Aducanumab is a human monoclonal antibody that works to reduce Aβ load in the brain; it is the first disease-modifying therapy to be approved for AD treatment. On 7 June 2021, the United States Food and Drug Administration (U.S. FDA) approved aducanumab via an accelerated approval pathway. Current AD treatment is centered on supportive care to manage the debilitating symptoms of dementia, and pharmacotherapy goals of mainstay classes of drugs, such as cholinesterase inhibitors (ChEIs) and N-methyl-D-aspartate (NMDA) receptor antagonists, do not modify the course of the disease. A substantial proportion of patients with Alzheimer’s disease live in low- and middle-income countries (LMICs), and the debilitating effects of this disease exerts burdens on patients and caregivers in addition to the significant economic strains many nations bear.
- Alzheimer’s disease
- aducanumab
- LMICs
- APOE
- burden of disease
- treatment cost
1. Aducanumab in Low- and Middle-Income Countries
| Monoclonal Antibodies | Cholinesterase Inhibitors (ChEIs) | N-methyl-D-aspartate (NMDA) Receptor Antagonists |
|---|
| Drug | Aducanumab | Donepezil, rivastigmine, and galantamine | Memantine | ||||||||||||
| Pharmacotherapy goal(s) | Disease-modifying treatment to reduce cognitive decline. | Symptomatic management of cognition and global functioning. | Symptomatic management of cognition and global functioning. |
||||||||||||
| Mechanism of action | Selectively targets and clears Aβ aggregates, Aβ fibrils, and soluble oligomers. A reduction in Aβ build up in the brain is expected to demonstrate a reduction in cognitive decline in patients | [10 | 14 | ] | . | Increases cholinergic transmission by inhibiting cholinesterase at the synaptic cleft, thereby improving cortical cholinergic function | [5 | 9 | ] | . | Exerts neuroprotective effects by blocking pathological stimulation of glutamate NMDA receptors, thereby decreasing excitotoxicity | [5 | 9 | ] | . |
| Indication | Mild cognitive impairment (MCI), mild AD | [1115] | Mild to moderate AD, advanced disease | [711] | Moderate to severe AD, mild AD (off-label) | [1216] | |||||||||
| Route of administration | Intravenous infusion | Oral, transdermal patch (rivastigmine only) | Oral | ||||||||||||
| Efficacy in terms of cognitive function | A statistically significant improvement in various scales of cognitive function was observed in the high-dose arm of EMERGE . | A statistically significant improvement in various scales of cognitive function was observed in the high-dose arm of EMERGE [4]. |
A meta-analysis of ChEIs revealed modest improvements | [1317] | . | A reduction in deterioration on different scales of clinical efficacy compared to placebo was observed in patients with moderate to severe AD | [12 | 16 | ] | . | |||||
