Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Carmen Teodosiu.
Municipal wastewater treatment plants (MWWTPs) face great challenges in optimizing technologies and ensuring environmental sustainability, in direct correlation with the increased pollution with emerging and priority compounds, wastewater quality discharged and climate changes challenges. The recent developments on the synthesis and characterization of composites based on polyelectrolytes, are discussed, and a correlation of their actual structure and properties with the adsorption mechanisms and removal efficiencies of various pollutants in aqueous media (priority and emerging pollutants or other model pollutants) are presented.
- advanced wastewater treatment
- polyelectrolytes
- composites
- sorption
- heavy metals
- organic pollutants
1. Introduction
Municipal wastewater treatment plants (MWWTPs) face great challenges in optimizing technologies to avoid ecological and human health problems and to ensure environmental sustainability, in direct correlation with the increased pollution due to economic and social growth, wastewater quality discharged into surface waters and climate changes. Industrial activities are responsible for the discharge of effluents with a wide range of inorganic and organic compounds that belong to the priority (PPs) and emerging pollutants (EPs) classes, pharmaceuticals and personal care products, pesticides, heavy metals, detergents, flame-retardants being only few examples of such pollutants. Through their presence, eco-toxicological and human health effects, bio-accumulative and degradation characteristics may influence aquatic biota and the performances and costs of water and wastewater treatment technologies [1]. Moreover, considering the new Circular Economy Action Plan and EU Green Deal there is a huge pressure nowadays on the regional water operators (running water and wastewater treatment plants) to decrease their operational costs and associated environmental impacts and carbon footprints (especially due to energy consumption), while introducing more viable alternatives for wastewater recycling for industries, agriculture/irrigation, aquacultures, trying to recover materials and energy from wastewater sludge [2,3][2][3].
MWWTPs collecting wastewater from combined sewers usually remove solids of different sizes, biodegradable organic and inorganic compounds based on conventional processes such as the following: mechanical (bar-screens, grit removal and sedimentation), biological (suspended or attached growth) and tertiary (nitrogen and phosphorous removal) treatment. The implementation of wastewater reuse in agriculture or industry requires the elimination of targeted priority and emerging organic & inorganic pollutants, microorganisms (bacteria, viruses, parasites) by means of advanced wastewater treatment (AWWT) (such as membrane processes, advanced oxidation, adsorption, filtration, disinfection, etc.), that complete the conventional treatment as presented in Figure 1.
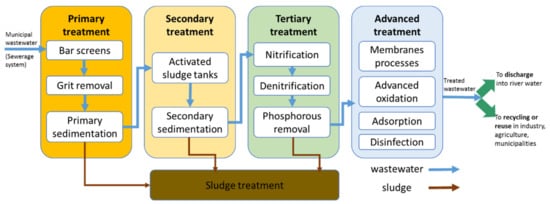
Figure 1. Municipal wastewater treatment outlines for various uses and pollutants removal.
Although, inorganic and organic pollutants classified as PPs or EPs are detected in wastewater at low concentrations (few micrograms/milligrams per liter), they are toxic, bio-accumulative, low biodegradable and very difficult to remove in terms of technological, energy and environmental efforts. In the European Union, both PPs and EPs are monitored in surface water [4,5[4][5][6],6], but at the moment, in Europe, only Switzerland enforces legal obligations to remove these compounds within MWWTPs [7]. The advanced wastewater treatment for EPs removal (for wastewater recycling and reuse) should consider at least the following criteria: (i) range of treated pollutants, treatment efficiency & removal mechanisms, (ii) environmental reduced impacts, (iii) simplicity of operation & maintenance, (iv) cost-effectiveness, (v) social acceptance [8].
In recent years, numerous classes of inorganic (metal oxides, zeolites, sand), organic (activated carbon, resins, covalent organic frameworks), or composite sorbents have been designed to sorb different classes of organic/inorganic pollutants [9,10,11,12,13,14,15][9][10][11][12][13][14][15]. Due to some disadvantages (high costs, low sorption capacities, low number of reusing cycles, non-degradable characteristics, secondary pollution) many types of these materials could be difficult to be used in practice at large scale. Recently, natural and synthetic polyelectrolytes were combined with some inorganic materials (SiO2, TiO2, graphene oxide, Fe3O4, clays, zeolites etc.) or other organic polymers (cellulose, wood, cyclodextrin, polystyrene (PS), etc.) to create new materials (composite type) as perfect candidates for sorption of toxic pollutants such as pharmaceuticals and heavy metal ions [16,17][16][17].
2. Composite Polyelectrolytes with Versatile Properties in Targeting Different Types of Pollutants Dissolved in Real/Simulated Aqueous Effluents
2.1. Why Composites Based on Polyelectrolytes?
Polyelectrolytes are charged/chargeable polymers whose repeated ionic/ionizable structural units are higher than 10–15 mol%. Based on their ability to partially/fully dissociate in aqueous environment, polyelectrolytes are usually classified in weak or strong and in anionic and cationic in dependence of charge type. These polymeric compounds have a great potential in water and wastewater treatment (such as coagulation-flocculation process) due to a particular characteristic: the high densities of functional groups bound to a flexible polymeric chain, allowing the intimate interaction at the atomic level with various types of pollutants.
Using polyelectrolyte characteristics, numerous types of composites can be fabricated by covalent bonding or non-covalent bonding (hydrogen bonding, π–π stacking, metal-ligand coordination, ionic, and hydrophobic interactions) between polyelectrolytes and different inorganic/organic partners (Table 1). Composite polyelectrolyte materials, which include mainly polymeric ionic chains, contain a large number and specific stimuli-responsive functional groups with controllable action (detection, immobilization, releasing) toward different classes of pollutants entities found in aqueous environment [18,19][18][19]. The main types of polyelectrolytes, polymers, and inorganics entities used in creation of composite sorbents, as well as the main tested pollutants, have been summarized in Table 1.
Table 1. Composite sorbents based on polyelectrolytes used for pollutants removal from aqueous media.
