papillary pathway (20% of cases), including carcinoma in situ (CIS) and infiltrating carcinomas, there is a loss of p53 or RB1 function. In 15% of cases with genetic instability and involvement of the RB1/p53 pathway, severe intraurothelial dysplasia/CIS (HGIN) developing in bladder mucosa adjacent to a low-grade papillary tumor may be responsible for switching the pathway and progression of some low-grade papillary tumors to high-grade invasive cancers [8][11]. In patients with advanced urothelial carcinoma, promising clinical activity has been shown for antibodies targeting the programmed cell death-1 (PD-1)/PD-ligand 1 (PD-1/PD-L1) checkpoint, but when different algorithms have been used to assess high vs. low/negative PD-L1 expression, the extent of concordance of the available PD-L1 immunohistochemical (IHC) assays has been poorly defined. The comparison of technical performance and characteristics of different assays and algorithms will allow a more accurate interpretation of outcomes associated with anti–PD-1/PD-L1 therapies in patients with urothelial cancer. When four commercially available PD-L1 assays (PD-L1 IHC 28–8 pharmDx, PD-L1 IHC 22C3 pharmDx), SP142, and SP263) were used in the analysis of biopsy samples from 335 tumors, in all assays except for SP142, the analytical concordance was high for both tumor cells and the proportion of tumor infiltrating area with PD-L1 staining [9][17]. In a Spanish multicenter study aimed at assessing PD-L1 expression in different tumor variants using CPS and SP142 assays, tumors with squamous cell or sarcomatoid differentiation and adenocarcinomas showed a high PD-L1 expression (accounting for 50% in some cases), whereas PD-L1 expression was almost absent in micropapillary, nested, plasmacytoid, clear cells and neuroendocrine variants (Figure 1). Tumors with squamous cell differentiation mostly belong to the basal molecular subtype, whereas micropapillary, nested and plasmacytoid variants are included in the luminal molecular subtype [10][18].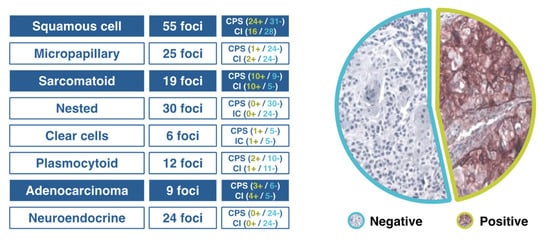
Figure 1. Results of a Spanish study of PD-L1 expression in different variants of urothelial cancer.
3. Role of Imaging Techniques in Staging, Assessment of Response, and Follow-Up
3.1. Nuclear Medicine
[18F]-Fluorodeoxyglucose (FDG)-positron emission tomography (PET) with multislice helical computed tomography (CT) (F18-FDG PET/CT) is currently a standard imaging tool used in the diagnosis and control of patients with MIBC. In 2017, the guidelines of the European Association of Urology (EAU) considered FDG-PET/CT to be an imaging procedure pending evaluation, but the National Institute for Health and Care Excellence (NICE) [14][63] has approved the use of FDG-PET/CT in high-risk patients with muscle-invasive or non-muscle-invasive bladder cancer (pTaG3, pT1G2, pT1G3, pTis), in the presence of aggressive variants (micropapillary or nested subtypes), indeterminate findings of CT and magnetic resonance imaging (MRI), or at high risk of metastatic disease (e.g., T3b). In 2018, an expert panel on urologic imaging recommended PET/CT for staging of MIBC in the initial patient assessment [15][64]. In the 2020 guidelines of the National Comprehensive Cancer Network (NCCN), FDG-PET/CT is recommended for staging ≥ IIIA (T3N0) (level of evidence 2b) [16][65].
Clinical studies have shown that FDG-PET/CT provides important additional staging information, which influences the treatment of MIBC in 18–68% of cases due to correct overstaging as compared to conventional imaging techniques [17][18][66,67]. FDG-PET/CT shows a similar accuracy to that of MRI in N-staging, with faster whole-body acquisition times and a high accuracy in M-staging. Data of systemic reviews and meta-analyses of the diagnostic accuracy of FDG-PET/CT for preoperative lymph node staging in newly diagnosed bladder cancer patients have shown moderate sensitivities similar to MRI and high specificities [19][20][21][22][68,69,70,71] (Table 1).