| Difference vs. placebo: −0.39 (95% CI 0.69 to −0.09) Score: CDR-SB * | Difference vs. placebo: −0.39 (95% CI 0.69 to −0.09) [4] Score: CDR-SB * |
Donepezil | MD −1.02, (95% CI −1.66 to −0.39, p = 0.002) [9] Scale: ADAS-Cog † | MD −1.02, (95% CI −1.66 to −0.39, p = 0.002) [13] Scale: ADAS-Cog † |
|||||||||||
| MD −2.67, (95% CI −3.31 to −2.02) | [610] | Scale: ADAS-Cog | † | ||||||||||||
| MD 1.05, (95% CI 0.73 to 1.37) | [610] | Score: MMSE | ‡ | ||||||||||||
| Difference vs. placebo: −1.400 (p = 0.00967) Scale: ADAS-Cog ** | Difference vs. placebo: −1.400 (p = 0.00967) [4] Scale: ADAS-Cog ** |
Galantamine | |||||||||||||
| MD −2.95, (95% CI −3.32, −2.57) | [812] | Scale: ADAS-Cog | † | ||||||||||||
| MD 2.50, (95% CI 0.86 to 4.15) | [812] | Score: MMSE | ‡ | ||||||||||||
| Difference vs. placebo: 0.6 (p = 0.05) Score: MMSE ‡ | Difference vs. placebo: 0.6 (p = 0.05) [4] Score: MMSE ‡ |
Rivastigmine | |||||||||||||
| MD −1.79, (95% CI −2.21 to −1.37) | [711] | Scale: ADAS-Cog | † | ||||||||||||
| MD 0.74, (95% CI 0.52 to 0.97) | [711] | Score: MMSE | ‡ |
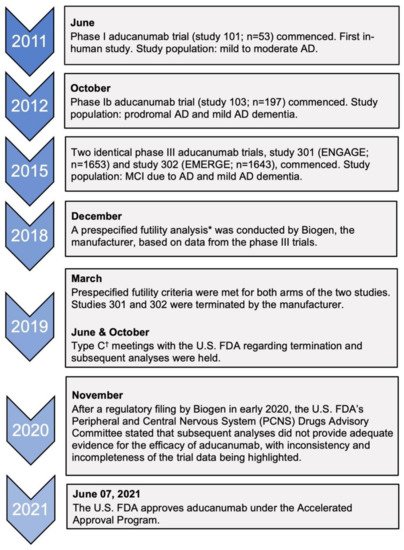
1.1. Accelerated Approval and the Efficacy of Aducanumab
1.1. Accelerated Approval and the Efficacy of Aducanumab
1.2. Treatment Challenges
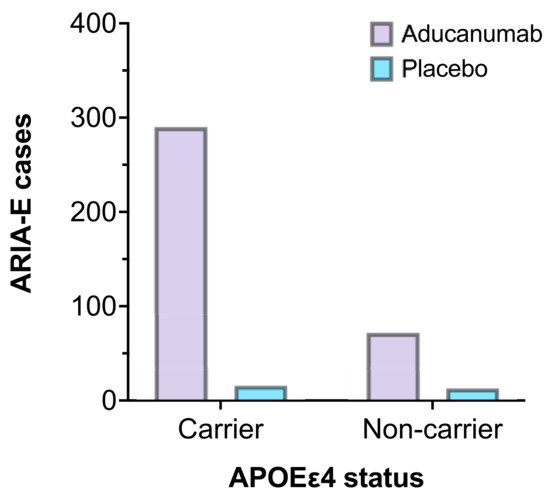
1.3. Adverse Effects
1.4. Apolipoprotein E and Interethnic Differences
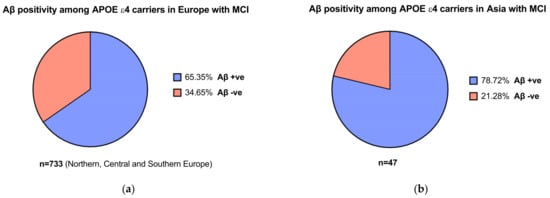
1.5. The Economic Burden
| Monoclonal Antibodies | Cholinesterase Inhibitors (ChEIs) | N-methyl-D-aspartate (NMDA) Receptor Antagonists | |
|---|---|---|---|
| Drug | Aducanumab | Donepezil, rivastigmine, and galantamine | Memantine |
| Functional outcomes | MD 1.70 (95% CI 0.71 to 2.69) (high-dose arm of EMERGE) Scale: ADCS-AD a |
Donepezil: SMD 0.22 (95% CI 0.12–0.33) [6 |
| Monoclonal Antibodies | Cholinesterase Inhibitors (ChEIs) | N-methyl-D-aspartate (NMDA) Receptor Antagonists | |||
|---|---|---|---|---|---|
| Drug | Aducanumab | Donepezil, rivastigmine, and galantamine | Memantine | ] | MD 0.95 (95% CI 0.22 to 1.76) [12] Scale: ADCS-AD c |
| Galantamine: SMD 0.19 (95% CI 0.01–0.37) [5] | |||||
| Rivastigmine: MD 1.80 (95% CI 0.20 to 3.40) [40] | |||||
| Scale: ADCS-AD b | |||||
| Entry to institutional or nursing care | Not assessed | No significant benefit in terms of delay of entry to institutional care [41]. | No effect on the rate of nursing home placement [42]. | ||
| Adverse effects | ARIA-E and ARIA-H Symptoms: headaches, confusion, dizziness, falls, vision changes, and nausea [16]. |
Donepezil: gastrointestinal symptoms (upset stomach, nausea, diarrhoea, and anorexia) |
Dizziness, confusion, weight gain, hallucinations [12]. | ||
| Galantamine: gastrointestinal symptoms | |||||
| Rivastigmine (patch): nausea, vomiting, anorexia, headaches, dizziness |
|||||
| General vagotonic symptoms: bradycardia and hypotension [13]. | |||||