| Weak/Strong Polyelectrolytes | Organic/Inorganic Partner | Pollutant Targeted | References | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chitosan (CS) | Poly(ethyleneimine), Poly(vinyl amine), Poly(vinyl alcohol), Poly( | N | , | N | -dimethylamino)ethyl methacrylate, Poly(methacrylic acid), Poly(sodium acrylate), carrageenan, carboxymethyl β-cyclodextrin, carboxyalkyl chitosan, Poly-hexamethylene guanidine, microcrystalline cellulose, sodium alginate, karaya gum, citric acid, itaconic acid, sodium dodecyl sulphate, Sodium lignosulfonate, graphene oxide (GO), Fe | 3 | O | 4 | , Fe, TiO | 2 | , Mesoporous silica structures (MCM-48), silicate rectorite, zeolite, succinic anhydride, maleic anhydride, itaconic acid, trans-aconitic acid, biochar | Congo Red (CR), Methyl Orange (MO), Methylene Blue (MB), Bromocresol Green (BCG), Reactive Black 5 (RB5), Acid blue-113, viruses, Fe(II), Fe(III), Cu(II), Ni(II), Co(II), Cr(III), Cr(VI), Zn(II) As(III), As(V), Cd(II), Pb(II), diclofenac, ciprofloxacin | [13,20,21,22,23,24,25,26,27,28,48, | [13][20] | 29, | [ | 30,31, | 21][ | 32, | 26][27][28][29][30][31][ | 33,34,35,36,37,38, | 32 | 39,40, | ] | 41,42, | [33] | 43, | [34] | 44, | [ | 45,46, | 35[39 | 47, | ][40][41][ | 49, | ]42][43][44 | 50, | ][45][46][47][48][49][50 | 51, | [22] | 52, | [23]][36][ | 53, | [24] | 54, | [ | 55,56, | 25 | 57] | 37][38]][51][52][53][54][55][56][57] |
| Quaternized chitosan (QCS) | Chitosan, 3-chloro-2-hydroxypropyl trimethyl ammonium chloride, Fe | 3 | O | 4 | , GO | MO, CR, Cu(II), Fe(III), Cr(VI) | [20,58, | 20][58] | 59, | [59] | 60] | [[60] | ||||||||||||||||||||||||||||||||||||||
| Sodium alginate (SA) | Activated carbon, bentonite, activated organo-bentonite, carboxy carbon nanotubes, pillared clay, Mauritanian clay, organo-illite/smectite clay, montmorillonite, nano-hydroxyapatite, carboxymethyl cellulose, microcrystalline cellulose, polyaniline, poly(acrylic acid) glutaraldehyde, Poly(hydroxybutyrate, biochar, CS, GO, Zr(IV), Fe | 3 | O | 4 | , MgAl-layered double hydroxide, SiO | 2 | , aluminum-based metal organic framework and chitosan | Nitrophenol, Pentachlorophenol, polychlorinated biphenyl, crystal violet (CV), MB, MO, As(V), Cu(II), Pb(II), Cd(II), Fe(III), F | - | , Cr(VI), bisphenol A | [14,33,56,59,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,] | [14] | 78, | [33] | 79, | [ | 80,81, | 56][59][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81] | 82 | [82] | ||||||||||||||||||||||||||||||
| Carboxyalkyl chitosan (CCS) | CS, salecan, citric acid, Fe | 3 | O | 4 | , SiO | 2 | , Cu(II), Al(III), hexamethylenediisocyanate | As(III), As(V), Ni(II), Pb(II), ciprofloxacin | [52,83,84,85] | [52][83][84][85] | ||||||||||||||||||||||||||||||||||||||||
| Modified Poly(Cyclodextrin) | 2,4-toluene diisocyanate, 1,6-hexamethylene diisocyanate, montmorillonite | 2,4-dinitrophenol, bisphenol A | [86,87] | [86][87] | ||||||||||||||||||||||||||||||||||||||||||||||
| Poly(ethyleneimine) (PEI) | Chitosan, Epichlorohydrin (ECH), Poly(acrylic acid) (PAA), poly(vinyl amine), Poly(ethylene glycol) diglycidyl ether, diglycidyl ether of 1,4-butandiol, PS nanoparticles, montmorillonite, cellulose acetate, diatomaceous earth, bacteria, SiO | 2 | , CaCO | 3 | , Fe | 3 | O | 4 | Formaldehyde, CR, BCG, Rhodamine B, Hg(II), UO | 2 | (II), Cd(II), Zn(II), Cu(II), Ni(II), As(III), Mn(II), Cr(III), Cr(VI), Co(II), Fe(II), Pb(II), Zn(II) | [24,36,88,89, | 89 | 90,91, | ][90 | 92, | ] | 93,94, | [91] | 95, | [ | 96,97, | 92][93][94][95][96 | 98,99,100,101,102,103] | [24][36][88][][97][98][99][100][101][102][103] | |||||||||||||||||||||||||
| Carboxymethyl cellulose (CMC) | SA, SiO | 2 | , | CR, MO, MB, BCG, Pb(II) | [24, | [24 | 79] | ][79] | ||||||||||||||||||||||||||||||||||||||||||
| Cationic cellulose | Cellulose nanocrystals, wood pulp | MB | [104] | |||||||||||||||||||||||||||||||||||||||||||||||
| Poly(sodium 4-styrene sulfonate) (PSS) | Octacalcium phosphate, gelatin, pineapple leaf fiber, ZnO, Fe | 3 | O | 4 | Cu(II), Cd(II), tetracycline, CR, MB | [105,106, | 105][106] | 107, | [107] | 108] | [[108] | |||||||||||||||||||||||||||||||||||||||
| Poly(vinyl amine) (PVAm) | CS, PEI | CR, BCG, Cu(II) | [54,109] | [54][109] | ||||||||||||||||||||||||||||||||||||||||||||||
| Poly(acrylic acid) (PAA) | PEI, CS, Nano ferrous sulfide, SA | Cd(II), Cr(III), Cr(IV), Cr(VI), Cu(II) | [49,71,97] | [49][71][97] | ||||||||||||||||||||||||||||||||||||||||||||||
| Carrageenan | chitosan, hybrid siliceous shells | CR, MB, metoprolol | [31,110] | [31][110] | ||||||||||||||||||||||||||||||||||||||||||||||
| Anionic polyacrylamide | Kaolinite, montmorillonite, xanthan gum, SiO | 2 | Cr(IV), Pb(II), oil | [111,112,113,114] | [111][112][113][114] | |||||||||||||||||||||||||||||||||||||||||||||
| Poly(allylamine hydrochloride) (PAH) | GO, diglycidyl ether of 1,4-butandiol | Cr(VI), Cu(II), Co(II), Zn(II), Ni(II) | [93,115,116] | [93][115][116] | ||||||||||||||||||||||||||||||||||||||||||||||
| Poly(2-acrylamido-2-methylpropane sulfonic acid) (PAMPS) | Ti | 3 | C | 2 | -MXenes, methacrylic acid, 2-hydroxyethyl-methacrylate, gelatin, Fe | 3 | O | 4 | , CuO with chitosan, alumina | MB, Hg(II), Cu(II), Cd(II), Ni(II), Pb(II), Zn(II), doxycycline, ciprofloxacin | [106,117,118,119,120,121] | [106][117][118][119][120][121] | ||||||||||||||||||||||||||||||||||||||
| Poly{[2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide- | co | -acrylic acid} (PDMAPS- | co | -AA) | Ti | 3 | C | 2 | -MXenes | MB | [117] | |||||||||||||||||||||||||||||||||||||||
| Poly(diallyldimethylammonium chloride) (PDDA) | pineapple leaf fiber, ZnO | CR | [107] | |||||||||||||||||||||||||||||||||||||||||||||||
| Quaternized4-vinyl-pyridine- | co | -acrylamide (QVP- | co | -AAm) | Fe | 3 | O | 4 | CR | [122] | ||||||||||||||||||||||||||||||||||||||||
| Humic acid (HA) | Fe | 3 | O | 4 | Cu(II) | [123] | ||||||||||||||||||||||||||||||||||||||||||||
| Poly{[2-(Methacryloyloxy)ethyl]trimethylammonium chloride} | N,N′ -Methylenebisacrylamide | Orange II, Remazol Brilliant Blue R | [124] |
The overview in Table 1 suggests the predominant use of heavy metals and dyes (HM&D) as models for inorganic and organic aqueous pollutants, respectively. From our testing experience, these pollutants present certain advantages as compared to more complex molecules, like emerging pollutants:
-
chemical structure—HM&D are usually present in solution in ionic form and have smaller molecular weights, allowing them to diffuse more easily through the sorbent’s pores and reach the active sites;
-
range of initial concentration during adsorption tests—HM&D are used up to hundreds of mg/L, while EPs usually are at most in the first few tens of mg/L;
-
analysis equipment—HM&D can be determined by using more affordable, less time consuming and potentially less sophisticated equipment (e.g., UV-Vis spectrophotometer, atomic absorption spectrometer).
Every sorption process of the active species implies physical (transport, flow, swelling, diffusion etc.) and chemical (interactions, immobilization) aspects. First of all, the pollutant molecules, dissolved in a certain medium, must be transported near to the active site of the composite sorbent. Then, based on physico-chemical interactions between sorbate and sorbent, the sorption process can take place. Therefore, the shape and size of each composite sorbent at macro/nanoscale will dictate the active specific surface available for subsequent chemical interactions. The swelling will influence the diffusion of sorbate through the dynamic pores and the composite particle size will dictate the potential applications at laboratory or industrial scale. For example, composites larger than 10 microns could be used in a column experimental set-up.
Thus, in this study all composites based on polyelectrolytes have been divided according to their macroscopic shape: (1) Beads—high porous materials with high accessibility to the functional moieties; (2) Core-shell, “hard-soft” composites with high surface area; (3) Gels (hydrogels, cryogel monoliths, sponges), stimuli-responsive systems with high surface area; (4) Nanofibers, presenting good mechanical properties with high specific area and (5) Membranes, with sorption and separation in one step (Figure 32).
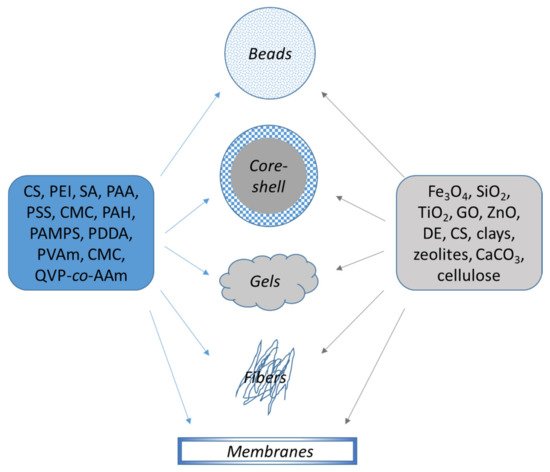

Figure 32. Organic/inorganic composite sorbents based on polyelectrolytes.
