Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Nora Tang and Version 1 by Michał Strankowski.
Smart materials are much discussed in the current research scenario. The shape memory effect is one of the most fascinating occurrences in smart materials, both in terms of the phenomenon and its applications. Many metal alloys and polymers exhibit the shape memory effect (SME).
- shape memory
- elastomer
- fixity
- recovery
- applications
1. Natural Rubber (NR) Based Materials with Shape Memory
The superiority of NR over synthetic polymers lies on the fact that they are capable of supporting large amounts of stress up to 30 MPa at a strain of more than 1000%.This is due to strain-induced crystallization (SIC) that occurs whenever cross-linked NR is stretched to large elongations. The crystals formed vanish when releasing the stretching force and regain their original amorphous condition. The effect of SIC was investigated by Katz and thermodynamically by Flory. Flory proposed a theory that considers the entropy change of molecular chains due to stretching. The entropy change is a factor that promotes the SIC and the formation of crystals with low surface energy promotes the enhancement of SIC. In a study by Tosaka et al., the surface energies of strain-induced NR crystals were found to be relatively small. The low surface energy crystallites usually exhibit a high melting point exceeding room temperature; this supports the existence of shape memory natural rubber [54][1].
2. Lightly Cross-Linked Shape Memory Natural Rubber
Lightly cross-linked NR networks have a cross-link density below 0.4%; the crystals do not disappear after releasing the stretching force but stabilize the network in a highly elongated state, up to 1000%, at room temperature. The recovery of this network can be triggered by heating it above its melting point, which is known as a trigger temperature. The trigger temperature of shape memory natural rubber (SMNR) is adjustable from −20–50 °C [55][2]. Lightly cross-linked NR, rapidly stretched and kept in this state, does not recover its original state. But when applying a small heat, such as body temperature, to the stretched material it recovers its original state. Thus, lightly cross-linked NR can be programmed below its triggered temperature, and the stretching of semi-crystalline polymer results in partial recoverability. The cross-linking of NR in-between thermoplastics and heat allow the formation of crystals under strain that can withstand the network in the high heat of elongation. A typical formulation for shape memory natural rubber is given in Table 1 [56][3].
Table 1.
Components to be considered when fabricating SME natural rubber.
| Components | Phr |
|---|
| Natural rubber | 100 |
| Sulphur | 0.2 |
| ZnO | 0.15 |
| Zinc diethyldithiocarbamate | 0.15 |
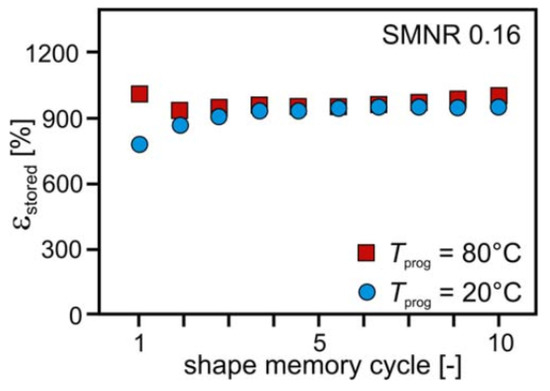
SMNR is capable of storing a high amount of strain. For an SMNR with a cross-link density of 0.12%, the strain stored was found to be 990%. Figure 31 shows the strain stored (εstored) during a 10 shape memory cycle at 20 and 80 °C, which also indicates the programming temperature slightly affects the storable strain [57,58][4][5].