| APOE genotyping | Not a requirement. However, genetic screening may help ascertain ARIA risk. |
Not required | Not required | ||
| Pre-treatment amyloid status | Amyloid-PET or CSF analysis may be conducted [11]. | Not applicable | Not applicable | ||
| Baseline MRI scan of the brain | Required (a recent scan within one year prior to initiating therapy) [11]. | For clinical diagnosis | For clinical diagnosis | ||
| Follow-up MRI scans of the brain | Two scans prior to the seventh and twelfth doses. Additional monitoring of ARIAs with MRI if symptomatic [11]. |
Not required | Not required | ||
| Average annual cost (U.S. $) | 56,000 * | 2796 † | 4096 ‡ | ||
| Generics or biosimilars | None | Available | Available |
2. Conclusions
1.2. Treatment Challenges
1.3. Adverse Effects
1.4. Apolipoprotein E and Interethnic Differences


1.5. The Economic Burden
| Functional outcomes | |||
| MD 1.70 (95% CI 0.71 to 2.69) (high-dose arm of EMERGE) [4] Scale: ADCS-AD a |
Donepezil: SMD 0.22 (95% CI 0.12–0.33) [10] | MD 0.95 (95% CI 0.22 to 1.76) [16] Scale: ADCS-AD c |
|
| Galantamine: SMD 0.19 (95% CI 0.01–0.37) [9] | |||
| Rivastigmine: MD 1.80 (95% CI 0.20 to 3.40) [44] | |||
| Scale: ADCS-AD b | |||
| Entry to institutional or nursing care | Not assessed | No significant benefit in terms of delay of entry to institutional care [45]. | No effect on the rate of nursing home placement [46]. |
| Adverse effects | ARIA-E and ARIA-H Symptoms: headaches, confusion, dizziness, falls, vision changes, and nausea [20]. |
Donepezil: gastrointestinal symptoms (upset stomach, nausea, diarrhoea, and anorexia) |
Dizziness, confusion, weight gain, hallucinations [16]. |
| Galantamine: gastrointestinal symptoms | |||
| Rivastigmine (patch): nausea, vomiting, anorexia, headaches, dizziness |
|||
| General vagotonic symptoms: bradycardia and hypotension [17]. | |||
| APOE genotyping | Not a requirement. However, genetic screening may help ascertain ARIA risk. |
Not required | Not required |
| Pre-treatment amyloid status | Amyloid-PET or CSF analysis may be conducted [15]. | Not applicable | Not applicable |
| Baseline MRI scan of the brain | Required (a recent scan within one year prior to initiating therapy) [15]. | For clinical diagnosis | For clinical diagnosis |
| Follow-up MRI scans of the brain | Two scans prior to the seventh and twelfth doses. Additional monitoring of ARIAs with MRI if symptomatic [15]. |
Not required | Not required |
| Average annual cost (U.S. $) | 56,000 * | 2796 † | 4096 ‡ |
| Generics or biosimilars | None | Available | Available |
References
- Hardy, J. A Hundred Years of Alzheimer’s Disease Research. Neuron 2006, 52, 3–13. Prince, M.; Guerchet, M.; Prina, M. WHO Thematic Briefing: The Epidemiology and Impact of Dementia—Current State and Future Trends; World Health Organization: Geneva, Switzerland, 2015.