The use of inorganic/organic or organic/organic composites with polyelectrolyte(s) as active part of the sorbent proved to be of high interest for immobilization of different pollutant types from aqueous environment, due to the large variety and high numbers of functional groups (carboxylic, sulphonic, amine, imine, hydroxyl) [125]. Immobilization efficiency and selectivity through complexation, ion exchange, electrostatic and hydrophobic interactions were linked to the nature of both functional groups and pollutants.
The nature of composite components, the cross-linking density of the synthesized material and the polyelectrolyte architecture significantly influence the sorption properties and kinetics. Thus, after the physical state of composites (shape, size, density, cross-linking degree etc.), the chemical interactions will be the second important parameter that dictates the material capacity for pollutant detection, immobilization, concentration in solid phase and subsequent releasing for a new starting sorption cycle. The sorption process of inorganic (Me2+) or organic pollutants (dyes, pharmaceuticals etc.) are driven mainly by electrostatic interactions, coordinative bonds formation, dipole-dipole or Yoshida H-bonding interactions, and ion exchange interactions. The schematic diagram of all these interactions is presented in Figure 43.
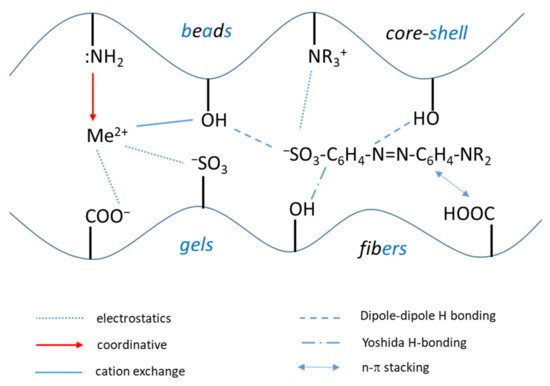

Figure 43. Principal types of interactions between inorganic (Me2+) and organic pollutants with composite polyelectrolyte sorbents functional groups.
This review brings a clear outlook on the benefits of using different moieties types as active sites of composites based polyelectrolytes for the removal by sorption mechanism of a wide range of toxic, undesired pollutants dissolved in water and wastewaters. The following subsections will be in accordance with the physical state of composite materials, such as beads, core-shells, gels, fibers and membranes, where the chemistry behind each pollutant retention is based only on sorptive mechanisms.
3.2. Beads Composites Based on Polyelectrolytes
2.2. Beads Composites Based on Polyelectrolytes
In the last years, numerous studies have been focused towards combination between organic polymers and organic/inorganic entities (see Table 1) due to their structural diversity, which can be successfully used in removal of inorganic/organic pollutants dissolved in aqueous media. Sorption processes in water treatment could require granular materials with certain size and, thus, the beads/microbeads composites based on polyelectrolytes could widen the possibilities of pollutants extraction in solid phases. Novel binary/ternary beads composites, where polyelectrolyte chain is the main component, could be mainly obtained by: (a) combination of CS with organic species (e.g., starches-g-polyacrylonitrile [53], carrageenan [31], PVAm [54], PEI [55], microcrystalline cellulose [56], carboxymethyl-β-cyclodextrin [42], QCS [20[20][60],60], citrate [52]) and/or inorganic (e.g., Fe3O4 [52], Fe [42]), (b) combination of SA with organic/inorganic entities, such as activated carbon [77], CMC [79], polyaniline [61], different types of clays (bentonite [63,78,80][63][78][80], ilite [76], kaolinite [76], montmorillonite [68,81,82][68][81][82], Fe3O4 [59[59][64][67],64,67], SiO2 [65], hydroxyapatite [66]), and (c) combination of other types of polyelectrolytes (PEI, PAA) [97] to form interpolyelectrolyte composite beads. The physico-chemical integrity of combined architectures inside the bead composite must be kept under different environmental conditions (pH, ionic strength, temperature, magnetic field, etc.); therefore, the cross-linking of organic/inorganic components is very important for beads stability/integrity and functional groups subsequent accessibility by the pollutant molecule. The cross-linking could be carried out in situ (emulsification method) during the bead formation or after the coagulation/precipitation or frozen of composite beads. The most important cross-links could be achieved by covalent or ionic cross-linking with bifunctional compounds (e.g., glutaraldehyde [53[53][62],62], ECH [20,31,54,55,96][20][31][54][55][96], poly(ethyleneglycol diglycidyl ether) [53], carbodiimide [42[42][52],52], 3-chloro-2-hydroxypropyl trimethyl ammonium chloride [60] etc.) and small ions (ionic gelation method), respectively, including Ca2+ [56,61,63,64,65,66[56][61][63][64][65][66][68][76][77][78][80][82],68,76,77,78,80,82], Ce3+ [67], Zr4+ [59], Fe3+), tripolyphoshate [54]. Bifunctional reagents (glutaraldehyde, ECH etc.) can react with primary amino groups (CS, PEI, PVAm) to form covalent cross-links, while small ions such as Ca2+, Fe3+, or Zr4+ can ionically interact with two, three, or four carboxyl groups of SA.
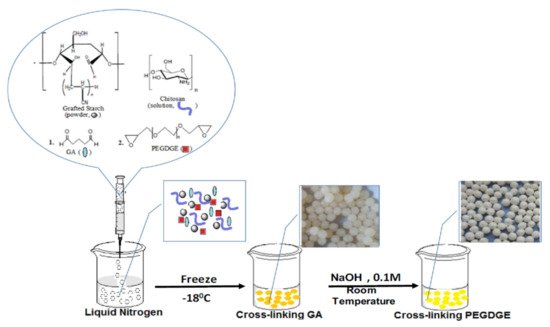
Dragan and Apopei Loghin [53] obtained biosorbents cryobeads from chitosan and starch, using glutaraldehyde and poly(ethylene glycol diglycidyl ether) as dual cross-linkers (Figure 54). It was shown that these composite cryobeads kept their integrity and sorption capacity toward three heavy metal ions (Cu2+, Ni2+ and Co2+) during five sorption/desorption cycles.

Numerous authors obtained composite beads with magnetic properties by embedding Fe3O4 or Fe inside the polymeric matrix, which can be CS/carrageenan [31], CS/carboxymethyl-β-cyclodextrin [42], carboxymethyl chitosan/chitosan/citrate [52], calcium alginate [64], cerium alginate [67] etc., feasible to be used both in batch and column sorption studies.
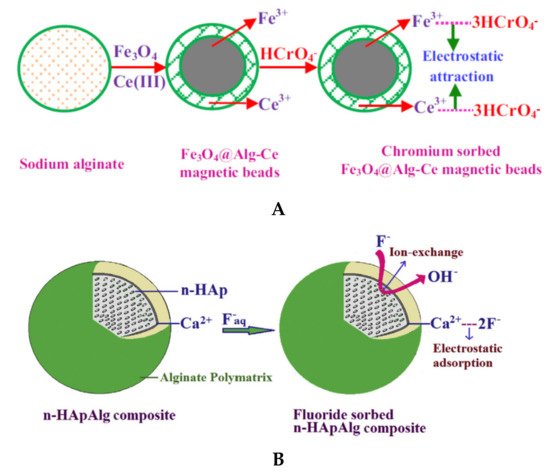
Magnetic composites beads based on natural polyelectrolytes have attracted the scientists’ attention due to the high sorption/selectivity capacity. Liang and co-workers [31] obtained composite beads with magnetic responsiveness, showing high sorption efficiency toward dyes (MB and CR) and heavy metal ions (Cu2+ and Cr3+). Gopalakannan and Viswanathan [67] obtained magnetic alginate composites beads by incorporating Fe3O4 into SA network followed by Ce3+ ionic gelation (Figure 65). The synthesized beads presented higher sorption capacity for chromate ions (14.29 mg/g) compared with beads without Fe3O4 (9.45 mg/g) and single Fe3O4 particles (9.72 mg/g). In this study it was shown the spontaneous and endothermic nature of chromium sorption.

To improve the mechanical stability and porosity/accessibility inside the composite beads, different types of clays (bentonite, kaolinite, montmorillonite), SiO2, hydroxyapatite etc., have been included during the beads synthesis [63,65[63][65][66][68][76][80],66,68,76,80], where the inorganic component could act as a cross-linker agent together with small ions, such as Ca2+. Pandi and Viswanathan [66] showed that defluorination capacity of hydroxyapatite (1296 mg F−/kg) and SA beads (680 mg F−/kg) increased to 3870 mg F−/kg for SA/hydroxyapatite composite beads. Belhouchat and co-workers [63] used bentonite to obtain SA/bentonite composite beads with high sorption capacity for anionic dyes, such as MB and MO. The authors observed that both anionic and cationic dyes could be immobilized by SA composite beads with bentonite inside. The MO sorption increased with bentonite content of SA composite, while MB sorption decreased.