On the other hand, diuretic FDG PET/CT is highly sensitive and specific and plays an important role in improving detection of the primary tumor and locoregional staging of urinary bladder tumors [23][72] (Figure 2).
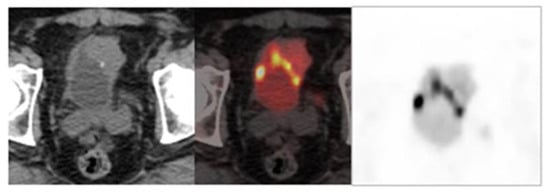
Table 1. Diagnostic accuracy of imaging techniques for initial lymph node staging in bladder cancer patients.
| First Author (Reference) |
Technique | Number of Studies (Number of Patients) |
Sensitivity, % (95% CI) |
Specificity, % (95% CI) |
|
|---|---|---|---|---|---|
| Ha [19] | Ha [68] | 18F-FDG-PET/CT | 14 (785) | 57 (49–64)) | 92 (87–95) |
| Kim [20] | Kim [69] | C-11 choline and C-11 acetate PET/CT | 10 (282) | 66 (54–75) | 89 (76–95) |
| Soubra [21] | Soubra [70] | 18F-FDG-PET/CT | Single-center (78) | 56 (29–80) | 98 (91–100) |
| Woo [22] | Woo [71] | MRI | 24 (2928) | 56 (42–69) (per-patient) | 94 (90–96) (per patient) |
| 57 (29–82) (per-lymph node |
97 (94–98) (per-lymph node) |

Figure 2. Image obtained with FDG-PET/CT after the administration of a diuretic.
3.2. Radiology
The role of imaging techniques in the diagnosis of MIBC, in general, is neither correctly defined nor standardized. Ultrasound is a well-accepted, cost-effective, and noninvasive diagnostic method for the screening of patients with suspicion of bladder cancer, with hematuria or symptoms of the low urinary tract, although ultrasound may be inconclusive in the detection of small tumors (<1 cm), flat lesions or with atypical morphology. Contrast-enhancement ultrasound (CEUS) improves detection of lesions in patients with acute hematuria and high suspicion of bladder tumor, and is useful to differentiate clots from parietal lesions as well as tumors located on the bladder base from intravesical prostatic protrusion. However, CEUS is still not routinely used in daily practice.
The usefulness of radiological techniques for staging is related to detection of local invasion of the bladder walls, local or retroperitoneal lymph nodes, involvement of the upper urinary tract, and hematogenous metastases. CT urography is recommended by guidelines [14][16][63,65] in TNM staging due to several advantages, including assessment o extension or perivesical fat, adjacent organs and pelvic wall; evaluation of the upper urinary tract to exclude synchronic tumors (technique of choice); number and location of lymph nodes; and distant spread (chest CT). CT urography has a higher spatial resolution and faster acquisition times as compared with MRI, although limitations include use of radiation, no differentiation of the three layers (important in pT3 tumors), and difficulties in assessing changes after a surgical procedure or radiotherapy. Evaluation of lymph nodes using CT urography is based on size, shape and density of nodes.
In relation to assessment of the clinical stage using MRI, the advantages as compared with CT include better tissue resolution with the option of using diffusion MRI (diffusion-weighted MRI) and differentiation of the layers of the urinary wall. Limitations, however, include the need to have a full bladder for this examination, lower accessibility compared to CT, and the fact that use of MRI is not generalized in the clinical setting.
It is important to determine accurately whether there is infiltration of the muscularis propria (T stage) for treatment decisions in bladder cancer patients, since the therapeutic approach is based on differentiating MIBC from non-invasive muscle bladder cancer. Data provided by CT and MRI are not sufficiently accurate to establish the clinical stage and guide treatment. The Vesical Imaging-Reporting and Data System (VI-RADS) reported in 2018, a new approach to assess muscularis bladder infiltration by malignant cells [24][25][79,80]. This method combines T2-weigthed imaging (T2W1), dynamic contrast enhanced (DCE), and diffusion-weighted imaging (DWI) sequences according to which the possibilities of muscle invasion can be established. The VI-RADS scoring system is based on a 5-point scale (from 1 = very unlikely to 5 = very likely). The new VI-RADS scoring system should be further validated and evaluated in different clinical scenarios, including prospective and randomized studies [26][81].