- Alves, L.; Correia, A.S.A.; Miguel, R.; Alegria, P.; Bugalho, P. Alzheimer’s disease: A clinical practice-oriented review. Front. Neurol. 2012, 3, 63. Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222.
- Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, A.M.; Winblad, B.; Jönsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dement. 2017, 13, 1–7. Alexander, G.C.; Karlawish, J. The Problem of Aducanumab for the Treatment of Alzheimer Disease. Ann. Intern. Med. 2021.
- Hung, S.-Y.; Fu, W.-M. Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 2017, 24, 1–12. Haeberlein, S.B.; von Hehn, C.; Tian, Y.; Chalkias, S.; Muralidharan, K.K.; Chen, T.; Wu, S.; Skordos, L.; Nisenbaum, L.; Rajagovindan, R.; et al. Emerge and Engage topline results: Phase 3 studies of aducanumab in early Alzheimer’s disease. Alzheimer’s Dement. 2020, 16, e047259.
- Li, D.-D.; Zhang, Y.-H.; Zhang, W.; Zhao, P. Meta-Analysis of Randomized Controlled Trials on the Efficacy and Safety of Donepezil, Galantamine, Rivastigmine, and Memantine for the Treatment of Alzheimer’s Disease. Front. Neurosci. 2019, 13, 472. Lin, G.A.; Whittington, M.D.; Synnott, P.G.; McKenna, A.; Campbell, J.; Pearson, S.D.; Rind, D.M. Aducanumab for Alzheimer’s Disease: Effectiveness and Value; Final Evidence Report and Meeting Summary. Institute for Clinical and Economic Review, August 5, 2021. Available online: https://icer.org/assessment/alzheimers-disease-2021/ (accessed on 5 August 2021).
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 10, 14651858. Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, M. World Alzheimer Report 2015—The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer’s Dis. Int. 2015.
- Birks, J.S.; Chong, L.Y.; Grimley Evans, J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015, 16, 295–315. Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, A.M.; Winblad, B.; Jönsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dement. 2017, 13, 1–7.
- Jiang, D.; Yang, X.; Li, M.; Wang, Y.; Wang, Y. Efficacy and safety of galantamine treatment for patients with Alzheimer’s disease: A meta-analysis of randomized controlled trials. J. Neural Transm. 2015, 122, 1157–1166. Hung, S.-Y.; Fu, W.-M. Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 2017, 24, 1–12.
- Kishi, T.; Matsunaga, S.; Oya, K.; Nomura, I.; Ikuta, T.; Iwata, N. Memantine for Alzheimer’s Disease: An Updated Systematic Review and Meta-analysis. J. Alzheimer’s Dis. 2017, 60, 401–425. Li, D.-D.; Zhang, Y.-H.; Zhang, W.; Zhao, P. Meta-Analysis of Randomized Controlled Trials on the Efficacy and Safety of Donepezil, Galantamine, Rivastigmine, and Memantine for the Treatment of Alzheimer’s Disease. Front. Neurosci. 2019, 13, 472.
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 10, 14651858.
- Cummings, J.; Aisen, P.; Apostolova, L.G.; Atri, A.; Salloway, S.; Weiner, M. Aducanumab: Appropriate Use Recommendations. J. Prev. Alzheimer’s Dis. 2021, 8, 1–13. Birks, J.S.; Chong, L.Y.; Grimley Evans, J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015, 16, 295–315.
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, CD003154. Jiang, D.; Yang, X.; Li, M.; Wang, Y.; Wang, Y. Efficacy and safety of galantamine treatment for patients with Alzheimer’s disease: A meta-analysis of randomized controlled trials. J. Neural Transm. 2015, 122, 1157–1166.