Uyar and co-workers [82] demonstrated that the drying method of composite beads could have a significant influence onto subsequent sorption of different types of pollutants. Composites that were deep-freezed at −21 °C presented a drastically modified morphology of beads and improved surface area and sorption capacity, compared with beads dried at room temperature. The pollutants sorption capacity and selectivity of synthesized composite beads could be drastically improved by subsequent grafting of different small molecules [98] or polymers (PEI [55], polyaniline [61]) onto the solid beads surface. The PEI beads modified with 3-chloropropanesulfonyl chloride exhibited high removal percentage for Hg2+ (>87%) and high selectivity in the presence of competing ions (Mn2+, Ni2+, Fe2+, Pb2+, Zn2+, and Cr3+) [98].
3.3. Core-Shell Composites
2.3. Core-Shell Composites
Many scientists conducted pollutant sorption studies on different types of small inorganic (SiO2, TiO2, Fe3O4, clays, minerals, etc.) and organic (active carbon, GO, biochar) solid surfaces [126]. The pollutant immobilization on a certain surface strongly depends on the nature, concentration, distribution and accessibility of material functional groups. Inorganic sorbents present very good kinetics but low pollutant sorption capacity relative to sorbent amount. Polyelectrolytes, with high number of functional groups on unit mass, present very high sorption capacities but low kinetics due to slow diffusion in the collapsed state.
The core-shell architecture could be achieved by direct deposition of different types of natural/synthetic polyelectrolytes (CS, PEI, humic acid, PAH, PVAm, PAA, PSS etc.) on 2D-cores, such as GO [21,22,23,25,115,116][21][22][23][25][115][116] and Ti3C2-MXenes [117], or 3D-cores, including Fe3O4 [57[57][108][122][123][126][127],108,122,123,126,127], SiO2 [24,28,83,90,100[24][28][83][90][100][101][103][109],101,103,109], SiO2/Fe3O4 [88[88][128],128], PS [91], FeS [129], clays [99[99][111][112],111,112], CaCO3 [102], mesoporous diatomite [89], natural fibers [107] and sand [123]. The direct deposition of the organic or organic/inorganic “shell” onto inorganic or organic “core” could be carried out in (i) one-step, by physisorption [21,89,108,111,112][21][89][108][111][112], grafting [23[23][83][88][100][115][117],83,88,100,115,117], ionic or solvent gelation/precipitation [25,27,57,69,128][25][27][57][69][128] or (ii) a multi-step procedure, such as layer-by-layer [24,90,101,102,103,109][24][90][101][102][103][109]. Using a strong polyelectrolyte (PSS), Chong and co-workers [108] obtained a stable magnetite nanoparticles with excellent dye removal efficiency (~94%). By simple PSS sorption onto Fe3O4, the dynamic light scattering and electrophoretic measurements showed a constant hydrodynamic diameter of 150 nm of the magnetic composites over 5 h measuring. This stable dispersion had 50% higher dye sorption capacity compared with bare magnetite nanoparticles. Also, Yao and co-workers [129] obtained stable core-shell colloids (~65–90 nm) based on FeS and PAA with increased sorption properties for Cr6+ sorption compared with unmodified FeS. Using direct deposition, by pH inversion precipitation of carboxymethylchitosan onto unmodified and modified silica particles, Aden and collaborators [83] obtained core-shell composites with excellent sorption properties for Ni2+ (Figure 76). After drying at 100 °C for 24 h, the composite particles were used without further modification steps as Ni2+ ion sorbent at pH 5 and 7.
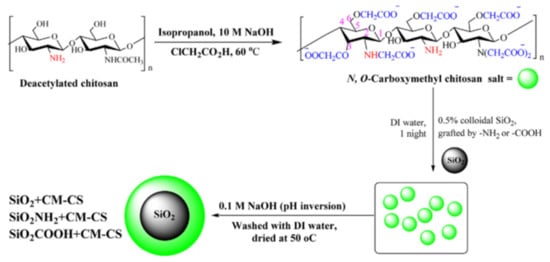

Figure 76. Synthesis of a core-shell composite based on carboxymethylchitosan (CM-CS) and silica particles (reprinted with permission from ref. [83]).
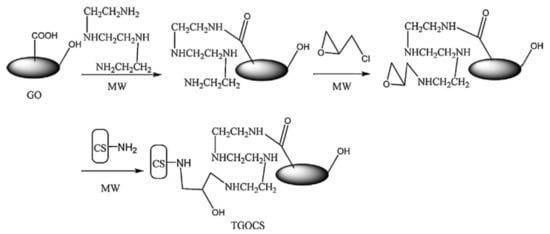
Sometimes, due to harsh environment conditions (extreme pH, high ionic strength, temperature, etc.), which can delaminate the “shell” of composite, the direct grafting of polymeric chains to the solid “core” or “shell” cross-linking after or during deposition, must be carried out. For more stable composites over wide ranges of environment stimuli, many authors anchor polyelectrolyte chains covalently to the inorganic core, which contains a linker molecule on surface [88,100,115][88][100][115]. In this way, the new created core-shell composite is more stable in aqueous media and more effective in sorption of different pollutants. The immobilization of “shell” around the “core” could be achieved by chemical cross-linking with bifunctional compounds, such as glutaraldehyde [89[89][102][103][109][115],102,103,109,115], epichlorohydrin [23], α,α’-dichloro-p-xylene [24], phtaldialdehyde [91]. Ge and Ma [23] obtained CS/GO composite microparticles by microwave irradiation method using GO, triethylenetetraamine, ECH and CS (Figure 87). Sorption of Cr(VI) onto composite particles showed high values, reaching 219 mg/g at pH 2. The results obtained by batch experiments showed that sorption capacity of synthesized composite increased with temperature and the sorbent material could be recyclable.
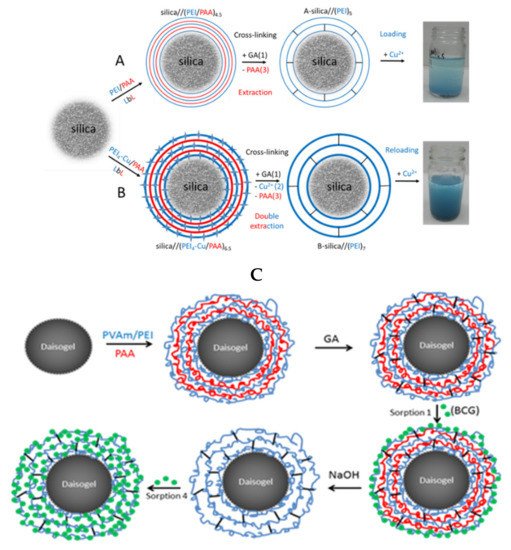
Bucatariu and co-workers used selective cross-linking to obtain core-shell silica composites based on PEI, PVAm, polylysine, and PAH. This type of composites has been utilized in the removal of dyes [109] and heavy metal ions [90,101,103][90][101][103] from real and simulated waters. The layer-by-layer technique involved in these studies allowed a controlled deposited polyelectrolyte amount onto spherical silica particles. Furthermore, the subsequent glutaraldehyde cross-linking stabilized the polycation layer onto each individual solid particle. To increase the multilayer flexibility and functional groups accessibility toward pollutant molecules, the polyanion has been removed from the cross-linked multilayer in extreme basic medium, as it can be seen in Figure 98. The fast kinetic of Cu2+ sorption and high sorbed amount of anionic dyes (BCG, CR) after polyanion extraction, confirmed the polyelectrolyte multilayer stability and flexibility after cross-linking and polyanion extraction. Based on distribution parameter and relative atomic concentration of elements on surface, the authors demonstrated that silica/(PEI)10 composite particles can clean (>95%) a simulated water contaminated with four heavy metal ions (Cu2+, Co2+, Ni2+, Cd2+), if the ratio between number of composite functional groups and number of ions is higher than ~9 (non-competitive regime). The competitive sorption between different metal ions showed that the composite had high selectivity for Cu2+. Subsequent chemical modification of stable cross-linked core-shell composites with small molecules, i.e., disodium ethylenediamine tetraacetate (EDTA-2Na), increased the sorption capacities by creation of new functional groups onto core-shell surface.