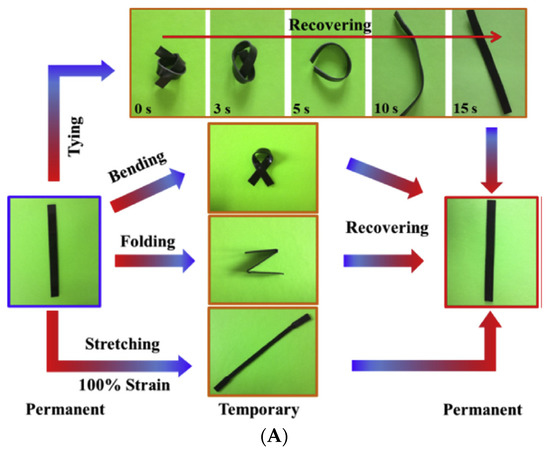
In a study by Chai et al., palmitic acid was used as a swelling agent for shape memory properties. Under a cooling effect, palmitic acid crystallizes onto natural rubber to form a platelet network. This network allows the fabricated shape memory of NR to deform and recover its original shape at room temperature [59][6].
Blends of epoxidized NR (ENR) with polylactic acid (PLA) and polycaprolactone (PCL) are an example of bio-triggered shape memory polymer. The driving force of recovery is the stored elastic energy of the ENR phase, which is elongated and restricted by the rigid PLA or PCL continuous phase in its temporary shape [60,61][7][8].
3. Synthetic Rubber-Based ShapeMemory Materials
The term synthetic rubber derives from the synthetic analogue to natural rubber, but a great variety of other rubbery materials are produced by chemical synthesis [7][9].The shape memory properties of synthetic rubbers, such as polyurethane, ethylene propylene diene monomer (EPDM), and silicone rubbers, were largely studied by scientists all over the world. The shape memory effect of zinc-neutralized sulfonated EPDM and fatty acid salts was studied by Weiss and others [62][10]. Here, ionic aggregates form the cross-linked network and fatty acid salts form the temporary network. The temporary shape was achieved by straining the sample above the melting point of the sample. The next candidates to show the SME are the silicon rubbers. Silicone elastomers were usually blended with paraffin wax or sodium acetate to generate shape memory polymer. Silicon paraffin wax blend showed a one-way shape memory effect, where deformation was achieved by reversible volume expansion/contraction of the wax during melting or crystallization [11,63][11][12]. In another study silicone, sodium acetate trihydrate (SAT) blends were prepared. The SAT formed supercooled liquids, where crystallization was obtained by tapping the sample. The deformation is formed at room temperature and the shape can be regained by heating above the melting point of SAT or immersing in water [64][13].
Polyurethane (PU) after cross-linking, using excess diisocyanate or glycerine, can be used as a shape memory material. The improvement in the increase of the recovery temperature was observed due to the introduction of cross-links. The thermoplastic PU with shape memory effect was analysed by graft polymerizing with the PU backbone [65,66][14][15].
4. Rubber Composites with Carbon-Based Fillers
Carbon-based fillers are good thermal conductive fillers. The incorporation of these fillers enhance thermal conductivity and improve the heat distribution between the shape memory device and heat source [67,68,69,70,71][16][17][18][19][20]. Carbon nanotubes have a high aspect ratio, which results in high mechanical strength. Fonseca et al. reported that the reduction of the particle size of the filler improves the thermomechanical properties of the material. They have improved the CNT dispersion in thermoplastic polyurethane by functionalization. The carboxylation of CNT established the linkage between CNT and the matrix and improved the thermal diffusivity of the nanocomposite. Reinforcement of CNT into the natural rubber matrix made the composite susceptible to near-infrared laser irradiation, which actsas a trigger to the shape memory process [72][21]. Lai et al. melted blended natural rubber/paraffin wax/CNT composite and studied the two-way shape memory effect, which involves melt-induced contraction and cooling-induced elongation behaviour. The measurements were conducted using a dynamic mechanic analyser [67][16].They heated the rubber/paraffin wax/CNT composite to the deformation temperature (Td) of 90 °C at a heating rate of 5 °C/min, then elongated it to the elongation value of 120 kPa, fixed the sample shape, and then cooled it to 10 °C. Then the load was removed, recovery was noted, and the procedure was repeated. Figure 42A shows the one-way shape memory cycle and Figure 42B shows the two-way memory cycle, in which the sample is again heated to a Td of 90 °C, elongated up to 450 kPa, cooled up to 10 °C, and repeated. Figure 42C(a–e) shows the near infrared laser-induced shape memory effects of the NR/paraffin wax/CNT sample and Figure 42D(a–e) shows the images of the sunlight-induced shape memory effects of the NR blend composites. However, applied external stress is needed for the vapour-triggered shape memory process; they overcame this issue by replacing the paraffin wax with beeswax. By adjusting the beeswax composition they have attained the solvent vapour-triggered process [23,31][22][23].