- Birks, J.S. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 1, CD005593. Kishi, T.; Matsunaga, S.; Oya, K.; Nomura, I.; Ikuta, T.; Iwata, N. Memantine for Alzheimer’s Disease: An Updated Systematic Review and Meta-analysis. J. Alzheimer’s Dis. 2017, 60, 401–425.
- Dunstan, R.; Bussiere, T.; Fahrer, D.; Quigley, C.; Zhang, X.; Themeles, M.; Engber, T.; Rhodes, K.; Arastu, M.; Li, M. Quantitation of beta-amyloid in transgenic mice using whole slide digital imaging and image analysis software. Alzheimer’s Dement. 2011, 7, S700. Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56.
- Liu, K.Y.; Howard, R. Can we learn lessons from the FDA’s approval of aducanumab? Nat. Rev. Neurol. 2021, 17, 715–722. Cummings, J.; Aisen, P.; Apostolova, L.G.; Atri, A.; Salloway, S.; Weiner, M. Aducanumab: Appropriate Use Recommendations. J. Prev. Alzheimer’s Dis. 2021, 8, 1–13.
- U.S. Food & Drug Administration. Briefing Document: Combined FDA and Applicant PCNS Drugs Advisory Committee. Available online: https://www.fda.gov/media/143502/download (accessed on 27 June 2021).McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, CD003154.
- U.S. Food & Drug Administration. Guidance for Industry: Formal Meetings between the FDA and Sponsors or Applicants. Available online: https://www.fda.gov/media/72253/download (accessed on 11 November 2021).Birks, J.S. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 1, CD005593.
- Retinasamy, T.; Shaikh, M.F. Aducanumab for Alzheimer’s disease: An update. Neurosci. Res. Notes 2021, 4, 17–20. Dunstan, R.; Bussiere, T.; Fahrer, D.; Quigley, C.; Zhang, X.; Themeles, M.; Engber, T.; Rhodes, K.; Arastu, M.; Li, M. Quantitation of beta-amyloid in transgenic mice using whole slide digital imaging and image analysis software. Alzheimer’s Dement. 2011, 7, S700.
- Yuan, J.; Maserejian, N.; Liu, Y.; Devine, S.; Gillis, C.; Massaro, J.; Au, R.; Bondi, M. Severity Distribution of Alzheimer’s Disease Dementia and Mild Cognitive Impairment in the Framingham Heart Study. J. Alzheimer’s Dis. 2021, 79, 807–817. Liu, K.Y.; Howard, R. Can we learn lessons from the FDA’s approval of aducanumab? Nat. Rev. Neurol. 2021, 17, 715–722.
- Anand, A.; Patience, A.A.; Sharma, N.; Khurana, N. The present and future of pharmacotherapy of Alzheimer’s disease: A comprehensive review. Eur. J. Pharmacol. 2017, 815, 364–375. U.S. Food & Drug Administration. Briefing Document: Combined FDA and Applicant PCNS Drugs Advisory Committee. Available online: https://www.fda.gov/media/143502/download (accessed on 27 June 2021).
- U.S. Food & Drug Administration. PCNS Drugs Advisory Committee: Aducanumab for the Treatment of Alzheimer’s Disease. Available online: https://www.fda.gov/media/143506/download (accessed on 2 August 2021).U.S. Food & Drug Administration. Guidance for Industry: Formal Meetings between the FDA and Sponsors or Applicants. Available online: https://www.fda.gov/media/72253/download (accessed on 11 November 2021).
- Bitton, A.; Fifield, J.; Ratcliffe, H.; Karlage, A.; Wang, H.; Veillard, J.H.; Schwarz, D.; Hirschhorn, L.R. Primary healthcare system performance in low-income and middle-income countries: A scoping review of the evidence from 2010 to 2017. BMJ Glob. Health 2019, 4, e001551. Retinasamy, T.; Shaikh, M.F. Aducanumab for Alzheimer’s disease: An update. Neurosci. Res. Notes 2021, 4, 17–20.