References
- Teodosiu, C.; Gilca, A.-F.; Barjoveanu, G.; Fiore, S. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. J. Clean. Prod. 2018, 197, 1210–1221.
- Changing How We Produce and Consume: New Circular Economy Action Plan Shows the Way to a Climate-Neutral, Competitive Economy of Empowered Consumers. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_20_420 (accessed on 4 October 2021).
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A review on wastewater sludge valorisation and its challenges in the context of circular economy. J. Clean. Prod. 2019, 228, 244–263.
- Directive 2008/105/CE. Available online: https://eur-lex.europa.eu/eli/dir/2008/105/oj (accessed on 31 September 2021).
- Decision 2015/495/EU. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2015.078.01.0040.01.ENG (accessed on 31 September 2021).
- Decision 2018/840/EU. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018D0840 (accessed on 31 September 2021).
- Bui, X.T.; Vo, T.P.T.; Ngo, H.H.; Guo, W.S.; Nguyen, T.T. Multicriteria assessment of advanced treatment technologies for micropollutants removal at large-scale applications. Sci. Total Environ. 2016, 563–564, 1050–1067.
- Shah, A.I.; Din Dar, M.U.; Bhat, R.A.; Singh, J.P.; Singh, K.; Bhat, S.A. Prospectives and challenges of wastewater treatment technologies to combat contaminants of emerging concerns. Ecol. Eng. 2020, 152, 105882.
- Yu, G.; Wang, X.; Liu, J.; Jiang, P.; You, S.; Ding, N.; Guo, Q.; Lin, F. Applications of Nanomaterials for Heavy Metal Removal from Water and Soil: A Review. Sustainability 2021, 13, 713.
- Cavallaro, G.; Micciulla, S.; Chiappisi, L.; Lazzara, G. Chitosan-based smart hybrid materials: A physico-chemical perspective. J. Mater. Chem. B 2021, 9, 594–611.
- Mohapi, M.; Sefadi, J.S.; Mochane, M.J.; Magagula, S.I.; Lebelo, K. Effect of LDHs and Other Clays on Polymer Composite in Adsorptive Removal of Contaminants: A Review. Crystals 2020, 10, 957.
- Zheng, B.; Lin, X.; Zhang, X.; Wu, D.; Matyjaszewski, K. Emerging Functional Porous Polymeric and Carbonaceous Materials for Environmental Treatment and Energy Storage. Adv. Funct. Mater. 2020, 30, 1907006.
- Chen, S.; Yang, K.; Leng, X.; Chen, M.; Novoselov, K.S.; Andreeva, D.V. Perspectives in the design and application of composites based on graphene derivatives and bio-based polymers. Polym. Int. 2020, 69, 1173–1186.
- Luo, J.; Huang, Z.; Liu, L.; Wang, H.; Ruan, G.; Zhao, C.; Du, F. Recent advances in separation applications of polymerized high internal phase emulsions. J. Sep. Sci. 2021, 44, 169–187.
- Abu Elella, M.H.; Goda, E.S.; Gab-Allah, M.A.; Hong, S.E.; Pandit, B.; Lee, S.; Gamal, H.; Rehman, A.U.; Yoon, K.R. Xanthan gum-derived materials for applications in environment and eco-friendly materials: A review. J. Environ. Chem. Eng. 2021, 9, 104702.
- Potaufeux, J.-E.; Odent, J.; Notta-Cuvier, D.; Lauro, F.; Raquez, J.-M. A comprehensive review of the structures and properties of ionic polymeric materials. Polym. Chem. 2020, 11, 5914–5936.
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456.
- Ghiorghita, C.-A.; Mihai, M. Recent developments in layer-by-layer assembled systems application in water purification. Chemosphere 2021, 270, 129477.
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, É.; Mangin, D.; Elaissari, A. pH-sensitive polymers: Classification and some fine potential applications. Polym. Adv. Technol. 2021, 32, 1455–1484.
- Cai, L.; Ying, D.; Liang, X.; Zhu, M.; Lin, X.; Xu, Q.; Cai, Z.; Xu, X.; Zhang, L. A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem. Eng. J. 2021, 410, 128404.
- Zhang, L.; Luo, H.; Liu, P.; Fang, W.; Geng, J. A novel modified graphene oxide/chitosan composite used as an adsorbent for Cr(VI) in aqueous solutions. Int. J. Biol. Macromol. 2016, 87, 586–596.
- Menazea, A.A.; Ezzat, H.A.; Omara, W.; Basyouni, O.H.; Ibrahim, S.A.; Mohamed, A.A.; Tawfik, W.; Ibrahim, M.A. Chitosan/graphene oxide composite as an effective removal of Ni, Cu, As, Cd and Pb from wastewater. Comput. Theor. Chem. 2020, 1189, 112980.
- Ge, H.; Ma, Z. Microwave preparation of triethylenetetramine modified graphene oxide/chitosan composite for adsorption of Cr(VI). Carbohydr. Polym. 2015, 131, 280–287.
- Ghiorghita, C.A.; Bucatariu, F.; Dragan, E.S. Novel silica/polyelectrolyte multilayer core-shell composite microparticles with selectivity for anionic dyes. Cellul. Chem. Technol. 2018, 52, 663–672.
- Yan, H.; Yang, H.; Li, A.; Cheng, R. pH-tunable surface charge of chitosan/graphene oxide composite adsorbent for efficient removal of multiple pollutants from water. Chem. Eng. J. 2016, 284, 1397–1405.
- Wang, Y.; Li, L.; Luo, C.; Wang, X.; Duan, H. Removal of Pb2+ from water environment using a novel magnetic chitosan/graphene oxide imprinted Pb 2+. Int. J. Biol. Macromol. 2016, 86, 505–511.
- Hosain, A.N.A.; El Nemr, A.; El Sikaily, A.; Mahmoud, M.E.; Amira, M.F. Surface modifications of nanochitosan coated magnetic nanoparticles and their applications in Pb(II), Cu(II) and Cd(II) removal. J. Environ. Chem. Eng. 2020, 8, 104316.
- Jiang, Y.; Abukhadra, M.R.; Refay, N.M.; Sharaf, M.F.; El-Meligy, M.A.; Awwad, E.M. Synthesis of chitosan/MCM-48 and β-cyclodextrin/MCM-48 composites as bio-adsorbents for environmental removal of Cd2+ ions; kinetic and equilibrium studies. React. Funct. Polym. 2020, 154, 104675.
- Habiba, U.; Siddique, T.A.; Joo, T.C.; Salleh, A.; Ang, B.C.; Afifi, A.M. Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo red and chromium(VI) by flocculation/adsorption. Carbohydr. Polym. 2017, 157, 1568–1576.
- Humelnicu, D.; Dragan, E.S.; Ignat, M.; Dinu, M.V. A Comparative Study on Cu2+, Zn2+, Ni2+, Fe3+, and Cr3+ Metal Ions Removal from Industrial Wastewaters by Chitosan-Based Composite Cryogels. Molecules 2020, 25, 2664.
- Liang, X.; Duan, J.; Xu, Q.; Wei, X.; Lu, A.; Zhang, L. Ampholytic microspheres constructed from chitosan and carrageenan in alkali/urea aqueous solution for purification of various wastewater. Chem. Eng. J. 2017, 317, 766–776.
- Humelnicu, D.; Lazar, M.M.; Ignat, M.; Dinu, I.A.; Dragan, E.S.; Dinu, M.V. Removal of heavy metal ions from multi-component aqueous solutions by eco-friendly and low-cost composite sorbents with anisotropic pores. J. Hazard. Mater. 2020, 381, 120980.
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020, 393, 124728.
- Ramakrishnan, R.K.; Padil, V.V.T.; Wacławek, S.; Černík, M.; Varma, R.S. Eco-Friendly and Economic, Adsorptive Removal of Cationic and Anionic Dyes by Bio-Based Karaya Gum—Chitosan Sponge. Polymers 2021, 13, 251.
- Dinu, M.V.; Dragan, E.S. Evaluation of Cu2+, Co2+ and Ni2+ ions removal from aqueous solution using a novel chitosan/clinoptilolite composite: Kinetics and isotherms. Chem. Eng. J. 2010, 160, 157–163.
- You, L.; Huang, C.; Lu, F.; Wang, A.; Liu, X.; Zhang, Q. Facile synthesis of high performance porous magnetic chitosan-polyethylenimine polymer composite for Congo red removal. Int. J. Biol. Macromol. 2018, 107, 1620–1628.