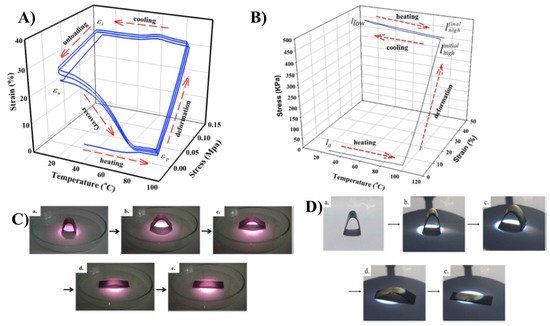
Figure 42. (A) One-way shape memory cycle for the natural rubber/paraffin wax 6:4 blend. (B) Two-way shape memory cycle for the NR. (C). Near-infrared laser-induced shape memory effects of NR/PW 6:4–0.5 CNT [(a) original sample, (b) irradiated for 15 s, (c) 30 s, (d) 90 s, and (e) 120 s]. (D) Sunlight-induced shape memory effects of NR/PW 6:4–0.5 CNT [(a) original sample, (b) irradiated for 30 s, (c) 60 s, (d) 90 s, and (e) for 120 s] (Reproduced with permission from [67][16], Elsevier. 2019).
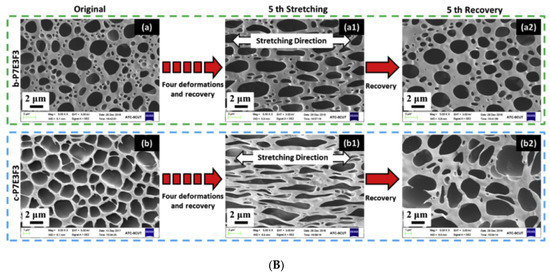
Graphene oxide is layered filler that shows high mechanical and thermal properties; when we reduce the surface oxygen group present in the graphene oxide, it becomes electrically conductive, reduced graphene oxide. Sarmadet et al. used the graphene oxide, reduced the graphene oxide (rGO), and functionalized the reduced graphene, as a filler in the polyurethane matrix. The shape memory effect was studied and a 99.1% of shape fixity value and 96.7% shape recovery value for 5 wt% TPU composite, reinforced with GO: rGO hybrid filler, was obtained [30,45][24][25]. Figure 53A illustrates the morphology of the GO-based shape memory material [(a) GO platelets, (b) rGO platelets, and (c) GO:rGO hybrid filler] and Figure 53B shows the shape fixity, recovery, and the molecular mechanism of the shape memory behaviour [(a) Shape memory thermo-mechanical cycle, (b) The molecular mechanism of Shape memory behavior (Blue lines: molecular chains with low mobility below Tg; red lines: molecular chains with high mobility above Tg), (c) shape fixity and (d) shape recovery on neat TPU, TPU/GO, TPU/rGO and hybrid TPU/GO:rGO composites with 1, 2 and 5 wt% filler content]
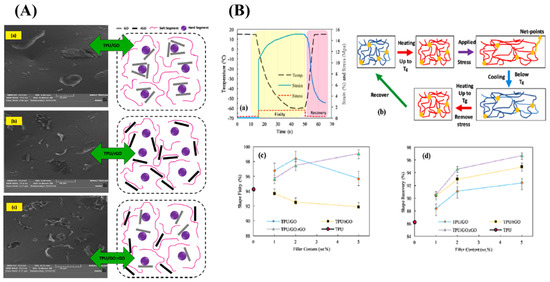
Figure 53. (A) Illustrated morphology of the GO-based shape memory material (TPU/GO: rGO) [(a) GO platelets, (b) rGO platelets, and (c) GO:rGO hybrid filler] and the (B) molecular mechanism of the shape memory behaviour, shape fixity, and shape recovery [(a) Shape memory thermo-mechanical cycle, (b) The molecular mechanism of Shape memory behavior (Blue lines: molecular chains with low mobility below Tg; red lines: molecular chains with high mobility above Tg), (c) shape fixity and (d) shape recovery on neat TPU, TPU/GO, TPU/rGO and hybrid TPU/GO:rGO composites with 1, 2 and 5 wt% filler content] (Reproduced with permission from [30][24], Elsevier, 2019).