- Schwarz, D.; Hirschhorn, L.R.; Kim, J.-H.; Ratcliffe, H.L.; Bitton, A. Continuity in primary care: A critical but neglected component for achieving high-quality universal health coverage. BMJ Glob. Health 2019, 4, e001435. Yuan, J.; Maserejian, N.; Liu, Y.; Devine, S.; Gillis, C.; Massaro, J.; Au, R.; Bondi, M. Severity Distribution of Alzheimer’s Disease Dementia and Mild Cognitive Impairment in the Framingham Heart Study. J. Alzheimer’s Dis. 2021, 79, 807–817.
- World Bank Data. Physicians (per 1000 People). Available online: https://data.worldbank.org/indicator/SH.MED.PHYS.ZS (accessed on 27 June 2021).Anand, A.; Patience, A.A.; Sharma, N.; Khurana, N. The present and future of pharmacotherapy of Alzheimer’s disease: A comprehensive review. Eur. J. Pharmacol. 2017, 815, 364–375.
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimer’s Dis. 2019, 67, 779–794. U.S. Food & Drug Administration. PCNS Drugs Advisory Committee: Aducanumab for the Treatment of Alzheimer’s Disease. Available online: https://www.fda.gov/media/143506/download (accessed on 2 August 2021).
- International Atomic Energy Agency (IAEA). IMAGINE—IAEA Medical Imaging and Nuclear Medicine Global Resources Database. Available online: https://humanhealth.iaea.org/HHW/DBStatistics/IMAGINEMaps3.html (accessed on 27 June 2021).Bitton, A.; Fifield, J.; Ratcliffe, H.; Karlage, A.; Wang, H.; Veillard, J.H.; Schwarz, D.; Hirschhorn, L.R. Primary healthcare system performance in low-income and middle-income countries: A scoping review of the evidence from 2010 to 2017. BMJ Glob. Health 2019, 4, e001551.
- Zhang, X.; Fu, Z.; Meng, L.; He, M.; Zhang, Z. The Early Events That Initiate β-Amyloid Aggregation in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 359. Schwarz, D.; Hirschhorn, L.R.; Kim, J.-H.; Ratcliffe, H.L.; Bitton, A. Continuity in primary care: A critical but neglected component for achieving high-quality universal health coverage. BMJ Glob. Health 2019, 4, e001435.
- Cai, Z.; Zhou, Y.; Xiao, M.; Yan, L.-J.; He, W. Activation of mTOR: A culprit of Alzheimer’s disease? Neuropsychiatr. Dis. Treat. 2015, 2015, 1015–1030. World Bank Data. Physicians (per 1000 People). Available online: https://data.worldbank.org/indicator/SH.MED.PHYS.ZS (accessed on 27 June 2021).
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimer’s Dis. 2019, 67, 779–794.
- Arimon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119. International Atomic Energy Agency (IAEA). IMAGINE—IAEA Medical Imaging and Nuclear Medicine Global Resources Database. Available online: https://humanhealth.iaea.org/HHW/DBStatistics/IMAGINEMaps3.html (accessed on 27 June 2021).
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Aalten, P.; Aarsland, D.; Alcolea, D.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938. Zhang, X.; Fu, Z.; Meng, L.; He, M.; Zhang, Z. The Early Events That Initiate β-Amyloid Aggregation in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 359.
- Li, C.; Loewenstein, D.A.; Duara, R.; Cabrerizo, M.; Barker, W.; Adjouadi, M.; Alzheimer’s Disease Neuroimaging, I. The Relationship of Brain Amyloid Load and APOE Status to Regional Cortical Thinning and Cognition in the ADNI Cohort. J. Alzheimer’s Dis. 2017, 59, 1269–1282. Cai, Z.; Zhou, Y.; Xiao, M.; Yan, L.-J.; He, W. Activation of mTOR: A culprit of Alzheimer’s disease? Neuropsychiatr. Dis. Treat. 2015, 2015, 1015–1030.