- Ghiorghita, C.-A.; Borchert, K.B.L.; Vasiliu, A.-L.; Zaharia, M.-M.; Schwarz, D.; Mihai, M. Porous thiourea-grafted-chitosan hydrogels: Synthesis and sorption of toxic metal ions from contaminated waters. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125504.
- Pan, J.; Zhu, J.; Cheng, F. Preparation of Sodium Lignosulfonate/Chitosan Adsorbent and Application of Pb2+ Treatment in Water. Sustainability 2021, 13, 2997.
- Milosavljević, N.B.; Ristić, M.Đ.; Perić-Grujić, A.A.; Filipović, J.M.; Štrbac, S.B.; Rakočević, Z.L.; Krušić, M.T.K. Removal of Cu2+ ions using hydrogels of chitosan, itaconic and methacrylic acid: FTIR, SEM/EDX, AFM, kinetic and equilibrium study. Colloids Surf. A Physicochem. Eng. Asp. 2011, 388, 59–69.
- Razzaz, A.; Ghorban, S.; Hosayni, L.; Irani, M.; Aliabadi, M. Chitosan nanofibers functionalized by TiO2 nanoparticles for the removal of heavy metal ions. J. Taiwan Inst. Chem. Eng. 2016, 58, 333–343.
- Tu, H.; Huang, M.; Yi, Y.; Li, Z.; Zhan, Y.; Chen, J.; Wu, Y.; Shi, X.; Deng, H.; Du, Y. Chitosan-rectorite nanospheres immobilized on polystyrene fibrous mats via alternate electrospinning/electrospraying techniques for copper ions adsorption. Appl. Surf. Sci. 2017, 426, 545–553.
- Tajuddin Sikder, M.; Tanaka, S.; Saito, T.; Kurasaki, M. Application of zerovalent iron impregnated chitosan-caboxymethyl-β-cyclodextrin composite beads as arsenic sorbent. J. Environ. Chem. Eng. 2014, 2, 370–376.
- Karim, M.R.; Aijaz, M.O.; Alharth, N.H.; Alharbi, H.F.; Al-Mubaddel, F.S.; Awual, M.R. Composite nanofibers membranes of poly(vinyl alcohol)/chitosan for selective lead(II) and cadmium(II) ions removal from wastewater. Ecotoxicol. Environ. Saf. 2019, 169, 479–486.
- Li, L.; Li, Y.; Cao, L.; Yang, C. Enhanced chromium (VI) adsorption using nanosized chitosan fibers tailored by electrospinning. Carbohydr. Polym. 2015, 125, 206–213.
- Li, C.; Lou, T.; Yan, X.; Long, Y.; Cui, G.; Wang, X. Fabrication of pure chitosan nanofibrous membranes as effective absorbent for dye removal. Int. J. Biol. Macromol. 2018, 106, 768–774.
- Haider, S.; Ali, F.A.A.; Haider, A.; Al-Masry, W.A.; Al-Zeghayer, Y. Novel route for amine grafting to chitosan electrospun nanofibers membrane for the removal of copper and lead ions from aqueous medium. Carbohydr. Polym. 2018, 199, 406–414.
- Chen, S.; Li, C.; Hou, T.; Cai, Y.; Liang, L.; Chen, L.; Li, M. Polyhexamethylene guanidine functionalized chitosan nanofiber membrane with superior adsorption and antibacterial performances. React. Funct. Polym. 2019, 145, 104379.
- Li, L.; Zhang, J.; Li, Y.; Yang, C. Removal of Cr (VI) with a spiral wound chitosan nanofiber membrane module via dead-end filtration. J. Memb. Sci. 2017, 544, 333–341.
- Jiang, M.; Han, T.; Wang, J.; Shao, L.; Qi, C.; Zhang, X.M.; Liu, C.; Liu, X. Removal of heavy metal chromium using cross-linked chitosan composite nanofiber mats. Int. J. Biol. Macromol. 2018, 120, 213–221.
- Tzereme, A.; Christodoulou, E.; Kyzas, G.Z.; Kostoglou, M.; Bikiaris, D.N.; Lambropoulou, D.A. Chitosan grafted adsorbents for diclofenac pharmaceutical compound removal from single-component aqueous solutions and mixtures. Polymers 2019, 11, 497.
- Afzal, M.Z.; Sun, X.-F.; Liu, J.; Song, C.; Wang, S.-G.; Javed, A. Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Sci. Total Environ. 2018, 639, 560–569.
- Mi, F.-L.; Wu, S.-J.; Chen, Y.-C. Combination of carboxymethyl chitosan-coated magnetic nanoparticles and chitosan-citrate complex gel beads as a novel magnetic adsorbent. Carbohydr. Polym. 2015, 131, 255–263.
- Dragan, E.S.; Loghin, D.F.A. Fabrication and characterization of composite cryobeads based on chitosan and starches-g-PAN as efficient and reusable biosorbents for removal of Cu2+, Ni2+, and Co2+ ions. Int. J. Biol. Macromol. 2018, 120, 1872–1883.
- Dragan, E.S.; Cocarta, A.I.; Dinu, M.V. Facile fabrication of chitosan/poly(vinyl amine) composite beads with enhanced sorption of Cu2+. Equilibrium, kinetics, and thermodynamics. Chem. Eng. J. 2014, 255, 659–669.
- Chatterjee, S.; Chatterjee, T.; Woo, S.H. Influence of the polyethyleneimine grafting on the adsorption capacity of chitosan beads for Reactive Black 5 from aqueous solutions. Chem. Eng. J. 2011, 166, 168–175.
- Vijayalakshmi, K.; Gomathi, T.; Latha, S.; Hajeeth, T.; Sudha, P.N. Removal of copper(II) from aqueous solution using nanochitosan/sodium alginate/microcrystalline cellulose beads. Int. J. Biol. Macromol. 2016, 82, 440–452.
- Zhang, H.; Tan, X.; Qiu, T.; Zhou, L.; Li, R.; Deng, Z. A novel and biocompatible Fe3O4 loaded chitosan polyelectrolyte nanoparticles for the removal of Cd2+ ion. Int. J. Biol. Macromol. 2019, 141, 1165–1174.
- Bai, B.; Mi, X.; Xiang, X.; Heiden, P.A.; Heldt, C.L. Non-enveloped virus reduction with quaternized chitosan nanofibers containing graphene. Carbohydr. Res. 2013, 380, 137–142.
- Li, X.; Qi, Y.; Li, Y.; Zhang, Y.; He, X.; Wang, Y. Novel magnetic beads based on sodium alginate gel crosslinked by zirconium(IV) and their effective removal for Pb2+ in aqueous solutions by using a batch and continuous systems. Bioresour. Technol. 2013, 142, 611–619.
- Ke, P.; Zeng, D.; Xu, K.; Cui, J.; Li, X.; Wang, G. Preparation of Quaternary Ammonium Salt-Modified Chitosan Microspheres and Their Application in Dyeing Wastewater Treatment. ACS Omega 2020, 5, 24700–24707.
- Jiang, N.; Xu, Y.; Dai, Y.; Luo, W.; Dai, L. Polyaniline nanofibers assembled on alginate microsphere for Cu2+ and Pb2+ uptake. J. Hazard. Mater. 2012, 215–216, 17–24.
- Lu, T.; Xiang, T.; Huang, X.-L.; Li, C.; Zhao, W.-F.; Zhang, Q.; Zhao, C.-S. Post-crosslinking towards stimuli-responsive sodium alginate beads for the removal of dye and heavy metals. Carbohydr. Polym. 2015, 133, 587–595.
- Belhouchat, N.; Zaghouane-Boudiaf, H.; Viseras, C. Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Appl. Clay Sci. 2017, 135, 9–15.
- Sun, J.; Chen, Y.; Yu, H.; Yan, L.; Du, B.; Pei, Z. Removal of Cu2+, Cd2+ and Pb2+ from aqueous solutions by magnetic alginate microsphere based on Fe3O4/MgAl-layered double hydroxide. J. Colloid Interface Sci. 2018, 532, 474–484.
- Soltani, R.D.C.; Khorramabadi, G.S.; Khataee, A.R.; Jorfi, S. Silica nanopowders/alginate composite for adsorption of lead (II) ions in aqueous solutions. J. Taiwan Inst. Chem. Eng. 2014, 45, 973–980.