The incorporation of hybrid fillers, such as CNT/GO, CNT/nano clay, and CNT/carbon black, was also explored by different researchers; there are plenty of hybrid filler combinations that need to be studied [29,73,74][26][27][28]. Liu et al. synthesized the graphene oxide/Trans-1,4-polyisoprene (GO/TPI) nanocomposite and improved the mechanical and thermal properties of the composites at 0.9 phr GO composition; they have also studied the effect of temperature on the rate of shape recovery. They find that the rate of shape recovery increases with temperature [75][29].
5. Composites with Metal and Metal Oxide Fillers
Magnetically sensitive shapememory materials have major applications in the intelligent biomedical field. Fe3O4 is a very good nanofiller used for magnetic property, which shows relatively high saturation magnetization, high initial permeability, and low connectivity. The dispersion of the magnetic filler is a major challenge and the in situ addition of magnetic filler could improve the dispersion within the matrix. Via the in situ polymerization reaction, Liu et al. designed a carboxylic styrene-butadiene rubber (XSBR)/ferriferous oxide (Fe3O4)/zinc dimethacrylate (ZDMA)-based shape memory material with a higher glass transition temperature (20.5 °C), a shape fixation ratio ~100% at room temperature, and a shape recovery ratio of~100%. Figure 64 shows the reaction of XSBR, Fe3O4, and ZDMA [76][30].
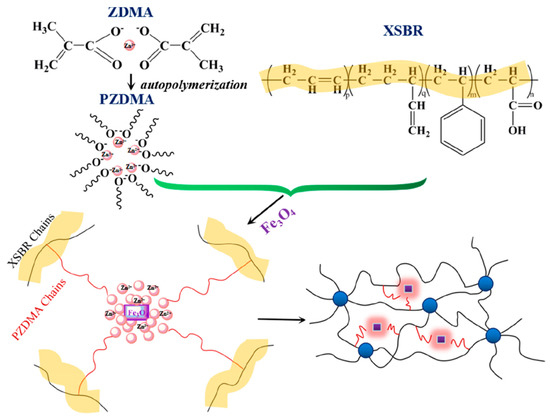
Huang et al. successfully synthesized a super tough and locally thermal/magnetic/light-triggered shape memory material with the highest Rf (~99%) and Rr (>90%) value using polylactide/epoxidized natural rubber thermoplastic vulcanizates by regulating the composition of ferriferous oxide (Fe3O4), using the dynamic vulcanization method. Figure 75A shows the recovery process, digitally photographed for a better understanding of the process. Figure 75B are the DCM-etched scanning electron microscopy images of the composite, which show very good recovery and have a potential application in intelligent biomedical areas [77][31].
References
- Cavicchi, K.A. Shape Memory Polymers from Blends of Elastomers and Small Molecule Additives. Macromol. Symp. 2015, 358, 194–201.
- Katzenberg, F.; Heuwers, B.; Tiller, J.C. Superheated rubber for cold storage. Adv. Mater. 2011, 23, 1909–1911.