- Kern, S.; Mehlig, K.; Kern, J.; Zetterberg, H.; Thelle, D.; Skoog, I.; Lissner, L.; Blennow, K.; Börjesson-Hanson, A. The Distribution of Apolipoprotein E Genotype Over the Adult Lifespan and in Relation to Country of Birth. Am. J. Epidemiol. 2015, 181, 214–217. Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249.
- Mattsson, N.; Groot, C.; Jansen, W.J.; Landau, S.M.; Villemagne, V.L.; Engelborghs, S.; Mintun, M.M.; Lleo, A.; Molinuevo, J.L.; Jagust, W.J.; et al. Prevalence of the apolipoprotein E ε4 allele in amyloid β positive subjects across the spectrum of Alzheimer’s disease. Alzheimer’s Dement 2018, 14, 913–924. Arimon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119.
- VandeVrede, L.; Gibbs, D.M.; Koestler, M.; La Joie, R.; Ljubenkov, P.A.; Provost, K.; Soleimani-Meigooni, D.; Strom, A.; Tsoy, E.; Rabinovici, G.D.; et al. Symptomatic amyloid-related imaging abnormalities in an APOE ε4/ε4 patient treated with aducanumab. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12101. Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Aalten, P.; Aarsland, D.; Alcolea, D.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938.
- Brickman, A.M.; Manly, J.J.; Honig, L.S.; Sanchez, D.; Reyes-Dumeyer, D.; Lantigua, R.A.; Lao, P.J.; Stern, Y.; Vonsattel, J.P.; Teich, A.F.; et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimer’s Dement. 2021, 17, 1353–1364. Li, C.; Loewenstein, D.A.; Duara, R.; Cabrerizo, M.; Barker, W.; Adjouadi, M.; Alzheimer’s Disease Neuroimaging, I. The Relationship of Brain Amyloid Load and APOE Status to Regional Cortical Thinning and Cognition in the ADNI Cohort. J. Alzheimer’s Dis. 2017, 59, 1269–1282.
- Howell, J.C.; Watts, K.D.; Parker, M.W.; Wu, J.; Kollhoff, A.; Wingo, T.S.; Dorbin, C.D.; Qiu, D.; Hu, W.T. Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimer’s Res. Ther. 2017, 9, 88. Kern, S.; Mehlig, K.; Kern, J.; Zetterberg, H.; Thelle, D.; Skoog, I.; Lissner, L.; Blennow, K.; Börjesson-Hanson, A. The Distribution of Apolipoprotein E Genotype Over the Adult Lifespan and in Relation to Country of Birth. Am. J. Epidemiol. 2015, 181, 214–217.
- Nguyen, T.A.; Knight, R.; Roughead, E.E.; Brooks, G.; Mant, A. Policy options for pharmaceutical pricing and purchasing: Issues for low- and middle-income countries. Health Policy Plan. 2015, 30, 267–280. Mattsson, N.; Groot, C.; Jansen, W.J.; Landau, S.M.; Villemagne, V.L.; Engelborghs, S.; Mintun, M.M.; Lleo, A.; Molinuevo, J.L.; Jagust, W.J.; et al. Prevalence of the apolipoprotein E ε4 allele in amyloid β positive subjects across the spectrum of Alzheimer’s disease. Alzheimer’s Dement 2018, 14, 913–924.
- Guerchet, M.; Mayston, R.; Lloyd-Sherlock, P.; Prince, M.; Aboderin, I.; Akinyemi, R.; Paddick, S.-M.; Wimo, A.; Amoakoh-Coleman, M.; Uwakwe, R.; et al. Dementia in Sub-Saharan Africa: Challenges and Opportunities; Alzheimer’s Disease International: London, UK, 2017. VandeVrede, L.; Gibbs, D.M.; Koestler, M.; La Joie, R.; Ljubenkov, P.A.; Provost, K.; Soleimani-Meigooni, D.; Strom, A.; Tsoy, E.; Rabinovici, G.D.; et al. Symptomatic amyloid-related imaging abnormalities in an APOE ε4/ε4 patient treated with aducanumab. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12101.