- Pandi, K.; Viswanathan, N. Synthesis of alginate bioencapsulated nano-hydroxyapatite composite for selective fluoride sorption. Carbohydr. Polym. 2014, 112, 662–667.
- Gopalakannan, V.; Viswanathan, N. Synthesis of magnetic alginate hybrid beads for efficient chromium (VI) removal. Int. J. Biol. Macromol. 2015, 72, 862–867.
- Barreca, S.; Orecchio, S.; Pace, A. The effect of montmorillonite clay in alginate gel beads for polychlorinated biphenyl adsorption: Isothermal and kinetic studies. Appl. Clay Sci. 2014, 99, 220–228.
- Do, X.-H.; Lee, B.-K. Removal of Pb2+ using a biochar–alginate capsule in aqueous solution and capsule regeneration. J. Environ. Manage. 2013, 131, 375–382.
- Zhuang, Y.; Yu, F.; Chen, J.; Ma, J. Batch and column adsorption of methylene blue by graphene/alginate nanocomposite: Comparison of single-network and double-network hydrogels. J. Environ. Chem. Eng. 2016, 4, 147–156.
- Wang, M.; Li, X.; Zhang, T.; Deng, L.; Li, P.; Wang, X.; Hsiao, B.S. Eco-friendly poly(acrylic acid)-sodium alginate nanofibrous hydrogel: A multifunctional platform for superior removal of Cu(II) and sustainable catalytic applications. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 228–241.
- Wang, Y.; Wang, W.; Wang, A. Efficient adsorption of methylene blue on an alginate-based nanocomposite hydrogel enhanced by organo-illite/smectite clay. Chem. Eng. J. 2013, 228, 132–139.
- Jiao, C.; Xiong, J.; Tao, J.; Xu, S.; Zhang, D.; Lin, H.; Chen, Y. Sodium alginate/graphene oxide aerogel with enhanced strength–toughness and its heavy metal adsorption study. Int. J. Biol. Macromol. 2016, 83, 133–141.
- Guo, J.; Zhang, Q.; Cai, Z.; Zhao, K. Preparation and dye filtration property of electrospun polyhydroxybutyrate–calcium alginate/carbon nanotubes composite nanofibrous filtration membrane. Sep. Purif. Technol. 2016, 161, 69–79.
- Karthik, R.; Meenakshi, S. Removal of Cr(VI) ions by adsorption onto sodium alginate-polyaniline nanofibers. Int. J. Biol. Macromol. 2015, 72, 711–717.
- Ely, A.; Baudu, M.; Kankou, M.O.S.O.; Basly, J.-P. Copper and nitrophenol removal by low cost alginate/Mauritanian clay composite beads. Chem. Eng. J. 2011, 178, 168–174.
- Hassan, A.F.; Abdel-Mohsen, A.M.; Elhadidy, H. Adsorption of arsenic by activated carbon, calcium alginate and their composite beads. Int. J. Biol. Macromol. 2014, 68, 125–130.
- Tan, W.S.; Ting, A.S.Y. Alginate-immobilized bentonite clay: Adsorption efficacy and reusability for Cu(II) removal from aqueous solution. Bioresour. Technol. 2014, 160, 115–118.
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb(II) removal using sodium alginate–carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr. Polym. 2016, 137, 402–409.
- Oladipo, A.A.; Gazi, M. Enhanced removal of crystal violet by low cost alginate/acid activated bentonite composite beads: Optimization and modelling using non-linear regression technique. J. Water Process Eng. 2014, 2, 43–52.
- Lezehari, M.; Baudu, M.; Bouras, O.; Basly, J.-P. Fixed-bed column studies of pentachlorophenol removal by use of alginate-encapsulated pillared clay microbeads. J. Colloid Interface Sci. 2012, 379, 101–106.
- Uyar, G.; Kaygusuz, H.; Erim, F.B. Methylene blue removal by alginate–clay quasi-cryogel beads. React. Funct. Polym. 2016, 106, 1–7.
- Aden, M.; Ubol, R.N.; Knorr, M.; Husson, J.; Euvrard, M. Efficent removal of nickel(II) salts from aqueous solution using carboxymethylchitosan-coated silica particles as adsorbent. Carbohydr. Polym. 2017, 173, 372–382.
- Privar, Y.; Shashura, D.; Pestov, A.; Modin, E.; Baklykov, A.; Marinin, D.; Bratskaya, S. Metal-chelate sorbents based on carboxyalkylchitosans: Ciprofloxacin uptake by Cu(II) and Al(III)-chelated cryogels of N-(2-carboxyethyl)chitosan. Int. J. Biol. Macromol. 2019, 131, 806–811.
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Self-assembly of binary oppositely charged polysaccharides into polyelectrolyte complex hydrogel film for facile and efficient Pb2+ removal. Chem. Eng. J. 2020, 388, 124189.
- Anne, J.M.; Boon, Y.H.; Saad, B.; Miskam, M.; Yusoff, M.M.; Shahriman, M.S.; Zain, N.N.M.; Lim, V.; Raoov, M. b-Cyclodextrin conjugated bifunctional isocyanate linker polymer for enhanced removal of 2,4-dinitrophenol from environmental waters. R. Soc. Open Sci. 2018, 5, 180942.
- Shabtai, I.A.; Mishael, Y.G. Polycyclodextrin-Clay Composites: Regenerable Dual-Site Sorbents for Bisphenol A Removal from Treated Wastewater. ACS Appl. Mater. Interfaces 2018, 10, 27088–27097.
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 2011, 281, 278–284.
- Nosrati, A.; Larsson, M.; Lindén, J.B.; Zihao, Z.; Addai-Mensah, J.; Nydén, M. Polyethyleneimine functionalized mesoporous diatomite particles for selective copper recovery from aqueous media. Int. J. Miner. Process. 2017, 166, 29–36.
- Bucatariu, F.; Schwarz, D.; Zaharia, M.; Steinbach, C.; Ghiorghita, C.-A.; Schwarz, S.; Mihai, M. Nanostructured polymer composites for selective heavy metal ion sorption. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125211.
- Tan, Y.Z.; Wu, D.; Lee, H.T.; Wang, H.; Honciuc, A.; Chew, J.W. Synthesis of ligand-carrying polymeric nanoparticles for use in extraction and recovery of metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 179–186.
- Privar, Y.; Malakhova, I.; Pestov, A.; Fedorets, A.; Azarova, Y.; Schwarz, S.; Bratskaya, S. Polyethyleneimine cryogels for metal ions sorption. Chem. Eng. J. 2018, 334, 1392–1398.
- Malakhova, I.; Privar, Y.; Parotkina, Y.; Mironenko, A.; Eliseikina, M.; Balatskiy, D.; Golikov, A.; Bratskaya, S. Rational Design of Polyamine-Based Cryogels for Metal Ion Sorption. Molecules 2020, 25, 4801.
- Chen, H.; Huang, M.; Liu, Y.; Meng, L.; Ma, M. Functionalized electrospun nanofiber membranes for water treatment: A review. Sci. Total Environ. 2020, 739, 139944.
- Pei, X.; Gan, L.; Tong, Z.; Gao, H.; Meng, S.; Zhang, W.; Wang, P.; Chen, Y. Robust cellulose-based composite adsorption membrane for heavy metal removal. J. Hazard. Mater. 2021, 406, 124746.
- Saad, D.M.; Cukrowska, E.M.; Tutu, H. Development and application of cross-linked polyethylenimine for trace metal and metalloid removal from mining and industrial wastewaters. Toxicol. Environ. Chem. 2011, 93, 914–924.
- Bediako, J.K.; Choi, J.-W.; Song, M.-H.; Lim, C.-R.; Yun, Y.-S. Self-coagulating polyelectrolyte complexes for target-tunable adsorption and separation of metal ions. J. Hazard. Mater. 2021, 401, 123352.
- Saad, D.M.G.; Cukrowska, E.M.; Tutu, H. Sulfonated cross-linked polyethylenimine for selective removal of mercury from aqueous solutions. Toxicol. Environ. Chem. 2012, 94, 1916–1929.
- Zvulunov, Y.; Ben-Barak-Zelas, Z.; Fishman, A.; Radian, A. A self-regenerating clay-polymer-bacteria composite for formaldehyde removal from water. Chem. Eng. J. 2019, 374, 1275–1285.
- Gemeay, A.H.; El-Halwagy, M.E.; Elsherbiny, A.S.; Zaki, A.B. Amine-rich quartz nanoparticles for Cu(II) chelation and their application as an efficient catalyst for oxidative degradation of Rhodamine B dye. Environ. Sci. Pollut. Res. 2021, 28, 28289–28306.