- Tosaka, M.; Shigeki, E. Triaxially oriented shape memory natural rubber. Polymer 2018, 157, 151–155.
- Katzenberg, F.; Tiller, J.C. Shape memory natural rubber. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1381–1388.
- Heuwers, B.; Beckel, A.; Krieger, A.; Katzenberg, F.; Tiller, J.C. Shape-memory natural rubber: An exceptional material for strain and energy storage. Macromol. Chem. Phys. 2013, 214, 912–923.
- Wee, J.S.H.; Chai, A.B.; Ho, J.H. Fabrication of shape memory natural rubber using palmitic acid. J. King Saud Univ.-Sci. 2017, 29, 494–501.
- Chang, Y.W.; Eom, J.P.; Kim, J.G.; Kim, H.T.; Kim, D.K. Preparation and characterization of shape memory polymer networks based on carboxylatedtelechelic poly(ε-caprolactone)/epoxidized natural rubber blends. J. Ind. Eng. Chem. 2010, 16, 256–260.
- Chen, Y.; Chen, K.; Wang, Y.; Xu, C. Biobased Heat-Triggered Shape-Memory Polymers Based on Polylactide/Epoxidized Natural Rubber Blend System Fabricated via Peroxide-Induced Dynamic Vulcanization: Co-continuous Phase Structure, Shape Memory Behavior, and Interfacial Compatibilization. Ind. Eng. Chem. Res. 2015, 54, 8723–8731.
- Hoffmann, W. Rubber Technology Handbook; Hanser Publishers: Munich, Germany, 1989.
- Nakayama, M.; Okano, T. Unique thermoresponsive polymeric micelle behavior via cooperative polymer corona phase transitions. Macromolecules 2008, 41, 504–507.
- Sun, L.; Huang, W.M.; Ding, Z.; Zhao, Y.; Wang, C.C.; Purnawali, H.; Tang, C. Stimulus-responsive shape memory materials: A review. Mater. Des. 2012, 33, 577–640.
- Ding, Z. Shape Memory Hybrids: Mechanism and Design for Tailored Properties; Nanyang Technological University: Singapore, 2012.
- Fan, K.; Huang, W.M.; Wang, C.C.; Ding, Z.; Zhao, Y.; Purnawali, H.; Liew, K.C.; Zheng, L.X. Water-responsive shape memory hybrid: Design concept and demonstration. Express Polym. Lett. 2011, 5, 409–416.
- Zia, K.M.; Zuber, M.; Barikani, M.; Bhatti, I.A.; Khan, M.B. Surface characteristics of chitin-based shape memory polyurethane elastomers. Colloids Surf. B Biointerfaces 2009, 72, 248–252.
- Basak, S. Thermoplastic elastomers in biomedical industry—Evolution and current trends. J. Macromol. Sci. Part A 2021, 58, 579–593.
- Lai, S.M.; Guo, G.L. Two-way shape memory effects of sulfur vulcanized natural rubber (NR) and NR/paraffin wax (PW)/carbon nanotube (CNT) nanocomposites. Polym. Test. 2019, 77, 105892.
- Bai, J.; Shi, Z. Dynamically Cross-linked Elastomer Hybrids with Light-Induced Rapid and Efficient Self-Healing Ability and Reprogrammable Shape Memory Behavior. ACS Appl. Mater. Interfaces 2017, 9, 27213–27222.
- Yu, K.; Liu, Y.; Leng, J. Conductive shape memory polymer composite incorporated with hybrid fillers: Electrical, mechanical, and shape memory properties. J. Intell. Mater. Syst. Struct. 2011, 22, 369–379.
- Kang, S.M.; Kwon, S.H.; Park, J.H.; Kim, B.K. Carbon nanotube reinforced shape memory polyurethane foam. Polym. Bull. 2013, 70, 885–893.