- Winblad, B.; Grossberg, G.; Frölich, L.; Farlow, M.; Zechner, S.; Nagel, J.; Lane, R. IDEAL: A 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease. Neurology 2007, 69, S14–S22. Brickman, A.M.; Manly, J.J.; Honig, L.S.; Sanchez, D.; Reyes-Dumeyer, D.; Lantigua, R.A.; Lao, P.J.; Stern, Y.; Vonsattel, J.P.; Teich, A.F.; et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimer’s Dement. 2021, 17, 1353–1364.
- Courtney, C.; Farrell, D.; Gray, R.; Hills, R.; Lynch, L.; Sellwood, E.; Edwards, S.; Hardyman, W.; Raftery, J.; Crome, P.; et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): Randomised double-blind trial. Lancet 2004, 363, 2105–2115. Howell, J.C.; Watts, K.D.; Parker, M.W.; Wu, J.; Kollhoff, A.; Wingo, T.S.; Dorbin, C.D.; Qiu, D.; Hu, W.T. Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimer’s Res. Ther. 2017, 9, 88.
- Howard, R.; McShane, R.; Lindesay, J.; Ritchie, C.; Baldwin, A.; Barber, R.; Burns, A.; Dening, T.; Findlay, D.; Holmes, C.; et al. Nursing home placement in the Donepezil and Memantine in Moderate to Severe Alzheimer’s Disease (DOMINO-AD) trial: Secondary and post-hoc analyses. Lancet Neurol. 2015, 14, 1171–1181. Nguyen, T.A.; Knight, R.; Roughead, E.E.; Brooks, G.; Mant, A. Policy options for pharmaceutical pricing and purchasing: Issues for low- and middle-income countries. Health Policy Plan. 2015, 30, 267–280.
- Consumers Union of U.S. Consumer Reports Best Buy Drugs. Evaluating Prescription Drugs Used to Treat: Alzheimer’s Disease. Available online: https://article.images.consumerreports.org/prod/content/dam/cro/news_articles/health/PDFs/Alzheimer’sDisease_fullreport.pdf (accessed on 1 August 2021).Guerchet, M.; Mayston, R.; Lloyd-Sherlock, P.; Prince, M.; Aboderin, I.; Akinyemi, R.; Paddick, S.-M.; Wimo, A.; Amoakoh-Coleman, M.; Uwakwe, R.; et al. Dementia in Sub-Saharan Africa: Challenges and Opportunities; Alzheimer’s Disease International: London, UK, 2017.
- Winblad, B.; Grossberg, G.; Frölich, L.; Farlow, M.; Zechner, S.; Nagel, J.; Lane, R. IDEAL: A 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease. Neurology 2007, 69, S14–S22.
- Courtney, C.; Farrell, D.; Gray, R.; Hills, R.; Lynch, L.; Sellwood, E.; Edwards, S.; Hardyman, W.; Raftery, J.; Crome, P.; et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): Randomised double-blind trial. Lancet 2004, 363, 2105–2115.
- Howard, R.; McShane, R.; Lindesay, J.; Ritchie, C.; Baldwin, A.; Barber, R.; Burns, A.; Dening, T.; Findlay, D.; Holmes, C.; et al. Nursing home placement in the Donepezil and Memantine in Moderate to Severe Alzheimer’s Disease (DOMINO-AD) trial: Secondary and post-hoc analyses. Lancet Neurol. 2015, 14, 1171–1181.
- Consumers Union of U.S. Consumer Reports Best Buy Drugs. Evaluating Prescription Drugs Used to Treat: Alzheimer’s Disease. Available online: https://article.images.consumerreports.org/prod/content/dam/cro/news_articles/health/PDFs/Alzheimer’sDisease_fullreport.pdf (accessed on 1 August 2021).