- Bucatariu, F.; Ghiorghita, C.-A.; Schwarz, D.; Boita, T.; Mihai, M. Layer-by-layer polyelectrolyte architectures with ultra-fast and high loading/release properties for copper ions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123704.
- Zaharia, M.-M.; Bucatariu, F.; Doroftei, F.; Loghin, D.-F.; Vasiliu, A.-L.; Mihai, M. Multifunctional CaCO3/polyelectrolyte sorbents for heavy metal ions decontamination of synthetic waters. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126084.
- Bucatariu, F.; Ghiorghita, C.-A.; Zaharia, M.-M.; Schwarz, S.; Simon, F.; Mihai, M. Removal and Separation of Heavy Metal Ions from Multicomponent Simulated Waters Using Silica/Polyethyleneimine Composite Microparticles. ACS Appl. Mater. Interfaces 2020, 12, 37585–37596.
- Harris, J.T.; McNeil, A.J. Localized hydrogels based on cellulose nanofibers and wood pulp for rapid removal of methylene blue. J. Polym. Sci. 2020, 58, 3042–3049.
- Zhu, J.; Shu, J.; Yue, X.; Su, Y. Hollow and porous octacalcium phosphate superstructures mediated by the polyelectrolyte PSS: A superior removal capacity for heavy metal and antibiotics. J. Mater. Sci. 2020, 55, 7502–7517.
- Ruiz, C.; Vera, M.; Rivas, B.L.; Sánchez, S.; Urbano, B.F. Magnetic methacrylated gelatin- g -polyelectrolyte for methylene blue sorption. RSC Adv. 2020, 10, 43799–43810.
- Deebansok, S.; Amornsakchai, T.; Sae-ear, P.; Siriphannon, P.; Smith, S.M. Sphere-like and flake-like ZnO immobilized on pineapple leaf fibers as easy-to-recover photocatalyst for the degradation of congo red. J. Environ. Chem. Eng. 2021, 9, 104746.
- Chong, W.H.; Ng, Q.H.; Lim, J.K.; Yeap, S.P.; Low, S.C. Study on the enhancement of colloidal stable poly(sodium 4-styrene sulfonate) coated magnetite nanoparticles and regeneration capability for rapid magnetophoretic removal of organic dye. J. Chem. Technol. Biotechnol. 2020, 95, 3093–3104.
- Bucatariu, F.; Ghiorghita, C.-A.; Dragan, E.S. Cross-linked multilayer films deposited onto silica microparticles with tunable selectivity for anionic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 53–60.
- Soares, S.F.; Simões, T.R.; António, M.; Trindade, T.; Daniel-da-Silva, A.L. Hybrid nanoadsorbents for the magnetically assisted removal of metoprolol from water. Chem. Eng. J. 2016, 302, 560–569.
- Fijałkowska, G.; Wiśniewska, M.; Szewczuk-Karpisz, K. Adsorption and electrokinetic studies in kaolinite/anionic polyacrylamide/chromate ions system. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125232.
- Fijałkowska, G.; Szewczuk-Karpisz, K.; Wiśniewska, M. Anionic polyacrylamide influence on the lead(II) ion accumulation in soil—The study on montmorillonite. J. Environ. Health Sci. Eng. 2020, 18, 599–607.
- Ghorai, S.; Sarkar, A.; Raoufi, M.; Panda, A.B.; Schönherr, H.; Pal, S. Enhanced Removal of Methylene Blue and Methyl Violet Dyes from Aqueous Solution Using a Nanocomposite of Hydrolyzed Polyacrylamide Grafted Xanthan Gum and Incorporated Nanosilica. ACS Appl. Mater. Interfaces 2014, 6, 4766–4777.
- Zhang, H.; Yang, H.; Sarsenbekuly, B.; Zhang, M.; Jiang, H.; Kang, W.; Aidarova, S. The advances of organic chromium based polymer gels and their application in improved oil recovery. Adv. Colloid Interface Sci. 2020, 282, 102214.
- Bao, S.; Yang, W.; Wang, Y.; Yu, Y.; Sun, Y. Highly efficient and ultrafast removal of Cr(VI) in aqueous solution to ppb level by poly(allylamine hydrochloride) covalently cross-linked amino-modified graphene oxide. J. Hazard. Mater. 2021, 409, 124470.
- Liu, H.; Li, S.; Sun, D.; Chen, Y.; Zhou, Y.; Lu, T. Layered graphene nanostructures functionalized with NH2-rich polyelectrolytes through self-assembly: Construction and their application in trace Cu(ii) detection. J. Mater. Chem. B 2014, 2, 2212.
- Lim, S.; Park, H.; Kim, J.H.; Yang, J.; Kwak, C.; Kim, J.; Ryu, S.Y.; Lee, J. Polyelectrolyte-grafted Ti 3 C 2 -MXenes stable in extreme salinity aquatic conditions for remediation of contaminated subsurface environments. RSC Adv. 2020, 10, 25966–25978.
- Baimenov, A.Z.; Berillo, D.A.; Moustakas, K.; Inglezakis, V.J. Efficient removal of mercury (II) from water by use of cryogels and comparison to commercial adsorbents under environmentally relevant conditions. J. Hazard. Mater. 2020, 399, 123056.
- Elgueta, E.; Rivas, B.L.; Mancisidor, A.; Núñez, D.; Dahrouch, M. Hydrogels derived from 2-hydroxyethyl-methacrylate and 2-acrylamido-2-methyl-1-propanesulfonic acid, with ability to remove metal cations from wastewater. Polym. Bull. 2019, 76, 6503–6528.
- Rahman, N.; Varshney, P. Effective removal of doxycycline from aqueous solution using CuO nanoparticles decorated poly(2-acrylamido-2-methyl-1-propanesulfonic acid)/chitosan. Environ. Sci. Pollut. Res. 2021, 28, 43599–43617.
- Dao, T.H.; Vu, T.Q.M.; Nguyen, N.T.; Pham, T.T.; Nguyen, T.L.; Yusa, S.I.; Pham, T.D. Adsorption characteristics of synthesized polyelectrolytes onto alumina nanoparticles and their application in antibiotic removal. Langmuir 2020, 36, 13001–13011.
- Atta, A.M.; Ezzat, A.O.; Moustafa, Y.M.; Sabeela, N.I.; Tawfeek, A.M.; Al-Lohedan, H.A.; Hashem, A.I. Synthesis of New Magnetic Crosslinked Poly (Ionic Liquid) Nanocomposites for Fast Congo Red Removal from Industrial Wastewater. Nanomaterials 2019, 9, 1286.
- Lin, S.; Shi, M.; Wang, Q.; Yang, J.; Zhang, G.; Liu, X.; Fan, W. Transport of Cu2+ in Unsaturated Porous Medium with Humic Acid/Iron Oxide Nanoparticle (Fe3O4) Amendment. Water 2021, 13, 200.
- Makrygianni, M.; Christofili, A.; Deimede, V. Emulsion-templated macroporous ammonium based polymers: Synthesis and dye adsorption study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125634.
- Beaugeard, V.; Muller, J.; Graillot, A.; Ding, X.; Robin, J.-J.; Monge, S. Acidic polymeric sorbents for the removal of metallic pollution in water: A review. React. Funct. Polym. 2020, 152, 104599.
- Nnadozie, E.C.; Ajibade, P.A. Multifunctional Magnetic Oxide Nanoparticle (MNP) Core-Shell: Review of Synthesis, Structural Studies and Application for Wastewater Treatment. Molecules 2020, 25, 4110.
- Yang, F.; Du, Q.; Sui, L.; Cheng, K. One-step fabrication of artificial humic acid-functionalized colloid-like magnetic biochar for rapid heavy metal removal. Bioresour. Technol. 2021, 328, 124825.
- Li, K.; Li, P.; Cai, J.; Xiao, S.; Yang, H.; Li, A. Efficient adsorption of both methyl orange and chromium from their aqueous mixtures using a quaternary ammonium salt modified chitosan magnetic composite adsorbent. Chemosphere 2016, 154, 310–318.
- Yao, Y.; Mi, N.; He, C.; Zhang, Y.; Yin, L.; Li, J.; Wang, W.; Yang, S.; He, H.; Li, S.; et al. A novel colloid composited with polyacrylate and nano ferrous sulfide and its efficiency and mechanism of removal of Cr(VI) from Water. J. Hazard. Mater. 2020, 399, 123082.
More