- Lu, H.; Yin, W.; Huang, W.M.; Leng, J. Self-assembled carboxylic acid-functionalized carbon nanotubes grafting onto carbon fiber for significantly improving electrical actuation of shape memory polymers. RSC Adv. 2013, 3, 21484–21488.
- Fonseca, B.L.; Fonseca, M.A.; Mónica, M.S. Thermo-mechanical characterization of shape-memory polyurethane nanocomposites filled with carbon nanotubes and graphene nanosheets. Polym. Compos. 2018, 39, E1216–E1223.
- Lai, S.M.; Guo, G.L.; Han, K.T.; Huang, P.S.; Huang, Z.L.; Jiang, M.J.; Zou, Y.R. Properties and characterization of near infrared-triggered natural rubber (NR)/carnauba wax (CW)/carbon nanotube (CNT) shape memory bio-nanocomposites. J. Polym. Res. 2019, 26, 86.
- Lai, S.M.; Guo, G.L.; Xie, Y.C.; Chen, J.M.; Xu, D.Y.; Wei, Y.E.; Cao, Z.R. A novel multi-triggered natural rubber (NR)/beeswax (BW)/carbon nanotube (CNT) shape memory bio-nanocomposite. J. Polym. Res. 2020, 27, 283.
- Panahi-Sarmad, M.; Goodarzi, V.; Amirkiai, A.; Noroozi, M.; Abrisham, M.; Dehghan, P.; Shakeri, Y.; Karimpour-Motlagh, N.; PoudinehHajipoor, F.; Ali Khonakdar, H.; et al. Programing polyurethane with systematic presence of graphene-oxide (GO) and reduced graphene-oxide (rGO) platelets for adjusting of heat-actuated shape memory properties. Eur. Polym. J. 2019, 118, 619–632.
- Panahi-Sarmad, M.; Abrisham, M.; Noroozi, M.; Goodarzi, V.; Arjmand, M.; Sadri, M.; Dehghan, P.; Amirkiai, A.; Khonakdar, H.A. Programing polyurethane with rational surface-modified graphene platelets for shape memory actuators and dielectric elastomer generators. Eur. Polym. J. 2020, 133, 109745.
- Wang, E.; Dong, Y.; Islam, M.Z.; Yu, L.; Liu, F.; Chen, S.; Qi, X.; Zhu, Y.; Fu, Y.; Xu, Z.; et al. Effect of graphene oxide-carbon nanotube hybrid filler on the mechanical property and thermal response speed of shape memory epoxy composites. Compos. Sci. Technol. 2019, 169, 209–216.
- Abrisham, M.; Panahi-Sarmad, M.; Mir Mohamad Sadeghi, G.; Arjmand, M.; Dehghan, P.; Amirkiai, A. Microstructural design for enhanced mechanical property and shape memory behavior of polyurethane nanocomposites: Role of carbon nanotube, montmorillonite, and their hybrid fillers. Polym. Test. 2020, 89, 106642.
- Arun, D.I.; Chakravarthy, P.; Santhosh Kumar, K.S. Synergy studies on polyurethane–carbon black, multi-walled carbon nanotube-based heterogeneous electroactive shape memory nanocomposite system. Bull. Mater. Sci. 2020, 43, 219.
- Mohan, D.G.; Gopi, S. Induction Assisted friction stir welding: A review. Aust. J. Mech. Eng. 2020, 18, 119–123.
- Liu, C.; Huang, J.; Yuan, D.; Chen, Y. Design of a High-Strength XSBR/Fe3O4/ZDMA Shape-Memory Composite with Dual Responses. Ind. Eng. Chem. Res. 2018, 57, 14527–14534.
- Huang, J.; Fan, J.; Yin, S.; Chen, Y. Design of remotely, locally triggered shape-memory materials based on bicontinuous polylactide/epoxidized natural rubber thermoplastic vulcanizates via regulating the distribution of ferroferric oxide. Compos. Sci. Technol. 2019, 182, 107732.
More